Abstract
Cardiac dynamics exhibit complex variability characterized by scale-invariant and nonlinear temporal organization related to the mechanism of neuroautonomic control, which changes with physiologic states and pathologic conditions. Changes in sleep regulation during sleep stages are also related to fluctuations in autonomic nervous activity. However, the interaction between sleep regulation and cardiac autonomic control remains not well understood. Even less is known how this interaction changes with age, as aspects of both cardiac dynamics and sleep regulation differ in healthy elderly compared to young subjects. We hypothesize that because of the neuroautonomic responsiveness in young subjects, fractal and nonlinear features of cardiac dynamics exhibit a pronounced stratification pattern across sleep stages, while in elderly these features will remain unchanged due to age-related loss of cardiac variability and decline of neuroautonomic responsiveness. We analyze the variability and the temporal fractal organization of heartbeat fluctuations across sleep stages in both young and elderly. We find that independent linear and nonlinear measures of cardiac control consistently exhibit the same ordering in their values across sleep stages, forming a robust stratification pattern. Despite changes in sleep architecture and reduced heart rate variability in elderly subjects, this stratification surprisingly does not break down with advanced age. Moreover, the difference between sleep stages for some linear, fractal, and nonlinear measures exceeds the difference between young and elderly, suggesting that the effect of sleep regulation on cardiac dynamics is significantly stronger than the effect of healthy aging. Quantifying changes in this stratification pattern may provide insights into how alterations in sleep regulation contribute to increased cardiac risk.
Keywords: Aging, cardiac dynamics, detrended fluctuations, fractal, nonlinear
I. Introduction
Physiological systems under spatially and temporally integrated neural regulation, such as the cardiac system, display continuous and seemingly erratic fluctuations [1], [2]. However, these “noisy” fluctuations in the intervals between consecutive heartbeats exhibit a temporal organization that is self-similar across a broad range of time scales, and is characterized by long-range power-law correlations [3]-[5] and nonlinear properties [6]-[9], indicating a complex fractal and multifractal structure [10], [11]. These fractal and nonlinear scaling features of cardiac dynamics change under sympathetic and parasym-pathetic blockade [12]-[14], and break down with pathologic conditions [15], [16]. The scaling exponent associated with these power-law correlations has been shown to be a sensitive marker for predicting mortality [17]. Moreover, the fractal and nonlinear organization of heartbeat fluctuations varies for different physiologic states, such as exercise and rest [18]-[20], wake and sleep [21]-[25], and with the circadian rhythm [26]-[28]. These findings indicate that the scale-invariant temporal organization of heartbeat fluctuations reflects the underlying mechanism of cardiac neuroautonomic regulation that changes with different physiologic states and pathologic conditions.
Recent studies have reported reduced heart rate variability [29] and alterations of the fractal and nonlinear properties of heartbeat fluctuations with healthy aging [30], [31], and these changes in cardiac dynamics have been associated with higher cardiac risk in elderly [26]. Sleep dynamics have also been found to change with advanced age—e.g., elderly subjects typically have more fragmented sleep with frequent arousals and reduced duration of deep sleep [32]. Sympathetic nerve activity measurements as well as spectral analysis of heart rate variability across sleep stages show dominant parasympathetic control during non-REM sleep, and activation of the sympathetic nervous system during REM and wake [33], [34] leading to different morphology in the heartbeat interval time series across sleep stages (Fig. 1). The intricate mechanism of interaction between sleep regulation and cardiac control, and whether this interaction declines with age, however, remains not well understood.
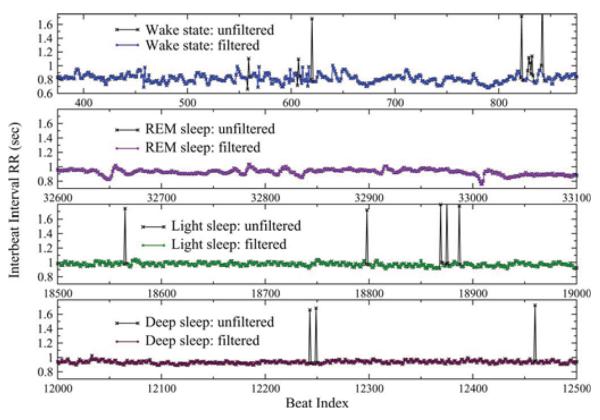
Fig. 1.
Representative recordings of segments of 500 RR intervals between consecutive normal heartbeats for a healthy young subject during deep sleep, light sleep, rapid eye movement (REM) sleep, and an intermediate wake state (from bottom to top), part of an 8 h polysomnographic recording. A small percentage of artefacts (e.g., spikes marked with ×) have been filtered before the actual analysis (see Section II). Data show ×(a) gradual decrease of the standard deviation, σRR (also denoted as SDNN) from wake to REM, to light, to deep sleep (see Fig. 2), and (b) that the heartbeat signal is more homogeneous in deep sleep compared to light sleep, REM sleep, and wake where it exhibits more irregular fluctuations (see Fig. 3).
To test how sleep dynamics affect the temporal fractal and nonlinear organization of heartbeat fluctuations across time scales, we compare heartbeat dynamics of healthy young and healthy elderly subjects for different sleep stages. One possible hypothesis is that there will be a well-pronounced stratification pattern in the values of static (e.g., mean, standard deviation) and dynamic (scaling and nonlinear) measures of heartbeat dynamics across sleep stages, and that this stratification will be present in both healthy young and healthy elderly subjects. An alternative hypothesis is that while there are significant differences in cardiac control with sleep stage transitions for young subjects, the static and dynamic features of heartbeat fluctuations in healthy elderly subjects will remain unchanged across sleep stages due to age-related loss of cardiac variability and decline in neuroautonomic responsiveness to changes in sleep regulation. Testing these hypotheses will address the question how sleep regulation influences cardiac dynamics, and whether this influence changes with healthy aging, as sleep disorders have been associated with increased cardiovascular risk [35]-[37]. Probing for changes in the complex temporal organization of heartbeat fluctuations during different sleep stages can help elucidate the mechanisms through which alterations of sleep regulation in elderly may contribute to cardiac risk. This approach is in line with earlier studies where combinations of heart rate variability (HRV) measures as outlined in the HRV task force 1996 [38] were utilized to increase their power in stratifying age- and disease-related cardiac risk [39].
In order to characterize heartbeat fluctuations during different sleep stages, as well as the effect of healthy aging on cardiac regulation, we employ static measures, such as the standard deviation σRR (also denoted as SDNN) of the consecutive RR interbeat intervals and the standard deviation σΔRR of the inter-beat increments ΔRR (also denoted as RMSSD). Further, we also analyze dynamic measures, such as the scaling exponent α which quantifies the long-term fractal correlations in the RR time series, as well as the scaling exponents αmag and αsign that quantify the long-term nonlinear and linear properties in the magnitude and sign of ΔRR, respectively [40]-[42].
Here, we demonstrate that key fractal, scale-invariant and nonlinear characteristics of heartbeat dynamics do not change and remain stable with advanced age, indicating that fundamental features of cardiac complexity do not break down and are not lost with healthy aging. Moreover, we find that key independent static and dynamic measures of cardiac control change significantly during different sleep stages, and exhibit a previously unknown stratification pattern similar in both young and elderly subjects. This pattern does not break down with aging, despite changes in sleep architecture and reduced heart rate variability in elderly subjects. Further, the difference in these measures between sleep stages far exceeds the difference between young and elderly subjects, suggesting that the effect of sleep regulation on cardiac dynamics may be significantly stronger than the effect of healthy aging.
II. Data and Methods
A. Subjects
We analyze 26 polysomnographic recordings obtained from 13 young subjects (average age 33.3 years; seven males and six females) during two consecutive nights of sleep in controlled laboratory conditions from the SIESTA project [43]. Continuous EEG and ECG signals were recorded during the habitual sleep periods of ≈8 h. Sleep stages were annotated in 30 s epocs following standard procedures [44].
We compare the results of the young group from the SIESTA database with healthy elderly subjects from the Sleep Heart Health Study (SHHS) database. Full details of the SHHS study design and cohort are provided in [45] and [46]. Details about obtaining the ECG and polysomnographic recordings are outlined in [47]. Sleep apnea episodes were annotated, and heart rate data during apnea (obstructive and central) were excluded from our analysis. From the SHHS database, we selected a subset of 24 subjects (six males; 18 females) average age of 78.4 years (youngest 72 years; oldest 89 years). These subjects were selected by age, body weight, cardiac conditions, sleep apnea, sleep disorders, and minimum drug intake. Subject with highest age and good health were selected. There is a selection bias towards healthy elderly subject meaning that the selected group is not representative of the average population with the same average age. The purpose of this selection is to separate the effect of healthy aging from the effects of pathologic deviations which become more pronounced with age.
B. Detrended Fluctuation Analysis
The detrended fluctuation analysis (DFA) method [48] has been developed to quantify dynamic characteristics of physiologic fluctuations embedded in nonstationary physiologic signals. Compared with traditional correlation analyses, such as autocorrelation, power spectrum analysis, and Hurst analysis, the advantage of the DFA method is that it can accurately quantify the correlation property of signals masked by polynomial trends, and is described in detail in [49]-[53]. The DFA method quantifies the detrended fluctuation function F (n) of a signal at different time scales n. A power-law functional form F (n) ~ nα indicates the presence of self-similar fractal organization in the fluctuations. The parameter α, called scaling exponent, quantifies the correlation properties of the heartbeat signal: if α = 0.5, there are no correlations (white noise); if α = 1.5 the signal is a random walk (Brownian motion); if 0.5 < α < 1.5 there are positive correlations, where large heartbeat intervals are more likely to be followed by large intervals (and vice versa for small heartbeat intervals); if α < 0.5 the signal is anticorrelated where large heartbeat intervals are likely to be followed by small intervals (with stronger anticorrelations when α is closer to 0).
One advantage of the DFA method is that it can quantify signals with α > 1, which cannot be done using the traditional autocorrelation and R/S analyses [54], as well as signals with strong anticorrelations [49]. In contrast to the conventional methods, the DFA method avoids spurious detection of apparent long-range correlations that are an artefact of nonstationary [55]. Thus, the DFA method is able to detect subtle temporal structures in highly heterogeneous physiologic time series.
An inherent limitation of the DFA analysis is the maximum time scale nmax for which the fluctuation function F(n) can be reliably calculated. To ensure sufficient statistics at large scales it was shown that nmax should be chosen nmax ≤ N/6, where N is the length of the signal [49], [52], [56].
C. Magnitude and Sign Analyses
Since the DFA method quantifies linear fractal characteristics related to two-point correlation, we employ the magnitude and sign analyses (MSA) method to probe for long-term nonlinear properties in the data. Specifically, it has been shown that signals with identical self-similar temporal organization, quantified by the DFA scaling exponent α, can exhibit very different nonlinear properties captured by the MSA method [40].
The MSA method [40]-[42] consists of the following steps: 1) given a RRi series we obtain the increment series ΔRRi = RRi+1 − RRi; 2) we decompose the increment series into a magnitude series |ΔRR| and a sign series sign(ΔRR); 3) because of limitations in the accuracy of the DFA method for estimating the scaling exponents of anticorrelated signals (α < 0.5), we integrate the magnitude and sign series; 4) we perform the DFA scaling analysis; 5) to obtain the scaling exponents for the magnitude and sign series, we measure the slope of F (n)/n on a log–log plot, where F(n) is the fluctuation function and n is the time scale.
This approach is sensitive to nonlinear features in signals encoded in the Fourier phases [57]. We find that positive correlations in the magnitude series (αmag > 0.5) are a reliable marker of long-term nonlinear properties, which are represented by the scaling behavior over a range of time scales of moments other than the second moment and relate to the width of the multifractal spectrum [42]. Thus, the MSA is a complementary method to the DFA, because it can distinguish physiologic signals with identical long-range fractal correlations, as quantified by the DFA method, but different nonlinear properties and different temporal organization for the sign (ΔRR) series.
D. Data Processing
From the annotated ECG recordings, only the intervals RR between consecutive normal beats are considered. Intervals containing nonnormal beats are disregarded. The RR time series are then segmented corresponding to sleep stages. Within each data segment, corresponding to a given sleep stage, outliers due to missed beat detection in the ECG (which would give rise to erroneously large intervals) are marked as gaps of size G and taken out. Heartbeat RR intervals are identified as outliers when outside the interval [0.5 s; 1.55 s] or when an interbeat increment ΔRR > 0.35 s (illustrated in Fig. 1). This results in removing on average 1.2% and no more than 5% of the data points for each record. Segments of RR intervals were concatenated when separated by gaps smaller than G = 70, 35, and 10 heartbeats for the analysis of the time series of RR, |ΔRR|, and sign (ΔRR), respectively. Segments separated by gaps larger than G were analyzed separately without concatenation.
We note that cutting out gaps in positively correlated fractal signals, such as the RR time series, does not effect the DFA and MSA scaling behavior at intermediate and large time scales [51]. For large values of G, in general, more segments of data are concatenated, which allows to explore larger time scales n in the DFA and MSA scaling analyses. We choose different values of G for the RR, |ΔRR|, and sign (ΔRR) time series as we estimate α, αmag, and αsign at large (n ∈ [50, 250]), intermediate (n ∈ [10, 150]), and short (n ∈ [7, 13] time scales, respectively (where n is in beat numbers). For larger values of G larger time scales n in the DFA and MSA scaling analyses are affected by the concatenation of adjacent data segments. The values for G are chosen conservatively small—our tests show that choosing larger values of G does not affect the scaling results for the fitting ranges mentioned earlier.
Because sleep stages were annotated in epocs of 30 s such coarse-graining leads to inaccuracy in determining the actual positions of sleep stage transitions. To assure that this inaccuracy does not affect our analysis, we disregard 40 heartbeats on each side of every sleep stage transition in the polysomnographic recordings. To avoid masking effects of occasional periodic breathing and sleep apnea episodes, which strongly affect cardiac dynamics [58]-[60], we disregard sections of heart rate data corresponding to central and obstructive apnea episodes, including additional 60 heartbeats before the beginning and after the end of each apnea episode to eliminate transient apnea effects.
Data segments separated by gaps due to sleep stage or apnea events transitions are never concatenated, and are always analyzed separately. For example, segments of heartbeat data during separate REM sleep stages throughout the night are treated separately in our analysis. Thus, when calculating the standard deviation σRR and σΔRR for these separate segments (each of which may have a different mean value), we subtract the global average for all segments (Fig. 2 and Table I). We note that subtracting the average in each segment separately leads to qualitatively the same results with the same relative difference in σRR and σΔRR between young and elderly and to the same stratification patterns across sleep stages as shown in Fig. 2.
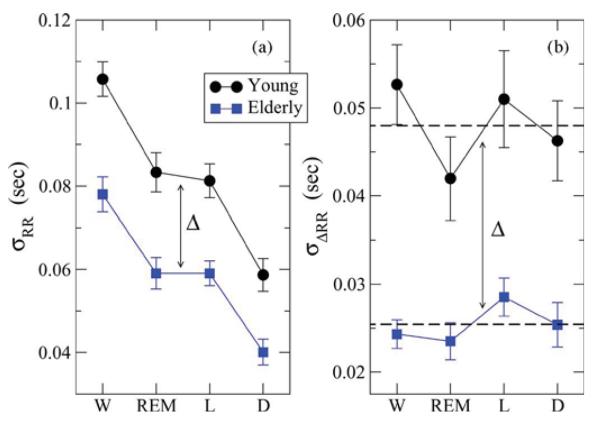
Fig. 2.
Static measures of heart rate variability across sleep stages for young and elderly. (a) Group average standard deviation of the interbeat RR intervals σRR (SDNN) exhibits a statistically significant stratification across sleep stages for both young and elderly (for young: p-value = 2.4 × 10−8 between wake and deep sleep, and p-value = 2.4 × 10−3 between REM and deep sleep). (b) Group average standard deviation σΔRR (RMSSD) of the interbeat increments ΔRR does not show a statistically significant dependence of sleep stages for both young and elderly. Average values across all stages are indicated by dashed lines for each group. For elderly both σRR and σΔRR exhibit a statistically significant (see Table I) vertical shift of Δ ≈ 0.02 s across all sleep stages. Error bars represent the standard error.
Table I.
Static and Scale-Invariant Dynamic Characteristics of Heartbeat Fluctuations for all Sleep Stages in Young and Elderly
|
static |
dynamic |
||||
|---|---|---|---|---|---|
| σRR | σΔRR | α | αmag | αsign | |
| Wake | |||||
| Young | .11±.03 | .05±.02 | .97±.14 | .65±.08 | .29±.22 |
| Elderly | .08±.03 | .02±.01 | 1.03±.13 | .60±.18 | .09±.17 |
| p-value | 1×10−4 | 1×10−9 | .075 | 0.20 | 1.6×10−4 |
| REM | |||||
| Young | .08±.03 | .04±.02 | .89±.13 | .69±.06 | .27±.16 |
| Elderly | .06±.02 | .02±.01 | 1.02±.18 | .62±.09 | .06±.18 |
| p -value | 8.9×10−4 | 2.8×10−4 | .015 | .003 | 1.6×10−5 |
| Light | |||||
| Young | .08±.03 | .05±.03 | .74±.17 | .61±.06 | .09±.18 |
| Elderly | .06±.02 | .03±.01 | .63±.15 | .59±.08 | .09±.16 |
| p-value | 2×10−4 | 7.4×10−5 | .011 | 0.22 | 0.97 |
| Deep | |||||
| Young | .06±.03 | .05±.02 | .61±.21 | .58±.l1 | −.04±.19 |
| Elderly | .04±.02 | .03±.02 | .57±.17 | .55±.12 | −.03±.16 |
| p-value | .002 | 8.1×10−5 | .465 | 0.36 | 0.77 |
Subjects for which the DFA maximum scale nmax < 220, 110, and 12 were not included in the calculation of the group average α, αmag, and αsign, respetively. (nmax ≡ N/6, where N is the signal length)
E. Statistical Tests
We apply the Student's t-test to test the statistical significance of differences between individual pairs of conditions (age or sleep stage). Further, we use the multiple analysis of variance (MANOVA) with 2 × 4 design for the two groups of young and elderly and for four physiologic states, wake, REM, light, and deep sleep with subjects as nested random effects (JMP version 5.1.2 software analysis package, SAS Institute, Cary, NC).
III. Results
A. Variability in Heartbeat Intervals and their Increments
To test whether heart rate variability changes according to sleep stage transitions (Fig. 1), and whether this variability is reduced in healthy elderly subjects for certain sleep stages, we first estimate for each subject and for each sleep stage the standard deviation of the RR interbeat intervals σRR (SDNN) and the standard deviation of the increments in the consecutive interbeat intervals σΔRR (RMSSD). For the group of healthy young subjects, we find that the group average σRR is highest for wake with , lower for REM and light sleep with , and lowest for deep sleep with A Student's t-test shows a significant difference between and for the young group, with a p-value = 2.4 × 10−8. A statistically significant difference, with a p-value = 2.4 × 10−3, we find also when comparing and with .
Compared to the young subjects, for the group healthy elderly subjects, we observe a very similar stratification of the values of σRR for the different sleep stages. Specifically, we find that for the elderly subjects is significantly different from with p-value = 2 × 10−3—very similar to the statistical significance with p-value = 6 × 10−3 of the difference between and for the young group [see Fig. 2(a)]. Further, we find that for the elderly subjects is statistically different from with p-value = 6.4 × 10−5. These observations demonstrate that the variability in cardiac dynamics in elderly subjects exhibits a remarkably similar stratification pattern across sleep stages as the one observed for young subjects [Fig. 2(a)].
We note that the elderly group average values for σRR are significantly lower (with a shift of Δ ≈ 0.02 s) for all sleep stages compared to the young group. The observed identical shift in σRR for all sleep stages [Fig. 2(a)], indicates significantly reduced heart rate variability in elderly subjects, in agreement with earlier studies [35], [59], [61], [62]. However, the response of cardiac dynamics to sleep stage transitions in healthy elderly subjects remains the same as in young subjects, as evident from the similar stratification patterns in σRR for both groups. A MANOVA-test shows that σRR changes significantly (p-value < 0.0001) with age and also with sleep stage (p-value < 0.0001).
We next consider σΔRR, another standard static measure which quantifies the variability in consecutive beat-to-beat increments. In contrast to σRR, we do not find a stratification in the values of σΔRR for different sleep stages in both young and elderly subjects [Fig. 2(b)]. However, we again observe a significant reduction in the values of σΔRR with a shift of Δ ≈ 0.02s for elderly subjects compared to the young group, consistent with earlier reports of lower heart rate variability in elderly [31]. This is also confirmed by a MANOVA-test which shows that σΔRR changes significantly (p-value < 0.0001) with age and but not with sleep stage (p-value =0.23).
B. Fractal Temporal Correlations
We next test whether the temporal organization of heartbeat fluctuations responds to sleep stage transitions, and whether it changes with advanced age. Previous studies have shown that heartbeat fluctuations exhibit self-similar fractal organization characterized by power-law correlations over a broad range of time scales from seconds to many hours [3]-[5]. The scaling exponent α quantifying these power-law correlations has been found to change significantly with transitions from sleep to wake state [21] and with the circadian rhythm [26], [27], indicating a complete reorganization of the fractal temporal structure in cardiac dynamics across all scales.
To probe how the scale-invariant organization in the interbeat intervals RR changes across sleep stages for both young and elderly subjects, we apply the DFA scaling analysis. We find a very pronounced difference in the scaling behavior of the fluctuation function F (n) for the different sleep stages (Fig. 3) with significantly different values for the DFA scaling exponent α [Fig. 4(a)]. Specifically, we find a clear stratification in the group average scaling exponent α, with highest value for the wake state, followed by a lower value for REM sleep and even lower values for light and deep sleep (Fig. 4(a) and Table I). Employing a Student's t-test for both young and elderly subjects we find a statistically significant difference between the group average exponent αW for wake and αD for deep sleep with p-value = 2 × 10−7 and p-value = 10−17, respectively (see Table I). We also find a statistically significant difference between αREM for REM sleep and αL for light sleep with p-value = 9 × 10−4 and p-value = 6.5 × 10−12, respectively for the young and elderly groups. Performing a MANOVA-test we find a significant change in αRR across sleep stages (p-value < 0.0001) but an insignificant change with age (p-value = 0.93).
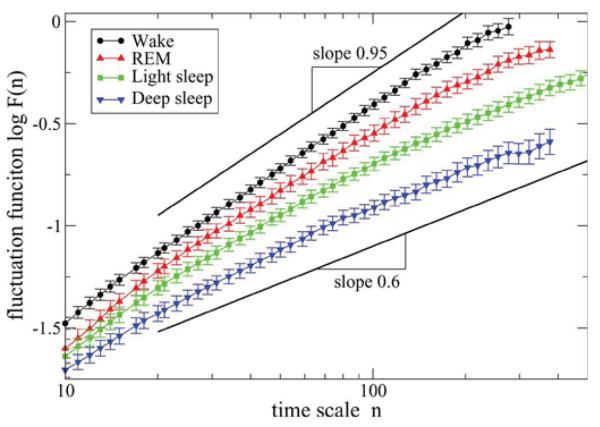
Fig. 3.
Log–log plots of the group average fluctuation functions F (n) versus time scale n (measured in beats) for group of young subjects using DFA-2 analysis of the heartbeat intervals RR during different sleep stages. Scaling curves F (n) for all sleep stages indicate presence of long-range power-law correlations characterized by scaling exponent α over a broad range of time scales. The exponent varies from α ≈ 1 for wake (indicating strong correlations) to α ≈ 0.6 for deep sleep (week correlations) suggesting different fractal temporal organization in heartbeat fluctuations during different sleep stages. Error bars represent the standard error. The scaling curves F (n) are vertically offset for clarity. As the durations of different stages vary among subjects and thus the maximum time scale nmax of the DFA analysis may be different for different subjects, only F (n) data points averaged over at least 14 recordings are presented for each stage.
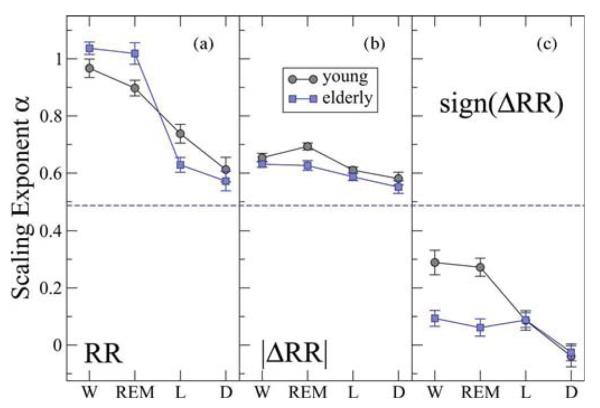
Fig. 4.
Scale-invariant dynamic measures of heart rate variability across sleep stages for young and elderly. (a) Group average scaling exponent α for the time series of heartbeat intervals RR exhibits a pronounced and statistically significant stratification across sleep stages for both young and elderly. High degree of fractal correlations is observed during wake an REM with α ≈ 1 and much weaker correlations during light and deep sleep with α ≈ 0.6. Uncorrelated (white noise) behavior with α = 0.5 is indicated by a dashed line. (b) Group average scaling exponent αmag for the time series of |ΔRR|. Both young and elderly exhibit long-term nonlinear behavior with αmag > 0.5 and with a very similar stratification pattern across all stages: higher αmag-values and higher degree of nonlinearity during wake and REM; lower αmag-values and closer to linear behavior during light and deep sleep. (c) Group average scaling exponent αsign for the time series of sign(ΔRR). A statistically significant stratification pattern with decreasing values of αsign across all stages is observed for the young group. Elderly subjects exhibit a different pattern with significantly lower values of αsign during wake and REM (see Table I). Data are obtained after fitting the DFA scaling curves F (n) for each individual recording in the range of time scales: n ∈ [50, 250] beats for α; n ∈ [10, 150] for αmag; and n ∈ [7, 13] for αsign. Error bars represent the standard error.
Our findings indicate 1) a significant change in the fractal temporal organization of heartbeat fluctuations in response to transitions across sleep stages, in agreement with [22] and [24], and surprisingly 2) that fractal correlations within each sleep stage remain robust and do not significantly change with healthy aging.
C. Magnitude and Sign Correlation
Scale-invariant fractal processes with identical long-range power-law correlation may exhibit very different dynamics for the magnitude and sign of their fluctuations [40]. It has been found that the information contained in the temporal organization of the magnitude and the sign time series is independent and complementary to the correlation properties of the original time series [41], [42]. For cardiac dynamics of healthy subjects during routine daily activity, it was shown that heartbeat intervals exhibit power-law correlations at intermediate and large time scales characterized by a scaling exponent α ≈ 1 [15], while at the same time scales the magnitude series of the increments ΔRR in the consecutive heartbeat intervals is characterized by αmag ≈ 0.75 [40]. Further, while the correlations in RR reflect the linear properties of cardiac dynamics, the temporal structure in the magnitude of heartbeat increments ΔRR relates to the nonlinear properties encoded in the Fourier phases [42], [57]. For certain pathologic conditions, such as congestive heart failure, which are associated with loss of nonlinearity in cardiac dynamics [6], [9], [11], previous studies have reported breakdown of the nonlinear multifractal spectrum [10] and reduced scaling exponent αmag [40] related to loss of Fourier-phase correlations.
To probe how the long-term nonlinear properties of heartbeat fluctuations change across sleep stages, we investigate the correlations in the magnitude of the interbeat interval increments ΔRR. For both groups of young and elderly subjects we find that for all sleep stages, the scaling exponent αmag is significantly higher than 0.5, indicating nonlinear dynamics during sleep [Fig. 4(b)]. We also find that both young and elderly subjects exhibit a clear stratification in the group average values of αmag across all sleep stages (although not so pronounced as in α). In particular, we find highest values for αmag during wake, followed by lower values during REM and light sleep, and lowest αmag-values during deep sleep (Fig. 4(b) and Table I), in agreement with [23].
Our results indicate a nonlinear behavior, consistent for both young and elderly subjects, where cardiac dynamics exhibit higher degree of nonlinearity during wake and REM sleep, and closer to linear behavior during light and deep sleep [Fig. 4(b)]. Furthermore, based on a paired Student's t-test, for the elderly subjects, we find a slight but not statistically significant shift to lower αmag-values for all sleep stages compared to the young group (Table I). Applying a MANOVA-test, we find a p-value = 0.005 with age and a significant change in αmag across sleep stages (p-value =0.0002).
For the sign of the interbeat increment ΔRR time series in young subjects we again find a clear and significant stratification in the values of the scaling exponent αsign (Fig. 4(c) and Table I). In particular, we find highest αsign-values during wake, followed by REM and light sleep, and lowest αsign-values during deep sleep—a stratification pattern very similar to the one we observe for σRR , α, and αmag. This indicates stronger anticorrelations in the sign (ΔRR) time series during light and deep sleep, and weaker anticorrelations during REM and wake. This stratification pattern is in contrast to the behavior we find for the elderly subjects, where there is no signifi-cant difference between wake, REM, and light sleep [Fig. 4(c)]. The values of αsign during light and deep sleep are almost the same for both young and elderly. In addition, we observe a significant difference between young and elderly subjects during wake (with a p-value = 1.6 × 10−4) and during REM (with a p-value = 1.6 × 10−5), with elderly subjects characterized by lower values for the exponent αsign [Fig. 4(c)]. The result of a MANOVA-test indicates a statistical significant difference between the two age groups (p-value = 0.0001) and a significant difference across sleep stages (p-value = 0.0001). These results suggests that the difference between young and elderly subjects is most pronounced in the sign (ΔRR) time series during wake and REM with stronger anticorrelations for elderly.
IV. Discussion
Our results show that key static and dynamic measures of heart rate variability exhibit a pronounced and statistically significant stratification pattern across sleep stages. We find a remarkably similar stratification pattern for both static and dynamic measures, with consistently higher values during wake, lower values during REM and light sleep, and lowest during deep sleep. This stratification of static and dynamic measures is surprisingly robust, as we observe very similar patterns for both young and elderly subjects, despite significantly reduced heart rate variability with age (Table I) [31], [35]. These results indicate that the influence of sleep regulation on the autonomic cardiac activity does not break down with progressive healthy aging.
A. Static Measures
We find that the standard deviation σRR of the heartbeat intervals, a static measure of cardiac dynamics, decreases significantly when comparing wake, REM, light, and deep sleep (Fig. 2). This indicates a strong responsiveness of cardiac dynamics to changes in sleep regulation across different sleep stages. The coupling between sleep and cardiac control appears to be robust, as we find the same stratification pattern in σRR for both healthy young and elderly subjects (Fig. 2). While the stratification patterns in σRR are the same for the young and the elderly groups, there is a significant vertical shift of size Δ ≈ 0.02 s to lower values of σRR in elderly subjects, consistently across all sleep stages [Fig. 2(a)]. These findings are in agreement with earlier reports of reduced heart rate variability with healthy aging during REM and non-REM sleep [59], [62]. However, the existence of a specific stratification pattern in the values of σRR, which remains unchanged with advanced age, has not been previously reported. We note, that for both young and elderly groups the difference in the group average standard deviation between wake and deep sleep s is approximately twice larger than the vertical shift Δ we find between young and elderly. These results indicate that the effect of sleep regulation on heart rate variability, as measured by 〈σRR〉, is stronger than the effect of aging (average age difference of 45 years between the young and elderly groups).
The pronounced stratification pattern we find in σRR across sleep stages relates to reduction in sympathetic tone during light and deep sleep compared to wake and REM [33], [34]. As the sympathetic activity is represented by the low-frequency range in the heart rate power spectrum [34], [63], bursts of sympathetic tone and parasympathetic withdrawal lead to increased nonstationarity of the interbeat interval time series characterized by higher values of σRR during wake and REM. With gradual decrease of sympathetic tone during light and deep sleep the degree of nonstationarity also decreases, and therefore, σRR is reduced. This is also observed for the group of healthy elderly subjects, however, with lower values of σRR for all sleep stages due to reduced parasympathetic tone in elderly.
In contrast to σRR, for the standard deviation σΔRR of the increments in the consecutive heartbeat intervals ΔRR, another static measure, we do not find a significant change across sleep stages for both young and elderly subjects. This measure is insensitive to nonstationarity in the interbeat time series as it filters out low-frequency trends associated with sympathetic activity. Thus, σΔRR does not capture the different degree of nonstationarity related to changes in the level of sympathetic activity during different sleep stages. However, σΔRR reflects the high-frequency variability in the interbeat time series related to parasympathetic activity. For the elderly subjects, we find a significant reduction of Δ ≈ 0.025 s in the values of σΔRR for all sleep stages, indicating a reduction in parasympathetic tone with aging (Fig. 2(b) and Table I).
There is a strong positive correlation between σRR and low-frequency heart rate variability, as well as between σΔRR and high-frequency variability [64]. As σΔRR represents high-frequency parasympathetic inputs and filters out low-frequency sympathetic inputs, the fact that we do not observe a significant reduction in σΔRR between REM and deep sleep suggests that parasympathetic tone does not significantly change. This, in turn, suggests that the statistically significant drop we find in σRR between REM and deep sleep in both healthy young and elderly subjects is due to a significant reduction of sympathetic activity. On the other hand, a similar drop of Δ ≈ 0.02 s between σRR for the young and σRR for the elderly subjects during REM must be caused by suppression of parasympathetic tone in elderly, as we also find a similar drop of Δ ≈ 0.02 s in σΔRR, which is a measure sensitive only to parasympathetic tone. Thus, while both σRR and σΔRR are static measures, they reflect fundamentally different aspects of cardiac control: while σRR captures both sympathetic and parasympathetic activity, and thus changes across sleep stages as well as with advanced age, the complementary measure σΔRR is only sensitive to changes in the parasympathetic activity, and thus changes only with age.
B. Dynamic Measures
Our results demonstrate that dynamic measures, such as α, αmag, and αsign, which probe the temporal fractal and nonlinear organization in heartbeat fluctuations, also change with transitions across sleep stages. Moreover, this change is very similar for both young and elderly subjects indicating a robust influence of sleep regulation on the temporal fractal correlations of heartbeat fluctuations. Specifically, we find significantly stronger correlations during wake and REM compared to light and deep sleep [Fig. 4(a)].
The scaling exponent α is determined over a range of long and intermediate time scales corresponding to low and intermediate frequencies in the power spectrum (Fig. 4). Thus, α mainly quantifies the contribution of the sympathetic component of neuroautonomic control to the fractal temporal organization of heartbeat fluctuations, as sympathetic tone affects low and intermediate frequencies [65]—i.e., higher values of α during wake and REM reflect significantly higher sympathetic activity compared to lower values of α during light and deep sleep. We note that although traditional heart rate variability measures, such as high and low frequency power [38], have been found to change significantly between healthy young and elderly subjects [59], [62], a consistent stratification pattern across sleep stages has not been previously found.
Our observation that two independent measures—the static measure σRR and the dynamic measure α that quantify different aspects of cardiac dynamics—change consistently across sleep stages and exhibit a very similar stratification pattern for both young and elderly subjects confirm our hypothesis of strong coupling between sleep and cardiac regulation. These results clearly indicate that heart rate variability changes significantly with transitions from one sleep stage to another in response to changes in sleep regulation during different sleep stages, where the values of σRR and α follow a particular order. In other words, our findings of a similar stratification pattern for both σRR and α indicates that the underlying regulatory mechanism affects two independent1 measures in a similar way. As the reflexive-responsiveness of cardiac dynamics in healthy young subjects is intact, the observed stratification pattern in the values of σRR and α—highest for wake, lower for REM and light sleep, and lowest for deep sleep—can only be attributed to the influence of different modes of sleep regulation during different sleep stages. As described in the Section III, the variability σRR is almost the same in REM and light sleep with significant differences to the wake and deep sleep states. In contrast, the α-values show most pronounced changes, specifically in elderly, between REM and light sleep.
We note that, compared to the young group, elderly subjects are characterized by higher values of the scaling exponent α during wake and REM, and slightly lower values for light and deep sleep. In contrast to σRR and σΔRR we do not find a significant shift to lower values for the scaling exponent α in elderly. The higher values of α for elderly subjects during wake and REM indicate higher degree of correlations in heartbeat fluctuations, and reflect lower parasympathetic tone in elderly compared to young subjects.
A similar, however, less pronounced stratification pattern we observe also for the scaling exponent αmag, which quantifies the long-term nonlinear properties of heartbeat dynamics encoded in the Fourier phases [42], [57]. This finding indicates higher degree of nonlinearity in cardiac dynamics during wake and REM, and closer to linear behavior during light and deep sleep. We find this tendency to hold for both young and elderly subjects [Fig. 4(b)]. We note that while there is a slightly lower degree of nonlinearity in elderly (lower αmag-values) for all sleep stages, the difference in αmag between wake/REM and light/deep sleep is larger compared to the difference between the young and elderly groups. This indicates that the influence of sleep regulation on the nonlinearity, as measured by αmag, is stronger than the effect of aging in healthy subjects.
Finally, for the scaling exponent αsign that quantifies the short-term correlations in the directionality of heart beat increments, we again find a pronounced stratification pattern with statistically significant differences between the values αsign for different sleep stages. As for σRR, α, and αmag, the values of αsign for young subjects are highest during wake, lower during REM and light sleep, and lowest during deep sleep [Fig. 4(c)]. In contrast to α and αmag, the values of αsign during wake and REM sleep are very different (much lower values) for elderly subjects (Table I).
Our findings have implications for comparative studies (healthy versus disease, young versus elderly, etc.) when ECG data during sleep are analyzed without prior knowledge of the sleep stages. The observation that certain measures of cardiac dynamics significantly change across sleep stages forming specific stratification patterns, may facilitate the development of automated detection of sleep stages based only on ECG recordings.
In summary, our results show that key static and dynamic measures of cardiac control change significantly in response to sleep stage transitions and exhibit a pronounced stratification pattern across sleep stages. For both static and dynamics measures this stratification pattern is characterized by highest values during wake, and decreasing values for REM, light and deep sleep. Our results indicate that not only the heart rate variability but also the fractal and nonlinear organization of heartbeat fluctuations follow the same stratification pattern, suggesting a fundamentally different cardiac dynamics during different sleep stages. This stratification pattern appears remarkably robust as we observe it both in young and elderly subjects. Moreover, for all sleep stages the elderly subjects exhibit very similar values for the linear and nonlinear scaling measures of cardiac dynamics compared to the young subjects, indicating that the fractal temporal organization of cardiac dynamics does not break down with healthy aging.
Further, our observations indicate that under healthy conditions the coupling between the mechanism of sleep regulation and the neuroautonomic cardiac control does not break down with progressive aging, despite significant reduction in heart rate variability and alterations of sleep architecture in elderly. Moreover, we find that the differences between the values of key static and dynamic measures of heart rate variability for different sleep stages exceed the differences between young and elderly subjects, suggesting that the effect of sleep regulation on cardiac dynamics is significantly stronger compared to the effect of healthy aging. Finally, these findings suggest a significant revision of the current theory of complexity loss with aging compared to disease, and provide new understanding of how sleep regulation affects cardiac dynamics in young and elderly. Quantifying changes in the stratification pattern, we uncover, across sleep stages can provide insights into how alterations in sleep regulation contribute to increased cardiac risk.
Acknowledgment
This paper represents the work of the authors and not the SHHS. This study was supported by National Institutes of Health (NIH) Grant 2 RO1 HL071972 and the Volkswagen Foundation.
Biographies
 Daniel T. Schmitt received the M.S. degree in theoretical physics from the University of Massachusetts at Dartmouth, North Dartmouth, in 2003, and the Ph.D. degree in statistical physics and biophysics from the University of Ulm, Ulm, Germany, in 2007.
Daniel T. Schmitt received the M.S. degree in theoretical physics from the University of Massachusetts at Dartmouth, North Dartmouth, in 2003, and the Ph.D. degree in statistical physics and biophysics from the University of Ulm, Ulm, Germany, in 2007.
He received a two-year research grant from the Volkswagen Foundation, which enabled him to work on physiological time series analysis at Boston University and Harvard Medical School.
 Phyllis K. Stein received the Ph.D. degree in health promotion from the University of Virginia, Charlottesville, VA, in 1990.
Phyllis K. Stein received the Ph.D. degree in health promotion from the University of Virginia, Charlottesville, VA, in 1990.
She is currently a Research Associate Professor of Medicine in the Division of Cardiology, Washington University School of Medicine, and where she is the Director of the Heart Rate Variability (HRV) Laboratory. Her current research interest includes the integration of heart-rate-based markers, including HRV, and information from polysomnography as markers of cardiovascular autonomic and central nervous system function, and as predictors of functional status and survival in the elderly.
 Plamen Ch. Ivanov received the M.S. degree in theoretical physics/condensed matter physics from Sofia University, Sofia, Bulgaria, in 1988, the Ph.D. degree in biophysics from Boston University, Boston, MA, in 1998, and the D.Sc. degree from the Bulgarian Academy of Sciences, Sofia, in 2007.
Plamen Ch. Ivanov received the M.S. degree in theoretical physics/condensed matter physics from Sofia University, Sofia, Bulgaria, in 1988, the Ph.D. degree in biophysics from Boston University, Boston, MA, in 1998, and the D.Sc. degree from the Bulgarian Academy of Sciences, Sofia, in 2007.
He is currently a Research Associate Professor at the Physics Department, Boston University, a Lecturer at Harvard Medical School, Brigham and Women's Hospital, Boston, and a Professor at the Institute of Solid State Physics, Bulgarian Academy of Sciences. His current research interests include methods of analysis and modeling of integrated biological and physiological systems and networks; multiscale dynamical properties emerging from cellular level interactions; neural regulation; stochastic processes and phase transitions. He has served as an editor of Fluctuation and Noise Letters (FNL) in the period 2000–2002, and he is currently on the Editorial Board of the Journal of Biological Physics (JOBP) and Europhysics Letters (EPL).
Footnotes
Note that σ is a static measure of the standard deviation of a signal, while α measures the temporal correlations of signal. In fact, signals with identical σ can exhibit completely different temporal correlations. Inversely, e.g., dividing all data point values in a correlated time series by a factor of 2 would reduce the σ by a factor of 2 but will leave the scaling exponent α, representing the temporal correlations unchanged.
References
- 1.Bassingthwaighte J, Liebovitch L, West B. Fractal Physiol. Oxford Univ. Press; London, U.K.: 1994. [Google Scholar]
- 2.Malik M, Camm AJ. Heart Rate Variability. Futura; Armonk, NY: 1995. [Google Scholar]
- 3.Kobayashi M, Musha T. 1/f fluctuation of heartbeat period. IEEE Trans. Biomed. Eng. 1982 Jun;BME-29(6):456–457. doi: 10.1109/TBME.1982.324972. [DOI] [PubMed] [Google Scholar]
- 4.Saul J, Albrecht P, Berger R, Cohen R. Analysis of long term heart rate variability: methods, 1/f scaling and implications. Comput. Cardiol. 1988;14:419–422. [PubMed] [Google Scholar]
- 5.Peng C-K, Mietus J, Hausdorff JM, Havlin S, Stanley HE, Goldberger AL. Long-range anticorrelations and non-Gaussian behavior of the heartbeat. Phys. Rev. Lett. 1993;70(9):1343–1346. doi: 10.1103/PhysRevLett.70.1343. [DOI] [PubMed] [Google Scholar]
- 6.Lefebvre JH, Goodings DA, Kamath MV, Fallen EL. Predictability of normal heart rhythms and deterministic chaos. Chaos. 1993;3(2):267–276. doi: 10.1063/1.165990. [DOI] [PubMed] [Google Scholar]
- 7.Kurths J, Voss A, Saparin P, Witt A, Kleiner HJ, Wessel N. Quantitative analysis of heart rate variability. Chaos. 1995;5(1):88–94. doi: 10.1063/1.166090. [DOI] [PubMed] [Google Scholar]
- 8.Sugihara G, Allan W, Sobel D, Allan K. Nonlinear control of heart rate variability in human infants. Proc. Nat. Acad. Sci. USA. 1996;93:2608–2613. doi: 10.1073/pnas.93.6.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poon C-S, Merrill CK. Decrease of cardiac chaos in congestive heart failure. Nature. 1997;389:492–495. doi: 10.1038/39043. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov P. Ch., Rosenblum MG, Amaral LAN, Struzik ZR, Havlin S, Goldberger AL, Stanley HE. Multifractality in human heartbeat dynamics. Nature. 1999;399:461–465. doi: 10.1038/20924. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov P. Ch., Amaral LAN, Goldberger AL, Havlin S, Rosenblum MG, Stanley HE, Struzik Z. From 1/f noise to multifractal cascades in heartbeat dynamics. Chaos. 2001;11:641–652. doi: 10.1063/1.1395631. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Hughson RL. On the fractal nature of heart rate variability in humans: Effects of data length and beta-adrenergic blockade. Amer.J.Physiol. 1994;266(1 Pt 2):R40–R49. doi: 10.1152/ajpregu.1994.266.1.R40. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Nakamura Y, Sato H, Yamamoto M, Kato K, Hughson R. On the fractal nature of heart rate variability in humans: Effects of vagal blockade. Amer. J. Physiol. Regul. Integr. Comp. Physiol. 1995;269:R830–R837. doi: 10.1152/ajpregu.1995.269.4.R830. [DOI] [PubMed] [Google Scholar]
- 14.Amaral LAN, Ivanov P. Ch., Aoyagi N, Hidaka I, Tomono S, Goldberger AL, Stanley HE, Yamamoto Y. Behavioral-independent features of complex heartbeat dynamics. Phys. Rev. Lett. 2001;86(26):6026–6029. doi: 10.1103/PhysRevLett.86.6026. [DOI] [PubMed] [Google Scholar]
- 15.Peng C-K, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5(1):82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 16.Ho KKL, Moody GB, Peng C-K, Mietus JE, Larson MG, Levy D, Goldberger AL. Predicting survival in heart failure cases and controls using fully automated methods for deriving nonlinear and conventional indices of heart rate dynamics. Circulation. 1997;96:842–848. doi: 10.1161/01.cir.96.3.842. [DOI] [PubMed] [Google Scholar]
- 17.Huikuri HV, Mäkikallio TH, Peng C-K, Goldberger AL, Hintze U, Moller M. Fractal correlation properties of R–R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation. 2000;101:47–53. doi: 10.1161/01.cir.101.1.47. [DOI] [PubMed] [Google Scholar]
- 18.Karasik R, Sapir N, Ashkenazy Y, Ivanov P. Ch., Dvir I, Lavie P, Havlin S. Correlation differences in heartbeat fluctuations during rest and exercise. Phys. Rev. E. 2002;66(6):062902. doi: 10.1103/PhysRevE.66.062902. [DOI] [PubMed] [Google Scholar]
- 19.Martinis M, Knezevic A, Krstacic G, Vargovic E. Changes in the Hurst exponent of heartbeat intervals during physical activity. Phys. Rev. E. 2004;70(1):012903-1–012903-9. doi: 10.1103/PhysRevE.70.012903. [DOI] [PubMed] [Google Scholar]
- 20.Echeverria JC, Aguilar SD, Ortiz MR, Alvarez-Ramirez J, González Camarena R. Comparison of RR-interval scaling exponents derived from long and short segments at different wake periods. Physiol. Meas. 2006;27(4):N19–N25. doi: 10.1088/0967-3334/27/4/N01. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov P. Ch., Bunde A, Amaral L, Havlin S, Fritsch Yelle J, Baevsky R, Stanley HE, Goldberger A. Sleep–wake differences in scaling behavior of the human heartbeat: Analysis of terrestrial and long-term space flight data. Europhys. Lett. 1999;48(5):594–600. doi: 10.1209/epl/i1999-00525-0. [DOI] [PubMed] [Google Scholar]
- 22.Bunde A, Havlin S, Kantelhardt JW, Penzel T, Peter J-H, Voigt K. Correlated and uncorrelated regions in heart-rate fluctuations during sleep. Phys. Rev. Lett. 2000;85(17):3736–3739. doi: 10.1103/PhysRevLett.85.3736. [DOI] [PubMed] [Google Scholar]
- 23.Kantelhardt JW, Ashkenazy Y, Ivanov P. Ch., Bunde A, Havlin S, Penzel T, Peter J-H, Stanley HE. Characterization of sleep stages by correlations in the magnitude and sign of heartbeat increments. Phys. Rev. E. 2002;65(5):051908. doi: 10.1103/PhysRevE.65.051908. [DOI] [PubMed] [Google Scholar]
- 24.Dvir I, Adler Y, Freimark D, Lavie P. Evidence for fractal correlation properties in variations of peripheral arterial tone during REM sleep. Amer. J. Physiol. Heart Circ. Physiol. 2002;283(1):H434–H436. doi: 10.1152/ajpheart.00336.2001. [DOI] [PubMed] [Google Scholar]
- 25.Penzel T, Wessel N, Riedl M, Kantelhardt JW, Rostig S, Glos M, Suhrbier A, Malberg H, Fietze I. Cardiovascular and respiratory dynamics during normal and pathological sleep. Chaos. 2007;17(1):015116. doi: 10.1063/1.2711282. [DOI] [PubMed] [Google Scholar]
- 26.Moelgaard H, Soerensen KE, Bjerregaard P. Circadian variation and influence of risk factors on heart rate variability in healthy subjects. Amer. J. Cardiol. 1991;68:777–784. doi: 10.1016/0002-9149(91)90653-3. [DOI] [PubMed] [Google Scholar]
- 27.Hu K, Ivanov P. Ch., Hilton MF, Chen Z, Ayers RT, Stanley HE, Shea SA. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. PNAS. 2004;101(52):18223–18227. doi: 10.1073/pnas.0408243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanov P. Ch., Hu K, Hilton MF, Shea SA, Stanley HE. Endogenous circadian rhythm in human motor activity uncoupled from circadian influences on cardiac dynamics. Proc. Nat. Acad. Sci. USA. 2007 Dec;104(52):20 702–20 707. doi: 10.1073/pnas.0709957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corino VDA, Matteuccib M, Cravelloc L, Ferraric E, Ferrarid AA, Mainardi LT. Long-term heart rate variability as a predictor of patient age. Comp. Methods Prog. Biomed. 2006;82(3):248–257. doi: 10.1016/j.cmpb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992;267(13):1806–1809. [PubMed] [Google Scholar]
- 31.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. J. Amer. College Cardiol. 1998;31(3):593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 32.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16(1):40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 33.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 1993;328(5):303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 34.Baharav A, Kotagal S, Gibbons V, Rubin BK, Pratt G, Karin J, Akselrod S. Fluctuations in autonomic nervous activity during sleep displayed by power spectrum analysis of heart rate variability. Neurology. 1995;45(6):1183–1187. doi: 10.1212/wnl.45.6.1183. [DOI] [PubMed] [Google Scholar]
- 35.Tsuji H, Venditti FJ, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The framingham heart study. Circulation. 1994;90:878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 36.Grote L, Ploch T, Heitmann J, Knaack L, Penzel T, Peter JH. Sleep-related breathing disorder is an independent risk factor for systemic hypertension. Amer. J. Respir. Crit. Care Med. 1999;160(6):1875–1882. doi: 10.1164/ajrccm.160.6.9811054. [DOI] [PubMed] [Google Scholar]
- 37.Mohsenin V. Sleep-related breathing disorders and risk of stroke. Stroke. 2001;32(6):1271–1278. doi: 10.1161/01.str.32.6.1271. [DOI] [PubMed] [Google Scholar]
- 38.Electrophysiology. Task Force of the European Society of Cardiology the North American Society of Pacing Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 39.Hallstrom AP, Stein PK, Schneider R, Hodges M, Schmidt G, Ulm K. Structural relationships between measures based on heart beat intervals: Potential for improved risk assessment. IEEE Trans. Biomed. Eng. 2004 Aug;51(8):1414–1420. doi: 10.1109/TBME.2004.828049. [DOI] [PubMed] [Google Scholar]
- 40.Ashkenazy Y, Ivanov P. Ch., Havlin S, Peng C-K, Goldberger AL, Stanley HE. Magnitude and sign correlations in heartbeat fluctuations. Phys. Rev. Lett. 2001;86(9):1900–1903. doi: 10.1103/PhysRevLett.86.1900. [DOI] [PubMed] [Google Scholar]
- 41.Ashkenazy Y, Ivanov P. Ch., Havlin S, Peng C-K, Yamamoto Y, Goldberger AL, Stanley HE. Decomposition of heartbeat time series: Scaling analysis of the sign sequence. Comput. Cardiol. 2000;27:139–142. [PubMed] [Google Scholar]
- 42.Ashkenazy Y, Havlin S, Ivanov P. Ch., Peng C-K, Schulte-Frohlinde V, Stanley HE. Magnitude and sign scaling in power-law correlated time series. Physica A. 2003;323:19–41. [Google Scholar]
- 43.Klösch G, Kemp B, Penzel T, Schlögl A, Rappelsberger P, Trenker E, Gruber G, Zeitlhofer J, Saletu B, Herrmann WM, Himanen SL, Kunz D, Barbanoj MJ, Röschke J, Värri A, Dorffner G. The siesta project polygraphic and clinical database. IEEE Eng. Med. Biol. Mag. 2001 May/Jun;20(3):51–57. doi: 10.1109/51.932725. [DOI] [PubMed] [Google Scholar]
- 44.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. U.S. Dept. of Health, Education, and Welfare; Bethesda, MD: 1968. [Google Scholar]
- 45.Quan S, Howard B, Iber C, Kiley J, Nieto F, O'Connor G, Rapoport D, Redline S, Robbins J, Samet J, Wahl P. The Sleep Heart Health Study: Design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 46.Lind BK, Goodwin JL, Hill JG, Ali T, Redline S, Quan SF. Recruitment of healthy adults into a study of overnight sleep monitoring in the home: Experience of the Sleep Heart Health Study. Sleep Breath. 2003;7(1):13–24. doi: 10.1007/s11325-003-0013-z. [DOI] [PubMed] [Google Scholar]
- 47.Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley JP. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. [PubMed] [Google Scholar]
- 48.Peng C-K, Buldyrev SV, Havlin S, Simons M, Stanley HE, Goldberger AL. Mosaic organization of DNA nucleotides. Phys. Rev. E. 1994;49(2):1685–1689. doi: 10.1103/physreve.49.1685. [DOI] [PubMed] [Google Scholar]
- 49.Hu K, Ivanov P. Ch., Chen Z, Carpena P, Stanley HE. Effect of trends on detrended fluctuation analysis. Phys. Rev. E. 2001;64(1):011114. doi: 10.1103/PhysRevE.64.011114. [DOI] [PubMed] [Google Scholar]
- 50.Kantelhardt JW, Koscielny-Bunde E, Rego HH, Havlin S, Bunde A. Detecting long-range correlations with detrended fluctuation analysis. Physica A. 2001;295:3–4. 441–454. [Google Scholar]
- 51.Chen Z, Ivanov P. Ch., Hu K, Stanley HE. Effect of nonstationarities on detrended fluctuation analysis. Phys. Rev. E. 2002;65(4):041107. doi: 10.1103/PhysRevE.65.041107. [DOI] [PubMed] [Google Scholar]
- 52.Xu L, Ivanov P. Ch., Hu K, Chen Z, Carbone A, Stanley HE. Quantifying signals with power-law correlations: A comparative study of detrended fluctuation analysis and detrended moving average techniques. Phys. Rev. E. 2005;71(5):051101. doi: 10.1103/PhysRevE.71.051101. [DOI] [PubMed] [Google Scholar]
- 53.Chen Z, Hu K, Carpena P, Bernaola Galvan P, Stanley HE, Ivanov P. Ch. Effect of nonlinear filters on detrended fluctuation analysis. Phys. Rev. E. 2005;71(1):011104. doi: 10.1103/PhysRevE.71.011104. [DOI] [PubMed] [Google Scholar]
- 54.Feder J. Fractals. Plenum; New York: 1988. [Google Scholar]
- 55.Taqqu M, Teverovsky V, Willinger W. Estimators for long-range dependence: An empirical study. Fractals. 1995;3(4):785–788. [Google Scholar]
- 56.Coronado AV, Carpena P. Size effects on correlation measures. J. Biol. Phys. 2005;31(1):121–133. doi: 10.1007/s10867-005-3126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Theiler J, Eubank S, Longtin A, Galdrikian B, Garmer DJ. Testing for nonlinearity in time series: The method of surrogate data. Physica D. 1992;58:77–94. [Google Scholar]
- 58.Penzel T, Kantelhardt J, Lo C, Voigt K, Vogelmeier C. Dynamics of heart rate and sleep stages in normals and patients with sleep apnea. Neuropsychopharmacology. 2003;28:S48–S53. doi: 10.1038/sj.npp.1300146. [DOI] [PubMed] [Google Scholar]
- 59.Brandenberger G, Viola AU, Ehrhart J, Charloux A, Geny B, Piquard F, Simon C. Age-related changes in cardiac autonomic control during sleep. J. Sleep Res. 2003;12(3):173–180. doi: 10.1046/j.1365-2869.2003.00353.x. [DOI] [PubMed] [Google Scholar]
- 60.Schmitt DT, Ivanov P. Ch. Fractal scale-invariant and nonlinear properties of cardiac dynamics remain stable with advanced age: a new mechanistic picture of cardiac control in healthy elderly. Amer. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293(5):R1923–1937. doi: 10.1152/ajpregu.00372.2007. [DOI] [PubMed] [Google Scholar]
- 61.Iyengar N, Peng C-K, Morin R, Goldberger AL, Lipsitz LA. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Amer. J. Physiol. Regul. Integr. Comp. Physiol. 1996;271(4):1078–1084. doi: 10.1152/ajpregu.1996.271.4.R1078. [DOI] [PubMed] [Google Scholar]
- 62.Crasset V, Mezzetti S, Antoine M, Linkowski P, Degaute JP, van de Borne P. Effects of aging and cardiac denervation on heart rate variability during sleep. Circulation. 2001;103(1):84–88. doi: 10.1161/01.cir.103.1.84. [DOI] [PubMed] [Google Scholar]
- 63.Bonnet MH, Arand DL. Heart rate variability: Sleep stage, time of night, and arousal influences. Electroencephalogr. Clin. Neurophysiol. 1997;102(5):390–396. doi: 10.1016/s0921-884x(96)96070-1. [DOI] [PubMed] [Google Scholar]
- 64.Otzenberger H, Gronfier C, Simon C, Charloux A, Ehrhart J, Piquard F, Brandenberger G. Dynamic heart rate variability: A tool for exploring sympathovagal balance continuously during sleep in men. Amer. J. Physiol. Heart Circ. Physiol. 1998;275(3):H946–950. doi: 10.1152/ajpheart.1998.275.3.H946. [DOI] [PubMed] [Google Scholar]
- 65.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]