Abstract
Apical distortion product otoacoustic emissions (DPOAEs) are comprised of at least two components, as evidenced by the interference pattern of alternating maxima and minima known as fine structure. DPOAE fine structure is produced by the shifting phase relationship in the ear canal, between the generator and characteristic frequency (CF) component of the response. Each component arises from a different cochlear region and, according to theory, reflects a distinct generation mechanism. The analysis of DPOAE components and phase in newborns may provide a window into targeted aspects of cochlear physiology during development. 2f1−f2 DPOAE fine structure was recorded from 15 adults and 14 newborns using a swept-tone technique. DPOAE group delay, as well as magnitude and phase of each component, was compared between age groups. Results show narrower fine structure spacing, a longer group delay (steeper phase gradient) in low frequencies, and a stronger relative contribution from the CF component in newborns. The prolonged group delay for low-frequency DPOAEs could indicate immature basilar membrane motion in the apex of the cochlea and warrants further investigation. The enhanced contribution from the CF component may have implications for clinical practice as well as for theories of cochlear maturation.
INTRODUCTION
It is now consensually accepted that ear canal recordings of apical (fdp<f1<f2) distortion product otoacoustic emissions (DPOAEs) include at least two components: one from the generator region, where traveling waves evoked by f1 and f2 overlap maximally (nearer f2), and one from the characteristic frequency (CF) region of the DPOAE. These two components not only arise from different sites on the cochlea but, according to recent theory (Shera and Guinan, 1999), also reflect two distinct generation mechanisms. The generator site near f2 produces inter-modulation distortion from nonlinearities in outer hair cell (OHC) function. The CF component is thought to be produced predominantly by irregularities along the basilar membrane, which scatter input energy via linear, coherent reflections. These two components will mainly be referred to throughout this report as the generator (i.e., distortion, overlap, and nonlinear sources) and the CF component (i.e., reflection and linear sources).
The existence of two DPOAE components from disparate cochlear regions (Heitmann et al., 1998; Talmadge et al., 1999; Mauermann et al., 1999a,1999b) and distinct generation processes (Shera and Guinan, 1999) suggests that the manner in which DPOAEs are interpreted should be re-examined and possibly refined. Each DPOAE component may be uniquely sensitive to cochlear pathologies of differing etiology. As such, the more we learn about these sources, their normal characteristics in the adult cochlea, and their maturational time course, the more likely their eventual application to effectively detect auditory pathology.
Interaction between the two DPOAE components, each with distinct phase behavior as a function of frequency, produces a pattern of alternating maxima and minima known as DPOAE fine structure. To our knowledge, only one other publication (from our joint laboratories) has defined fine structure in newborns. Dhar and Abdala (2007) described newborn fine structure and found it to be more prevalent, deeper, and with slightly broader frequency spacing. They did not compare DPOAE phase between the two age groups or examine individual DPOAE components. Although it was a preliminary study using a less than optimal recording protocol (i.e., discrete frequency versus swept tone), the age differences reported, nevertheless, suggest a peripheral auditory immaturity in newborns.
DPOAE age effects can only be fully interpreted after considering the impact of immature outer and middle ear functions. Past work has used the DPOAE input∕output function to model the effects the immature middle ear has on forward transmission (of stimulus) and reverse transmission (of DPOAE) (Abdala and Keefe, 2006; Keefe and Abdala, 2007). For high frequencies (4000–6000 Hz), these models have shown that a significant portion of the immaturity in peripheral auditory function can be accounted for by an immature conductive system that attenuates sound energy as it passes through the middle ear cavity toward the cochlea. The model also suggests that forward transmission through the middle ear should be relatively adult-like by six months, yet at this age, DPOAE ipsilateral suppression is still not adult-like (Abdala et al., 2007). This inconsistency suggests residual DPOAE immaturities not easily explained by outer and middle ear factors.
Given the early morphological maturity of OHCs (Pujol et al., 1998) and relatively normal OAEs in newborns (once the middle ear is considered), it is not likely that this peripheral immaturity involves cochlear micromechanics. However, passive motion of the basilar membrane determined by its physical properties may be later developing and could account for residual immaturities, as noted in laboratory animals (Mills and Rubel, 1996; Overstreet et al., 2002). Additionally, modulation of the cochlea by the medial efferent system may show postnatal immaturity, as OHC innervation by medial olivocochlear fibers does not commence until sometime around the third trimester and may not be refined until later (Lavigne-Rebillard and Pujol, 1988). The measurement of DPOAE phase and the examination of individual DPOAE components in newborns provide a window into cochlear function during development.
Three research areas were addressed in this study: (1) Earlier findings were replicated, evaluating basic fine structure features in newborns with an innovative swept-tone methodology. (2) Phase characteristics of the DPOAE were compared in newborns and adults. (3) Generator and CF components of the DPOAE were evaluated individually in both age groups to assess whether source contribution to the composite DPOAE is mature at birth.
METHODS
Subjects
15 normal-hearing adults and 14 newborn infants participated in this study. The 15 normal-hearing adults had a mean age of 25.4 years (range=18–32 years): 6 were males and 9 were females. One ear was tested per subject including eight right and seven left ears. Adults had normal audiograms [<15 dB hearing level (HL) between 500 and 8000 Hz] and type A tympanograms with static compliance between 0.4 and 1.5 cm3 and peak pressure between −50 and 150 daPa. The neonates were term born (delivered between 38 and 41 weeks of gestation) and were tested on average within 43.2 h of birth (range=17–72 h). Nine were females and five were males. Mean birthweight was 3132 g (range=2520–4425 g). All newborn subjects included in this study passed the click-evoked auditory brainstem response hearing screen at 30 dB HL and had no high-risk factors for hearing loss.
Signal analysis and instrumentation
Signal generation and recording were controlled using custom software developed by Dr. Carrick Talmadge and run on an Apple Macintosh G4 computer via a MOTU 828 Mk II input∕output device (24 bits∕44 100 Hz). Stimulus tones were presented to the subjects’ ear canal via ER2 insert phones and DPOAEs were recorded with the ER10B+ microphone. The output of the microphone was preamplified and then passed through an analog high-pass filter with 300 Hz cutoff frequency before being digitized by the MOTU and stored on disk. DPOAE recordings were made over 2f1−f2 frequencies spanning three-octaves, between 500 (f2=782 Hz) and 4000 Hz (f2=6256 Hz), using fixed stimulus levels of 65 (L1) and 55 (L2) dB sound pressure level (SPL) and a constant stimulus frequency ratio of f2∕f1=1.22. The stimulus tones were swept at a rate of 8 s∕octave for optimal definition of DPOAE fine structure (Long et al., 2008). For newborns, eight such sweeps were averaged in each condition, whereas six sweeps were averaged in adults.
DPOAE level and phase estimates were obtained using a least-squares-fit (LSF) algorithm as described by Long et al. (2008) and yielded estimates at every 2 Hz around 500–1000 Hz and every 6 Hz around 4000 Hz. The noise floor was estimated similarly except that every other temporal window was inverted and the pair summed to cancel the signal. Stimulus levels were calibrated in a Zwislocki coupler. The two transducers in the ER10B+ were individually equalized to produce flat constant drive voltage frequency responses of up to 7000 Hz. System distortion was below 30 dB SPL for the stimulus levels used in these recordings. The DPOAE phase estimate was corrected for variations in primary tone phase by subtracting 2ϕ1−ϕ2, where ϕ1 and ϕ2 are phases of the lower- and higher-frequency stimulus tones, respectively.
Protocol
All adult subjects were tested at the House Ear Institute (Los Angeles, CA). They received an audiogram and standard tympanometry as well as a brief questionnaire to screen for study inclusion. Following this, they were seated for DPOAE testing in a cushioned arm chair within a sound-attenuated booth. Six three-octave primary tone sweeps (each lasting 24 s) were recorded for off-line analysis. Duration of the entire protocol, including preliminary screening, was approximately 60 min.
Newborn subjects were tested at University of Southern California+Los Angeles County Medical Center. They were swaddled and fed, if necessary, prior to testing, and then positioned on their side or back in an Eckels (ABC) sound-attenuated isolette (www.ekel.ca). A soft rubber-tip probe was fit into the meatus of the ear canal and taped to the pinna securely with surgical tape. The test did not commence until the infant was sleeping soundly. Eight three-octave primary tone sweeps (each lasting 24 s) were recorded for off-line analysis. Sweeps were manually paused if the infant moved significantly or if there was any atypical increase in ambient room noise, and then repeated once the noise returned to normal baseline. While one experimenter monitored infant status visually, another monitored the peak activity recorded by the microphone on the computer screen. Either experimenter could initiate the software pause feature as needed. These necessary delays and sweep repetitions prolonged test sessions significantly, which led to a relatively low 30%–40% success rate with newborn infants. The entire infant protocol including preparation and calming of the infant averaged approximately 90 min but never exceeded 2 h.
Data analysis
Fine structure classification
Fine structure features were extracted with an automatic algorithm implemented previously by Dhar and Abdala (2007) and detailed below.
-
(a)
DPOAE level. The median values for DPOAE level and the noise floor were computed over every three successive data points. Data points where the signal-to-noise ratio (SNR) between level and noise floor medians was less than 6 dB were eliminated.
-
(b)
Maxima. Fine structure maxima were identified based on the first and second derivatives of the DPOAE level function and the relationship between them. Data points where the first derivative was equal to zero were identified as extrema, and then further classified as a maximum or minimum based on the second derivative being negative (maxima) or positive (minima). This process was checked by one observer familiar with the morphology of DPOAE fine structure. The visual check was implemented to eliminate peaks associated with noise that were erroneously identified by the program as maxima. These errors typically included maxima that were artificially tall and narrow and mimicked the noise floor configuration in the low-frequency range. In newborns, 78% of the maxima eliminated during visual analysis were in the low-frequency region below 1000 Hz, and in adults, 73% were <1000 Hz. Prevalence of DPOAE fine structure was quantified by counting the number of maxima in each 1∕3 octave interval.
-
(c)
Depth and spacing. The depth of each identified fine structure period was computed as 20 log10(Pmax∕Pav_min), where Pmax is the DPOAE level at a maximum and Pav_min is the average DPOAE level of the preceding and following minima. Frequency spacing of fine structure was computed both in hertz and as f∕Δf, where f is the geometric mean between two adjacent minima and Δf is the frequency separation between them (Shera, 2003). Only fine structure periods with depth ≥2.5 dB and spacing ratio ≤25 were accepted. Additionally, values that were more than 2 standard deviations from the mean were eliminated.
DPOAE phase
DPOAE phase as a function of frequency was characterized as group delay (i.e., phase gradient delay), which is the negative of the slope of phase. Starting with the initial phase value at the lowest frequency, a linear regression function was fitted to five consecutive phase values with a two-point overlap. The slope of the line was calculated to yield the group delay in milliseconds.
Component separation
DPOAE level was separated into its two constituent components using an inverse fast Fourier transform (IFFT) algorithm. A MATLAB-based analysis software (NIPR) developed by Talmadge et al. (1999) uses a variant of the IFFT algorithms described by Dhar et al., 2002 and Withnell et al., 2003 to separate the DPOAE generator and CF components based on their respective group delays. During IFFT, DPOAE complex amplitude measured in the frequency domain is multiplied by a moving Welch window (400 Hz window width and 50 Hz steps). The IFFT converts each windowed data set into the time domain, after which a fixed time window filter is applied to extract the desired delay component in the time domain. These filtered windows of data are then transformed back to the frequency domain by FFT and the complex amplitudes of the generator (around f2) and CF component (at 2f1−f2) are reconstructed.
Statistical analysis
Although the swept-tone paradigm provided DPOAE estimates every 2–6 Hz, resulting in 400–500 values of level and phase across the three-octave range, all variables were averaged into 500-Hz-wide frequency bins for statistical analysis and display.
-
(a)
Composite DPOAE. DPOAE level, phase (group delay), fine structure prevalence, spacing, and depth were computed for each individual subject and compared across frequency and age using Analyses of Variance (ANOVAs) with repeated measures on frequency.
-
(b)
DPOAE components. Level was compared across component, age, and frequency using a three-way ANOVA with repeated measures on component and frequency. Phase of each DPOAE component as a function of frequency was calculated with a linear regression function. For phase measurements, only values from 1000 to 4000 Hz were included because data <1000 were variably present in each subject. The resulting slope of phase measurements were compared across age and component.
RESULTS
DPOAE fine structure
Newborns showed more fine structure periods per given frequency interval than adults (f=6.76;p=0.01). There was an interaction between frequency and age (f=3.27;p=0.007) indicating that the age difference was greatest in the higher-frequency range above 2000 Hz. Overall, newborns had 2.0 fine structure periods per 1∕3 octave while adults had 1.44.
DPOAE level did not show an age effect. However, an interaction between frequency and age was present (f=3.76;p=0.003). As evident from the data presented in Fig. 1, infants showed a trend toward higher levels than adults below 3000 Hz. Figures 2a, 2b, 2c, 2d show an example of DPOAE fine structure from two randomly selected newborn and adult subjects.
Figure 1.
DPOAE level as a function of DPOAE frequency for 15 adults and 14 newborns. Level was averaged into 500-Hz-wide frequency bands. The frequency displayed represents the upper limit of this band. Error bars=±1 SD.
Figure 2.
[(a)–(d)] DPOAE fine structure (thick line), phase (thin line), and noise floor (gray line) from two newborn and two adult subjects. The range of values is the same in each graph, though absolute values vary. Note that DPOAE phase is referenced to the right vertical axis.
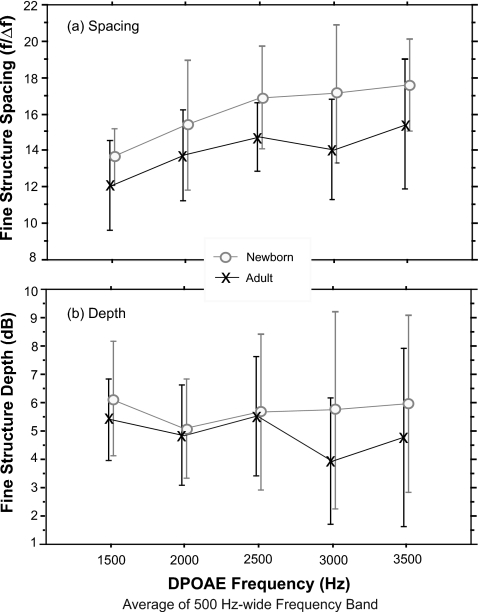
For both adults and infants, around 1500 Hz, the frequency width of fine structure periods was about 100 Hz; at 3000 Hz, spacing ranged from 175 to 225 Hz. Frequency spacing values were converted to spacing ratio values (f∕Δf) to accommodate the logarithmic frequency scale of the cochlea. Results of a two-way ANOVA (frequency×age) on spacing ratio showed an effect of both age and frequency on DPOAE fine structure spacing [Fig. 3a]. Infants had narrower fine structure spacing than adults (f=13.07;p=0.0005) and the spacing ratio increased as frequency increased (f=5.06;p=0.001).
Figure 3.
Mean DPOAE fine structure features from 15 adults and 14 newborns: (a) frequency spacing between fine structure periods and (b) depth of fine structure periods. Data were averaged into 500-Hz-wide frequency bands. The frequency displayed represents the upper limit of this band. Error bars=±1 SD.
The infant fine structure data were strongly biased toward high-frequency values relative to adult data because fine structure periods were more prevalent from 2500 to 4000 Hz in newborns. In this high-frequency range, infants had 29 values compared to the 15 data points available from the adults. A secondary ANOVA was also conducted, examining age effects on spacing for the frequency range through 2500 Hz only (similar to the frequency range tested in a previous publication from our joint laboratories, Dhar and Abdala, 2007). This analysis showed no effect of age on the spacing between DPOAE fine structure periods. Although there was a general trend for deeper fine structure in newborns (p=0.08) especially in the high frequencies as evident in Fig. 3b, neither age nor frequency produced significant effects on depth [Fig. 3b].
DPOAE phase
The slope of DPOAE phase was quantified by calculating a measure of group delay or phase gradient. This index represents the rate or slope of phase as it changes across frequency. Group delay was near 0 ms for both age groups in the mid- to high frequencies with a typical break from this near-zero constant at frequencies below 2000 Hz (Fig. 4). There was a significant difference in group delay between newborns and adults (f=9.77;p=0.004). This difference was present in low frequencies only, as seen in Fig. 4. The mean delay for newborns for the lowest-frequency band was 2.35 ms, while for adults, it was 1.74 ms. In the frequency interval between 1000 and 1500 Hz, the newborn group delay was double the adult value (1.91 versus 97). This finding indicates a steeper DPOAE phase gradient in the newborns at the lowest frequencies.
Figure 4.
Mean DPOAE group delay (negative of the slope of the phase) as a function of DPOAE frequency for 15 adults and 14 newborns. Data were averaged into 500-Hz-wide frequency bands. The frequency displayed represents the upper limit of this band. Error bars=±1 SD.
DPOAE components
Component magnitude
Once the DPOAE data were analyzed with an IFFT, the magnitude and phase of the two components comprising the ear canal response were examined separately. The generator component showed much higher levels than the CF component, which is typical in normal-hearing adults (Fig. 5, f=433;p<0.0001). There was also an interaction between age and component (f=9.19;p=0.0053). Posthoc analysis showed that newborns had a larger CF component than adults (f=12.1;p=0.001), but that generator component levels were comparable between age groups. The generator component averaged across many subjects should approximate the composite DPOAE level. This correspondence can be observed when comparing the data in Fig. 5a to data in Fig. 1.
Figure 5.
[(a) and (b)] DPOAE level as a function of frequency for each component and age group separately. The smaller inset graph shows the level difference between components (generator level subtracted from CF level) for each age group. Data were averaged into 500-Hz-wide frequency bands. The frequency displayed represents the upper limit of these bands. Error bars=±1 SD.
The difference between component levels was further analyzed by calculating a difference score: CF component level subtracted from generator component level (Fig. 5, inset). Adults had a greater difference score than newborns (f=10.52;p=0.0031), but only for frequencies >2500 Hz. Since the generator component level is comparable between age groups, the reduction in adult CF level produces the larger difference scores noted in Fig. 5.
Phase
Component phase was analyzed by fitting a linear regression to the phase×frequency function for generator and CF component separately. Results shown in Fig. 6 manifest the expected constant phase (i.e., shallow slope) for the distortion component and rapidly cycling phase (i.e., steep slope) for the CF component. This figure shows individual phase×frequency functions for each newborn and adult subject for both components. Clearly, there was a significant effect of component on slope of phase (f=806;p<0.0001) but there was not a significant effect of age on slope of phase for either component, although there was a trend for steeper slope of phase in infants for the CF component only (p<0.08).
Figure 6.
DPOAE phase for the generator (gray) and CF component (black) as a function of DPOAE frequency for both age groups. Lines represent phase from one adult subject, whereas each newborn subject has two such traces displayed. The mean slope of phase values, calculated with a linear regression function, are shown for each age and component.
DISCUSSION
These data show that infants have more prevalent DPOAE fine structure than adults in high frequencies, narrower spacing between fine structure periods, and a steeper DPOAE phase gradient (longer group delay) than adults at frequencies below 2000 Hz. While the greater prevalence of fine structure in infants is consistent with our previous report (Dhar and Abdala, 2007), the narrower spacing in infants is not. Possible reasons for this and other inconsistencies among studies of DPOAE fine structure are further discussed in Sec. 4B.
Cochlear physiology
Steep phase gradient
Spacing of DPOAE fine structure is primarily determined by the slope of CF component phase since the phase of the generator component is largely invariant as a function of frequency (the special case of frequencies below 2000 Hz is discussed separately). Given similarly flat slopes of phase for the generator component in adults and newborns (Fig. 6), the narrower fine structure spacing found in infants implies a steeper phase gradient for the newborn CF component. Past work from the first author’s laboratory has, in fact, found steeper CF component slope of phase for infants when frequencies between 500 and 1000 Hz were included in analysis; however, in the present study, slope of phase calculations for data lower than 1000 Hz were eliminated because the number of observations in this interval (500–1000 Hz) varied widely among newborn subjects due to signal-to-noise ratio (SNR) issues. Thus, the steepest part of the phase×frequency function was eliminated in the present analysis and may have contributed to the finding of borderline steeper slope in the phase of the DPOAE CF component measured from newborns.
Some have argued that DPOAE group delay is reflective of travel times in the cochlea (Moulin and Kemp, 1996a,1996b). Others have extended this interpretation to imply an underlying relationship with the sharpness of mechanical tuning in the cochlea (Bowman et al., 1997). However, these interpretations are complicated by the dependence of the delay on the DPOAE recording conditions (Tubis et al., 2000). Some have questioned the connection between estimates of cochlear travel times and DPOAE delays (Ruggero, 2004; Siegel et al., 2005), while others have shown that group delays measured from SFOAEs are correlated with the sharpness of psychophysical tuning curves (Shera et al., 2000). Slope of phase of the DPOAE CF component would be most closely related to the slope of phase of SFOAEs. Thus, the observation of narrower fine structure spacing in newborns could be interpreted to be indicative of sharper tuning. Consistent with this idea, DPOAE suppression tuning has been shown to be excessively sharp in human infants at high frequencies (Abdala et al., 2007). However, the link between OAE suppression and mechanical tuning of the basilar membrane is not straightforward (Howard et al., 2003).
Scaling symmetry
Consistent with previous observations, group delay was near 0 ms in the mid- to high frequencies with a typical break from this near-zero constant at frequencies below 2000 Hz. The invariance of DPOAE phase as a function of frequency above some mid-frequency boundary has been attributed to cochlear scaling symmetry. Since the most sensitive region for high to low frequencies is arranged from the base to the apex of the cochlea, vibrations caused by signals of different frequency “travel” different distances before reaching their best or CF region. Scaling symmetry simply implies that the number of cycles to the CF site is relatively independent of frequency, and mechanical vibrations produced by signals of all frequencies accumulate approximately the same amount of total phase (cycles) at their unique CF site.
Evidence supporting scaling symmetry has been observed in laboratory animals and human adults for a relatively large frequency range, with deviations from perfect scale invariance at lower frequencies, i.e., nearer the cochlear apex (Dhar et al., 2002; Shera and Guinan, 1999; Shera et al., 2000; Talmadge et al., 1999). This break from symmetry has not previously been shown in the developing human auditory system, but is clearly present in the newborn data presented here. For the lowest frequencies, the prolongation of group delay values in newborns was significantly greater than adult delays, possibly indicating more marked deviation from scaling invariance in the apex of the newborn cochlea.
Violations of perfect scaling invariance (defined by DPOAE data) may be due to multiple sources, some related to cochlear physiology and others related to cochlear roughness, wave reflection, and source interference (Dhar et al., 2002; Shera et al., 2000). Deviations from scaling symmetry can also be produced by shifting transfer functions of the outer∕middle ear. For example, Moulin and Kemp (1996a, 1996b) demonstrated the influence of middle ear pressure on DPOAE phase at low frequencies. Clearly, the relationship between DPOAE group delay and cochlear mechanics must be interpreted while considering this complexity and the varied sources contributing to scale invariance.
If newborns show a greater break from scaling symmetry than adults (i.e., a more prolonged group delay only at low frequencies), it could indicate that any of the above-named factors are immature around the time of birth. Morphologically, the mammalian cochlear apex is the last region to develop and segments of the apex remain non-adult-like well beyond the basal cochlea (Bredberg, 1968; Lavigne-Rebillard and Pujol, 1986, 1987; Pujol et al., 1998). Such immaturity in the newborn cochlea, regardless of its exact nature, could be responsible for the comparatively larger deviation from scaling symmetry. The larger CF component observed in the newborns in the present study points to the possibility of increased cochlear roughness, which could either be the cause or simply a result of the deviation from scale invariance. Moreover, the observed break from scaling symmetry might suggest immaturities in frequency representation in the apical region of the newborn cochlea. These questions are of significant interest to appropriately define the source of infant immaturities in peripheral auditory system function and warrant further research.
CF component level
Newborns show higher relative levels of the CF component than adults, suggesting a larger contribution from the 2f1−f2 site. This finding could be interpreted in two ways: The newborn cochlea is immature and∕or the adult cochlea is compromised by normal aging and exposure. The low-level CF component (reflection) is more sensitive to integrity of the cochlear amplifier and thus would be quite robust and optimally functional in the pristine neonatal cochlea. Several models of DPOAE generation including coherent reflection filtering (Shera and Zweig, 1993b; Talmadge et al., 1998; Zweig and Shera, 1995) depend on wave scattering from random, physical irregularities along the basilar membrane. Irrespective of the source of the roughness, the output of all such models depends critically on the height and breadth of the traveling wave. A more active cochlear amplifier would result in greater magnitude of vibration near the CF (a “taller” traveling wave), thereby leading to greater reflection and, subsequently, a larger DPOAE component from the CF region. Consistent with this hypothesis, newborns have larger (Burns et al., 1992) and greater numbers of spontaneous OAEs (SOAEs) per ear (Abdala, 1996). SOAEs are thought to be amplitude-stabilized cochlear standing waves originating from the same reflection process as the CF component of DPOAEs (Shera, 2003). The combination of the pristine infant cochlea and natural adult exposure to damaging noise and ototoxins may explain the larger CF component in newborns, arguing for early “auditory aging” in the adults rather than immaturity in the newborns.
A second possibility to explain a larger relative contribution from the CF component in newborns involves the medial olivocochlear (MOC) effect. Although cochlear micromechanics may be mature in the infant, modulation of OHC function by the medial efferent tract may remain immature around the time of term birth. MOC fibers are present early in gestational life (Moore et al., 1999), but it is not clear when MOC function becomes adult-like. There are limited reports of the MOC reflex in infants and most suggest the reflex is immature in prematurely born neonates and is approaching maturity around the time of term birth (Abdala et al., 1999; Ryan and Piron, 1994; Morlet et al., 1993). However, methods for probing the medial efferent system have changed significantly since these reports and they may not provide the most complete picture. It is possible that residual MOC immaturities in newborns influence the relative contribution of the CF component to the ear canal DPOAE. Recent work has, in fact, shown that the MOC suppresses the CF component more than the generator component in the adult cochlea (Abdala et al., 2008). Its immaturity, then, might be expected to influence the CF component disproportionately.
Finally, an immature middle ear∕cochlear junction would produce robust, multiple reflections within the cochlea, which may, in turn, contribute to an increase in the level of the CF component (Dhar et al., 2002; Dhar and Abdala, 2007). Additionally, there is ample evidence to indicate that middle ear transmission is immature in newborns and affects both stimulus and response level (Abdala and Keefe, 2006; Keefe and Abdala, 2007). Lower effective stimulus levels could produce a relatively enhanced CF component in newborns, consistent with deeper fine structure for lower level stimuli in adult ears (Dhar et al., 2005; He and Schmiedt, 1993; Konrad-Martin et al., 2001, 2002). While it is true that deeper DPOAE fine structure reflects relatively equal component levels and could well be achieved by a reduced generator component, we did not observe significantly deeper fine structure in newborns, nor was the newborn generator component reduced, arguing against middle ear immaturities playing a major role in our findings.
The larger CF component in newborns and the similarity of the generator component between the two age groups suggest different maturational time lines for generator and CF components and the physiological mechanisms responsible for their generation. Differences in the composition of the ear canal DPOAE between newborns and adults may have clinical implications. If the CF component (largely generated by a reflection mechanism) is relatively prominent in newborns, should either transient or stimulus frequency OAEs, also generated by the reflection mechanism, be more applicable in this population? If DPOAEs are then to be used, should the stimulus parameters be altered to bias the ear canal DPOAE toward the CF component? Of course, the oft-speculated greater sensitivity of reflection-source OAEs to cochlear pathology will have to be definitively demonstrated to support these types of clinical modifications.
Variability among reports
In a past report (Dhar and Abdala, 2007), we found slightly broader frequency spacing in the DPOAE fine structure of newborns compared to adults, which is in contrast to the present findings. Other aspects of the studies, such as the increased fine structure prevalence in newborns and the higher DPOAE levels, were in good agreement. There are some significant methodological differences between the two studies that might help explain these discrepancies, as well as inconsistencies in all reports of DPOAE fine structure among laboratories.
Range of test frequencies
In the present report, there is a marked difference in the number of observations available in the three highest-frequency bands tested (2500–3000, 3000–3500, and 3500–4000 Hz) between adults and infants. There is roughly twice the number of data points for newborns in the higher-frequency intervals, where the mean spacing ratio (f∕Δf) is 17.2. Thus, the newborn data in the current study have a biased distribution toward the high frequencies and narrower spacing relative to adults. These high-frequency bands were not evaluated in our previous report, which was tested only through 2500 Hz. When the high-frequency data are removed, there is no age difference in DPOAE fine structure frequency spacing between ages in this data set.
Analysis scheme
Algorithms and analysis strategies for the classification of DPOAE fine structure vary from laboratory to laboratory and report to report. Clearly, the type of analysis routine applied can influence the measurements of fine structure prevalence, spacing, and depth significantly. Some fine structure features, such as prevalence and spacing, have been relatively consistent across laboratories for human adults (Abdala et al., 2008; Dhar and Abdala, 2007; Engdahl and Kemp, 1996; He and Schmiedt, 1993; Heitmann et al., 1998; Reuter and Hammershoi, 2006). However, there are too few reports of infant fine structure to know whether this same stability is to be expected. The higher and more erratic noise floors associated with infant testing may increase the likelihood that fine structure features are less stable in newborns across reports.
More importantly, since the early report from our joint laboratories (Dhar and Abdala, 2007), we have continued to streamline and strengthen our analysis techniques. The classification of DPOAE fine structure appears to be an evolving effort, as noted by reports dedicated solely to this task (Heise et al., 2008). Whereas we processed all DPOAE magnitude and phase data through an automatic algorithm and did not treat the data further in our previous study, our current protocol includes additional steps of analysis (a visual check and the elimination of outliers). Any of these changes in our analysis routine may have contributed to differences among reports. Until there is a universally accepted strategy and algorithm for quantifying the features of DPOAE fine structure, these differences among studies will be inevitable.
Other methodological factors
A newer, swept-tone methodology was employed with infants in the current study giving 2–6 Hz frequency resolution, whereas discrete frequency recording was conducted with infants in the 2007 report. Although these two methods have been shown to be comparable in adults (Long et al., 2008), such equivalence has not been demonstrated in infants. Lastly, an ER10C probe microphone was used for neonates only by Dhar and Abdala (2007), whereas an ER10B+ was used for both adults and infants in this report. Although anecdotal data from our laboratories suggest that this probe difference will not affect fine structure spacing calculations, differences between the ER10B+ and ER10C have been documented in our laboratories with respect to several DPOAE features such as fine structure prevalence and DPOAE level.
Separating DPOAE components
Various methods of separating DPOAE components have been proposed and reported in literature. None of these methods are perfect mostly due to the complexity of DPOAE generation and its variability among individuals and across frequency. For example, the suppressor method, where a suppressor tone proximal in frequency to 2f1−f2 is used to eliminate the CF component and isolate the generator component, is critically dependent on finding the optimal suppressor level for each individual and each frequency within individuals (Dhar and Shaffer, 2004; Johnson et al., 2006, 2007). The method of time windowing used in this report relies on the differences in phase behavior between the two DPOAE components, with a steep phase gradient linked to the CF component. Scaling symmetry ensures that the phase of the generator component is relatively invariant as a function of frequency and, hence, can be distinguished from the CF component. A critical complication may arise when scaling symmetry breaks down, as is the case below 2000 Hz in our data set. Note from Fig. 4 that the phase gradient of the ear canal DPOAE is steep in low frequencies, even when the generator component is dominant in the ear canal. Consequently, at the output of an inverse FFT, some undefined portion of the generator component would be grouped with the CF component. This could produce underestimation of the generator and overestimation of the CF component. It could also produce a more shallow CF phase gradient. Given this complication, the suppressor method of separating the DPOAE components might be better suited in the low-frequency range. However, this would require stimulus sweeps with and without the suppressor in newborns, making a challenging test protocol even more difficult. It appears warranted to consider alternative means of component separation in infants where the low-frequency break from scaling symmetry may be more marked.
CONCLUSIONS
Newborns show narrower fine structure spacing, a steeper DPOAE phase gradient (prolonged group delay) in the more apical regions of the cochlea, and enhanced contribution from the CF component relative to adults. These results strongly suggest an immaturity in the peripheral auditory system of newborns, although its source is not clear. It is likely that partly it can be explained by immaturity in the conductive system of newborns. Findings may also suggest immaturity in passive vibration of the basilar membrane and frequency representation in the cochlear apex of newborns. Future work would do well to scrutinize the infant apical region to further define these non-adult-like features. Additionally, it will be important to continue investigating the relative source contribution to infant DPOAEs and possibly refine OAE protocol for more effective evaluation in this age group. Finally, DPOAE fine structure is not being classified in a consistent manner across studies and laboratories. Although the features of fine structure will continue to be of some interest, it may be more productive to separate and analyze components contributing to the DPOAE, rather than focus on the interference pattern produced by their interaction. However, methods of component separation in newborns should be carefully considered before application, as complexities associated with DPOAE generation make their utility less than straightforward.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health, NIDCD (Grant No. DC003552), the House Ear Institute, and Northwestern University. Authors would like to thank Tracy Williams and Srikanta Mishra for their assistance with data collection and management, Dr. Rangasamy Ramanathan for his continued support of neonatal hearing research at the University of Southern California Medical Center and Ping Luo for his technical assistance. Authors would also like to thank Chris Shera for his contributions with respect to phase calibration and correction. Data collection was conducted using software developed by Carrick L. Talmadge.
References
- Abdala, C. (1996). “Distortion product otoacoustic emission (2f1−f2) amplitude as a function of f2∕f1 frequency ratio and primary tone level separation in human adults and neonates,” J. Acoust. Soc. Am. 100, 3726–3740. 10.1121/1.417234 [DOI] [PubMed] [Google Scholar]
- Abdala, C., and Keefe, D. H. (2006). “Effects of middle-ear immaturity on distortion product otoacoustic emission suppression tuning in infant ears,” J. Acoust. Soc. Am. 120, 3832–3842. 10.1121/1.2359237 [DOI] [PubMed] [Google Scholar]
- Abdala, C., Keefe, D. H., and Oba, S. (2007). “A longitudinal study of DPOAE suppression tuning and acoustic admittance in human infants from birth through six months of age,” J. Acoust. Soc. Am. 121, 3617–3627. 10.1121/1.2734481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala, C., Ma, E., and Sininger, Y. (1999). “Maturation of medial efferent system function in humans,” J. Acoust. Soc. Am. 105, 2392–2402. 10.1121/1.426844 [DOI] [PubMed] [Google Scholar]
- Abdala, C., Mishra, S. K., and Williams, T. L. (2009). “Considering distortion product otoacoustic emission fine structure in measurements of the medial olivocochlear reflex,” J. Acoust. Soc. Am. 125, 1584–1594. 10.1121/1.3068442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, D. M., Brown, D. K., Eggermont, J. J., and Kimberley, B. P. (1997). “The effect of sound intensity on f1-sweep and f2-sweep distortion product otoacoustic emissions phase delay estimates in human adults,” J. Acoust. Soc. Am. 101, 1550–1559. 10.1121/1.418129 [DOI] [PubMed] [Google Scholar]
- Bredberg, G. (1968). “Cellular pattern and nerve supply of the human organ of Corti,” Acta Oto-Laryngol., Suppl. 236, 1–135. [PubMed] [Google Scholar]
- Burns, E. M., Arehart, K. H., and Campbell, S. L. (1992). “Prevalence of spontaneous otoacoustic emissions in neonates,” J. Acoust. Soc. Am. 91, 1571–1575. 10.1121/1.402438 [DOI] [PubMed] [Google Scholar]
- Dhar, S., and Abdala, C. (2007). “A comparative study of DPOAE fine structure in human newborns and adults with normal hearing,” J. Acoust. Soc. Am. 122, 2191–2202. 10.1121/1.2770544 [DOI] [PubMed] [Google Scholar]
- Dhar, S., Long, G. R., Talmadge, C. L., and Tubis, A. (2005). “The effect of stimulus frequency ratio on distortion product otoacoustic emission components,” J. Acoust. Soc. Am. 117, 3766–3776. 10.1121/1.1903846 [DOI] [PubMed] [Google Scholar]
- Dhar, S., and Shaffer, L. A. (2004). “Effects of a suppressor tone on distortion product otoacoustic emissions fine structure: Why a universal suppressor level is not a practical solution to obtaining single-generator DP-grams,” Ear Hear. 25, 573–585. 10.1097/00003446-200412000-00006 [DOI] [PubMed] [Google Scholar]
- Dhar, S., Talmadge, C. L., Long, G. R., and Tubis, A. (2002). “Multiple internal reflections in the cochlea and their effect on DPOAE fine structure,” J. Acoust. Soc. Am. 112, 2882–2897. 10.1121/1.1516757 [DOI] [PubMed] [Google Scholar]
- Engdahl, B., and Kemp, D. T. (1996). “The effect of noise exposure on the details of distortion product otoacoustic emissions in humans,” J. Acoust. Soc. Am. 99, 1573–1587. 10.1121/1.414733 [DOI] [PubMed] [Google Scholar]
- He, J., and Schmiedt, R. A. (1993). “Fine structure of the 2f1−f2 acoustic distortion product: Changes with primary level,” J. Acoust. Soc. Am. 94, 2659–2669. 10.1121/1.407350 [DOI] [PubMed] [Google Scholar]
- Heise, S. J., Verhey, J. L., and Mauermann, M. (2008). “Automatic screening and detection of threshold fine structure,” Int. J. Audiol. 47, 520–532. 10.1080/14992020802089473 [DOI] [PubMed] [Google Scholar]
- Heitmann, J., Waldmann, B., Schnitzler, H. U., Plinkert, P. K., and Zenner, H. P. (1998). “Suppression of distortion product otoacoustic emissions (DPOAE) near f1−f2 removes DP-gram fine structure—Evidence for a secondary generator,” J. Acoust. Soc. Am. 103, 1527–1531. 10.1121/1.421290 [DOI] [Google Scholar]
- Howard, M. A., Stagner, B. B., Foster, P. K., Lonsbury-Martin, B. L., and Martin, G. K. (2003). “Suppression tuning in noise-exposed rabbits,” J. Acoust. Soc. Am. 114, 279–293. 10.1121/1.1577555 [DOI] [PubMed] [Google Scholar]
- Johnson, T. A., Neely, S. T., Kopun, J. G., Dierking, D. M., Tan, H., Converse, C., Kennedy, E., and Gorga, M. P. (2007). “Distortion product otoacoustic emissions: Cochlear-source contributions and clinical test performance,” J. Acoust. Soc. Am. 122, 3539–3553. 10.1121/1.2799474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, T. A., Neely, S. T., Kopun, J. G., and Gorga, M. P. (2006). “Reducing reflected contributions to ear-canal distortion product otoacoustic emissions in humans,” J. Acoust. Soc. Am. 119, 3896–3907. 10.1121/1.2200048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe, D. E., and Abdala, C. (2007). “Theory of forward and reverse middle-ear transmission applied to otoacoustic emissions in infant and adult ears,” J. Acoust. Soc. Am. 121, 978–993. 10.1121/1.2427128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad-Martin, D., Neely, S. T., Keefe, D. H., Dorn, P. A., Cyr, E., and Gorga, M. P. (2002). “Sources of DPOAEs revealed by suppression experiments, inverse fast Fourier transforms, and SFOAEs in impaired ears,” J. Acoust. Soc. Am. 111, 1800–1809. 10.1121/1.1455024 [DOI] [PubMed] [Google Scholar]
- Konrad-Martin, D., Neely, S. T., Keefe, D. H., Dorn, P. A., and Gorga, M. P. (2001). “Sources of distortion product otoacoustic emissions revealed by suppression experiments and inverse fast Fourier transforms in normal ears,” J. Acoust. Soc. Am. 109, 2862–2879. 10.1121/1.1370356 [DOI] [PubMed] [Google Scholar]
- Lavigne-Rebillard, M., and Pujol, R. (1986). “Development of the auditory hair cell surface in human fetuses. A scanning electron microscopy study,” Anat. Embryol. (Berl) 174, 369–377. 10.1007/BF00698787 [DOI] [PubMed] [Google Scholar]
- Lavigne-Rebillard, M., and Pujol, R. (1987). “Surface aspects of the developing human organ of Corti,” Acta Oto-Laryngol., Suppl. 436, 43–50. 10.3109/00016488709124975 [DOI] [PubMed] [Google Scholar]
- Lavigne-Rebillard, M., and Pujol, R. (1988). “Hair cell innervation in the fetal human cochlea,” Acta Oto-Laryngol. 105, 398–402. 10.3109/00016488809119492 [DOI] [PubMed] [Google Scholar]
- Long, G. R., Talmadge, C. L., and Lee, J. (2008). “Measuring distortion product otoacoustic emissions using continuously sweeping primaries,” J. Acoust. Soc. Am. 124, 1613–1626. 10.1121/1.2949505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauermann, M., Uppenkamp, S., van Hengel, P. W. J., and Kollmeier, B. (1999a). “Evidence for the distortion product frequency place as a source of distortion product otoacoustic emission (DPOAE) fine structure in humans. II. Fine structure for different shapes of cochlear hearing loss,” J. Acoust. Soc. Am. 106, 3484–3491. 10.1121/1.428201 [DOI] [PubMed] [Google Scholar]
- Mauermann, M., Uppenkamp, S., van Hengel, P. W. J., and Kollmeier, B. (1999b). “Evidence for the distortion product frequency place as a source of distortion product otoacoustic emission (DPOAE) fine structure in humans. I. Fine structure and higher-order DPOAE as a function of the frequency ratio f2∕f1,” J. Acoust. Soc. Am. 106, 3473–3483. 10.1121/1.428200 [DOI] [PubMed] [Google Scholar]
- Mills, D. M., and Rubel, E. W. (1996). “Development of the cochlear amplifier,” J. Acoust. Soc. Am. 100, 428–441. 10.1121/1.415857 [DOI] [PubMed] [Google Scholar]
- Moore, J. K., Simmons, D. D., and Guan, Y. (1999). “The human olivocochlear system: Organization and development,” Audiol. Neuro-Otol. 4, 311–325. 10.1159/000013855 [DOI] [PubMed] [Google Scholar]
- Morlet, T., Collet, L., Salle, B., and Morgon, A. (1993). “Functional maturation of cochlear active mechanisms and of the medial olivocochlear system in humans,” Acta Oto-Laryngol. 113, 271–277. 10.3109/00016489309135808 [DOI] [PubMed] [Google Scholar]
- Moulin, A., and Kemp, D. T. (1996a). “Multicomponent acoustic distortion product otoacoustic emission phase in humans. I. General characteristics,” J. Acoust. Soc. Am. 100, 1617–1639. 10.1121/1.416063 [DOI] [PubMed] [Google Scholar]
- Moulin, A., and Kemp, D. T. (1996b). “Multicomponent acoustic distortion product otoacoustic emission phase in humans. II. Implications for distortion product otoacoustic emissions generation,” J. Acoust. Soc. Am. 100, 1640–1662. 10.1121/1.416064 [DOI] [PubMed] [Google Scholar]
- Overstreet, E. H., III, Temchin, A. N., and Ruggero, M. A. (2002). “Passive basilar membrane vibrations in gerbil neonates: Mechanical bases of cochlear maturation,” J. Physiol. 545, 279–288. 10.1113/jphysiol.2002.025205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol, R., Lavigne-Rebillard, M., and Lenoir, M. (1998). “Development of sensory and neural structures in the mammalian cochlea,” in Development of the Auditory System, edited by Rubel E., Popper A., and Fay R. (Springer, New York: ), pp. 146–192. [Google Scholar]
- Reuter, K., and Hammershoi, D. (2006). “Distortion product otoacoustic emission fine structure analysis of 50 normal-hearing humans,” J. Acoust. Soc. Am. 120, 270–279. 10.1121/1.2205130 [DOI] [PubMed] [Google Scholar]
- Ruggero, M. A. (2004). “Comparison of group delays of 2f1−f2 distortion product otoacoustic emissions and cochlear travel times,” ARLO 5, 143–147. 10.1121/1.1771711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, S., and Piron, J. (1994). “Functional maturation of the medial efferent olivocochlear system in human neonates,” Acta Oto-Laryngol. 114, 485–489. 10.3109/00016489409126091 [DOI] [PubMed] [Google Scholar]
- Shera, C. A. (2003). “Mammalian spontaneous otoacoustic emissions are amplitude-stabilized cochlear standing waves,” J. Acoust. Soc. Am. 114, 244–262. 10.1121/1.1575750 [DOI] [PubMed] [Google Scholar]
- Shera, C. A., and Guinan, J. J. (1999). “Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs,” J. Acoust. Soc. Am. 105, 782–798. 10.1121/1.426948 [DOI] [PubMed] [Google Scholar]
- Shera, C. A., Talmadge, C., and Tubis, A. (2000). “Interrelations among distortion-product phase-gradient delays: Their connection to scaling symmetry and its breaking,” J. Acoust. Soc. Am. 108, 2933–2948. 10.1121/1.1323234 [DOI] [PubMed] [Google Scholar]
- Shera, C. A., and Zweig, G. (1993b). “Order from chaos: Resolving the paradox of periodicity in evoked otoacoustic emission,” in Biophysics of Hair Cell Sensory Systems, edited by Duifhuis H., Horst J. W., van Dijk P., and van Netten S. M. (World Scientific, Singapore: ), pp. 54–63. [Google Scholar]
- Siegel, J. H., Cerka, A. J., Recio, A., Temchin, A. N., and Ruggero, M. A. (2005). “Delays of stimulus-frequency otoacoustic emissions and cochlear vibrations contradict the theory of coherent reflection filtering,” J. Acoust. Soc. Am. 118, 2434–43. 10.1121/1.2005867 [DOI] [PubMed] [Google Scholar]
- Talmadge, C. L., Long, G. R., Tubis, A., and Dhar, S. (1999). “Experimental confirmation of the two-source interference model for the fine structure of distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 105, 275–292. 10.1121/1.424584 [DOI] [PubMed] [Google Scholar]
- Talmadge, C. L., Tubis, A., Long, G. R., and Piskorski, P. (1998). “Modeling otoacoustic emission and hearing threshold fine structures,” J. Acoust. Soc. Am. 104, 1517–1543. 10.1121/1.424364 [DOI] [PubMed] [Google Scholar]
- Tubis, A., Talmadge, C. L., Tong, C., and Dhar, S. (2000). “On the relationships between the fixed-f1, fixed-f2, and fixed-ratio phase derivatives of the 2f1−f2 distortion product otoacoustic emission,” J. Acoust. Soc. Am. 108, 1772–1785. 10.1121/1.1310666 [DOI] [PubMed] [Google Scholar]
- Withnell, R. H., Shaffer, L. A., and Talmadge, C. L. (2003). “Generation of DPOAEs in the guinea pig,” Hear. Res. 178, 106–117. 10.1016/S0378-5955(03)00064-9 [DOI] [PubMed] [Google Scholar]
- Zweig, G., and Shera, C. A. (1995). “The origin of periodicity in the spectrum of evoked otoacoustic emissions,” J. Acoust. Soc. Am. 98, 2018–2047. 10.1121/1.413320 [DOI] [PubMed] [Google Scholar]