Abstract
Sensitivity to binaural cues was studied in 11 bilateral cochlear implant users, all of whom received both of their cochlear implants as adults, but who varied in the age at onset of deafness, from pre-lingual to childhood-onset to adult-onset. Sensitivity to interaural timing difference (ITD) and interaural level difference (ILD) cues was measured at basal, middle, and apical pitch-matched places of stimulation along the cochlear arrays, using a stimulation rate of 100 Hz. Results show that there is a trend for people whose onset of deafness occurred during adult life or late childhood to retain at least some sensitivity to ITDs, whereas people with onset of deafness earlier in life were insensitive to ITDs. In contrast, ILD cue sensitivity was present in all subjects. There were no effects of place of stimulation on binaural sensitivity, suggesting that there is no indication of a dependence of ITD sensitivity on apical vs basal electrode location.
INTRODUCTION
Cochlear implants (CIs) offer a means of providing hearing to deaf individuals through electrical stimulation of the auditory nerve. Traditionally, a single implant in either ear was provided; however, in recent years increasing numbers of patients have received bilateral cochlear implants (van Hoesel, 2004; Nopp et al., 2004; Litovsky et al., 2006a). Bilateral implantation has been motivated by the fact that, in normal-hearing people, binaural hearing plays an important role, for example, by facilitating sound localization and speech understanding in noise. Studies done in the sound field using loudspeakers have shown that at least some of the advantages of having two ears extend to bilateral cochlear implant users. For example, on tasks of sound source location identification, root-mean-square errors can be as low as 10°–20° when listening with bilateral implants and ≥60° when listening monaurally (van Hoesel and Tyler, 2003; Litovsky et al., 2004; Nopp et al., 2004). Similarly, the ability to understand speech in the presence of either noise or competing speech is improved in the majority of patients when they use both of their implants vs one implant alone (Nopp et al., 2004; Schleich et al., 2004; van Hoesel, 2004; Litovsky et al., 2006a, 2009).
Despite these notable improvements, both sound localization and speech understanding in noise abilities of bilateral cochlear implant users are overall significantly worse than performance measured in normal-hearing people. There may be a biological limitation at fairly peripheral levels in the system due to degradation of neural ganglion cells following a prolonged period of auditory deprivation (Leake et al., 1999; Coco et al., 2007), and thus loss of fidelity with which information can be transmitted to binaural circuits. Another factor that is likely constraining performance is hardware-based limitation of access to binaural information.
In normal-hearing people, sound localization abilities in the horizontal plane depend on the extent to which listeners are able to extract and utilize differences in arrival time and level of stimuli at the two ears. Localization of un-modulated signals up to approximately 1500 Hz is known to depend on the interaural time difference (ITD) arising from disparities in the fine-structure of the waveform (for review see Blauert, 1997). The prominent cue for localization of high-frequency signals is the interaural level difference (ILD) cue (Blauert, 1997). However, it has also now been well established that for higher-frequency signals ITD information can be transmitted by imposing a slow modulation, or envelope, on the high-frequency carrier (e.g., Bernstein, 2001). The use of such modulated signals with high-frequency carriers is highly relevant to the coding of speech in cochlear implant processors (Seligman et al., 1984; McDermott et al., 1992; Wilson et al., 1991; Skinner et al., 1994; Wilson and Dorman, 2008a).
Preservation and presentation of binaural cues to bilateral cochlear-implant users pose a unique challenge. Today’s clinical processors are not engineered in a way that allows for coordination between the devices in the two ears; thus the binaural cues that would be available to normal-hearing people are not preserved and presented to the auditory system with fidelity. Bilateral cochlear implant users are essentially fitted with two separate monaural systems. Speech processing strategies in clinical processors utilize pulsatile, non-simultaneous multi-channel stimulation, whereby a bank of bandpass filters is used to filter the incoming signal into numerous frequency bands (ranging from 12 to 22) and to send specific frequency ranges to individual electrodes. The envelope of the signal is extracted from the output of each band and is used to set stimulation levels for each frequency band; however, fine-structure information is discarded. An additional factor is that the rate at which electrical pulses are presented to selected electrodes is fixed, and not necessarily related to the characteristics of the acoustic stimulus. While it is possible that the envelope cues can provide some interaural difference cues that are usable and that lead to binaural advantages, interaural difference cues that arise from fine-structure information in the signal are not available (for review see van Hoesel, 2004; Wilson and Dorman, 2008b).
We investigated binaural processing in electrical hearing using an approach that bypasses the clinical processors and provides direct control over stimuli that are provided to each electrode, and the relative timing and levels of these stimuli. Previous experiments with electrical pulse-trains applied to electrode pairs, in which implant users were required to detect changes in ITD, have reported that discrimination thresholds for stimuli presented at low rates of about 100 Hz can be as low as 50–200 μs in some patients but are up to an order of magnitude larger in others (van Hoesel et al., 1993; Lawson et al., 1998; Majdak et al., 2006; van Hoesel, 2007). Thresholds from best-performers in the cochlear implant population overlap with thresholds seen in moderately trained normal-hearing people (∼70 μs) presented with low-frequency tones carrying ITD information (Blauert, 1997; Bernstein, 2001; Wright and Zhang, 2006).
Our work differs from prior studies in a number of ways. First, rather than testing discrimination abilities for stimuli presented to the right vs left, binaural parameters that are associated with a range of locations in space in acoustic hearing were used in a lateralization task in which listeners reported a perceived intracranial position of the sound source for various ITD or ILD values. This approach, which has been used to some extent over the years by van Hoesel and colleagues (van Hoesel et al., 1993; van Hoesel and Tyler, 2003; van Hoesel, 2008), offers a more direct estimate of the degree to which binaural cues contribute to perceived source locations.
Second, prior studies in this area have utilized small numbers of patients. Furthermore, most recent studies have the added criterion of deliberate exclusion of subjects with poorer than average ITD sensitivity, the reason being that effects of electrical stimulation per se, rather than unknown individual factors, were of interest (e.g., Laback et al., 2007; van Hoesel, 2008). Although these criteria are reasonable for studies that seek to focus on best performance achieved by CI users, they offer less opportunity for understanding the applicability of such findings to the general population of bilateral CI users. Individual variability in performance is one of the most challenging hallmarks of research in this area. One of the known sources of variability is the age at which the onset of deafness occurred. Patients can vary from having experienced onset of deafness prior to language acquisition, after language acquisition but still during childhood, or during adulthood. In addition, in the childhood- and adult-acquired groups, the activation of hearing with CIs can often occur many years after onset of deafness; thus patients vary in the amount of auditory deprivation experienced between the time of cessation of acoustic hearing and time of activation of electric hearing. Typically, patients with adult-onset of deafness have been studied more extensively with regard to binaural sensitivity. In the present study, by also including people with early-onset and childhood-onset of deafness, we were able to examine the extent to which early vs late onset of deafness is an important determinant for successful use of binaural cues with electric hearing.
Third, few published data are available on the importance of place of stimulation along the cochlear electrode array for binaural sensitivity. It may be possible that variation in place of stimulation is a potential factor in the variability observed in ITD sensitivity in prior studies. In this study, place of stimulation was varied to include basal, middle, and apical regions along the electrode array. Since the electrode arrays are essentially confined to the basal turn, the use of the terms apical, middle, and basal indicate relative positions within the basal turn. This approach enabled us to evaluate whether ITD coding at different places of stimulation is processed similarly. In normal-hearing listeners “transposed stimuli” have been used to compare ITD sensitivity for different places of stimulation along the cochlea (Bernstein and Trahiotis, 2002; Oxenham et al., 2004), although mechanical frequency coding differences may preclude sufficient matching of the spatio-temporal response patterns in different regions, an issue that cannot be easily resolved with acoustic stimulation. The use of electric stimulation circumvents this issue by eliminating systematic difference in peripheral responses at high- and low-frequency regions along the cochlea (van Hoesel et al., 2009).
METHODS
Subjects
Eleven adults participated in the study. All were successful users of bilateral cochlear implants who relied on their implants for everyday communication and provided verbal reports regarding noticeable changes in quality of life with bilateral implants compared to single-implant use. All subjects wore Nucleus-24 or 24M devices in both ears. Table 1 shows demographic information for each subject, including age at the time of testing, etiology, age at onset of hearing loss, and duration of cochlear implant use in each ear, or of hearing aid use prior to implantation. All subjects received both of their cochlear implants as adults, and as Table 1 indicates, many of the subjects had experienced hearing loss for numerous years prior to being implanted. The most relevant category for this study is that of age at the onset of deafness, divided into sub-groups of adult, mid-childhood, and pre-lingual. All subjects were native speakers of English and had bilateral listening experience for a minimum of 6 months. Speech processors in everyday use were either bilateral body-worn SPrint or bilateral ear-level ESPrit processors. All subjects traveled to Madison, WI where testing took place for 6–8 h a day for 2–3 days at a time.
Table 1.
Subject details are organized by age-at-deafness group. For each subject the following details are included, if available: age at time of testing, age at onset of hearing loss in each ear, type of hearing loss, etiology if known, duration of cochlear implant use in each ear, and whether a hearing aid was used in each ear.
| Age at deafness | Subject code | Age at testing (yr) | Age at onset of hearing loss | Type of hearing loss | Etiology | Duration of cochlear implant use | Hearing aid use | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |||||
| Adult | IAD | 48 | 18 yrs | 46 yrs | Sudden | Unknown | 16 mo | 16 mo | No | No |
| IAK | 58 | 27 yrs | 27 yrs | Sudden | Ototoxicity | 14 yr | 2.2 yr | Yes | Irregular | |
| IAN | 55.5 | 38 yrs | 38 yrs | Progressive | Hereditary | 6 mo | 7 yr | Yes | Yes | |
| IAP | 64 | 24 yrs | 24 yrs, deaf by 35 yrs | Progressive | Meniere’s syndrome, hereditary | 5 yr | 5 yr | Yes | Yes | |
| Mid- childhood | IAF | 58 | Mid- childhood, deaf by mid-20s | Mid- childhood, deaf by mid-20s | Progressive | Mumps∕measles∕ chicken pox | 1 yr | 7 yr | Until age 47 | Until age 47 |
| IAH | 48.5 | Mid- childhood | Mid- childhood | Progressive | Hereditary | 2.3 yr | 2.3 yr | Yes | Yes | |
| IAJ | 59 | Mid- childhood to adolescence | Mid- childhood to adolescence | Progressive | Unknown | 9 mo | 7.7 yr | Until age 15 | Until age 15 | |
| IAR | 50 | Mid- childhood | Mid- childhood | Unknown | Unknown | 3.3 yr | 4.7 yr | Yes | Yes | |
| Pre- lingual | IAE | 45 | 3 yrs | 3 yrs | Unknown | Unknown | 7 mo | 6 mo | Yes | 10–12 yrs |
| IAG | 49 | At birth | At birth | N∕A | Unknown | 11 mo | 5.7 yr | Yes | Yes | |
| IAI | 49 | At birth | At birth | N∕A | Unknown | 3 yr | 7.8 yr | Yes | Yes | |
Stimuli
All the tests were done by directly activating the electrodes using current levels appropriate for each individual subject. Stimulation was achieved using a custom built research processor (Spear III, The Hearing CRC) that transmits electrical stimulation to the patient’s receiver coils and that is able to control binaural pulse timing within 2.5 μs for chosen pairs of electrodes. Figure 1 shows a schematic of the electrode numbers, 1 and 22 being the most basal and apical electrodes on each electrode array, respectively. In the cochlear implant system used here, physical spacing between adjacent electrodes is 0.75 mm. Stimuli consisted of monopolar constant-amplitude biphasic current-pulses with extra-cochlear reference electrodes. Current pulses were gated on and off instantaneously and presented at a rate of 100 pulses per second (pps) for a total duration of 300 ms per stimulus. Pulse width was 25 μs per phase for 8∕11 subjects (40 for IAI and 50 for IAF and IAG). The 100 pps stimuli were selected based on previous research showing that ITD sensitivity is likely to be strongest at lower stimulation rates (e.g., van Hoesel et al., 2009). It is likely that at the low rate of 100 pps used here ongoing cues provide a strong cue for ITD discrimination in listeners displaying sensitivity to ITDs (e.g., van Hoesel, 2007; Laback et al., 2007; van Hoesel et al., 2009).
Figure 1.
Schematics of the stimuli used in this study are shown. Panel (A) shows the left and right cochlear arrays with 22 intracochlear electrodes. The potential combinations of stimulation places along the array (high-frequency at the base, mid-frequency in the middle, and low-frequency at the apex) are denoted by the arrows. Dashed arrows indicate that pitch-matched electrodes could consist of pairs of electrodes that were not always the same electrode number along the left and right arrays. Panel (B) shows a schematic of the electrically pulsed signals that were presented on each trial. Left- and right-ear stimuli are shown on the top and bottom rows as a function of time. Stimuli were 300 ms in duration, with biphasic pulses presented at a rate of 100 Hz. The interaural time difference can be seen in the difference between onsets of the left- and right-ear pulses.
General procedure
All experimental procedures followed the regulations set by NIH and were approved by the University of Wisconsin’s Human Subject IRB. Subjects reside outside of the Madison, WI area and were thus required to travel to Madison, at which point they stayed in town for several days and spent the majority of each day in the laboratory. Testing was conducted in blocks lasting 20–40 min, with breaks between blocks and long breaks for lunch.
Each experiment began by establishing threshold and maximal comfort levels before discomfort for 20 electrodes (even-numbered electrodes in the two cochleae). Current levels in the 20 electrodes were adjusted to yield equal loudness at a stimulation level near 90% of the dynamic range, comparable to everyday speech levels according to subjective reports. Pairs of electrodes in the two cochleae were selected based on subjective pitch-matching. Subjects were trained to perform a pitch-magnitude-estimation (PME) task, with training lasting approximately 1–2 h, whereby they labeled the perceived pitch of electrical stimulation at various cochlear locations on a numerical scale. Once it was established that PME performance was stable and consistent, PME was measured experimentally for the even-numbered electrodes in each ear, ranging from 2 to 22. A blocked randomized design was used (2 ears×10 electrodes per ear×10 repetitions per electrode). Once testing was completed, all PME data were sorted, and the electrodes with the most similar PME values in the right and left cochleae were selected as pitch-matched electrode pairs. Prior work by van Hoesel (van Hoesel et al., 1993; van Hoesel and Tyler, 2003; van Hoesel et al., 2009) has shown that this method for selecting electrode pairs yields similar results for binaural sensitivity to methods in which place-matching based on x-ray views are used. Selection of best-matched electrode pairs was further adjusted and sharpened using feedback from the participants about pitch similarity for various electrode combinations. The outcome of this elaborate procedure was the identification of three pairs of pitch-matched electrodes for each subject located in the basal, middle, or apical regions of the electrode array within the basal turn. For each electrode pair subsequently used in testing a second stage of stimulus selection took place. When pairs of electrodes that were subjectively reported to be loudness-balanced were activated simultaneously with an ITD of 0 μs, the extent to which the subject heard a fused auditory image with an intracranial position at the center of the head was determined. In the event that the image was un-fused testing was not conducted using that electrode pair. When there was an audible fused image, the perceived location was typically displaced intra-cranially toward the right or left. This perceived position could be shifted by lowering the amplitude of the electrode on the side of the head dominating perception. For instance, if the sound was perceived as right of the midline and the current level unit (CLU) on the right was then lowered by a few current level units the auditory image would shift toward center. Once a fused centered image was established for a given electrode pair, non-zero ITDs or ILDs were imposed, and lateralization judgments were obtained for each cue separately.
Prior to initiating testing on the lateralization task, subjects received training lasting approximately 1 h. There was emphasis placed on determining whether stimulation of each electrode pair produced an auditory image whose positions varied for different values of ITDs and ILDs. Initially, stimuli with large values of ITDs or ILDs were presented one at a time, and subjects were asked to report the perceived intracranial location of the auditory image. This was repeated for smaller values of ITDs and ILDs so that subjects understood that perceived locations could vary. However, feedback was not provided since there is no correct or incorrect response on these types of measures. As is described below, some subjects under some conditions were unable to perceive fused auditory images whose intracranial position could be reported.
During testing, on each trial, the perceived location of the auditory image within the head was measured using a visual pointer on the screen. Subjects faced a computer monitor and viewed a display depicting a horizontal line spanning about 20 cm across the center of the screen. The horizontal line was bisected in the middle with a small vertical line and was marked with the letters L and R on the left-most and right-most edges. Subjects were instructed to treat the horizontal line as the range of intracranial auditory images that span from their left ear to the center of the head and over to the right ear. Subjects used a computer mouse to click on a spot of their choice on the horizontal line that corresponded to perceived intracranial image. The response options on the horizontal lines were continuous, and responses were stored as numerical values that ranged from 0 (perceived near left ear) to 50 (perceived in center of the head) to 100 (perceived near right ear).
Testing sensitivity to binaural cues
Testing was conducted in blocks of trials in which the pair of electrodes being stimulated was held constant. The order for selecting electrode pairs and for testing ITD and ILD sensitivity for a given electrode pair was random. At the beginning of testing with each electrode pair, the general procedure described above for ensuring a fused, centered auditory image near 90% of the dynamic range was followed. For each electrode pair, either ITD or ILD values were varied within a block, with 20 trials per cue value. Prior to testing with the randomized block design, extensive pilot testing was conducted to determine what range of ITDs and ILDs would maximize listeners’ perception of lateralized images with each pair of electrodes. It was sometimes observed that values that were completely lateralized to one ear or the other prior to testing were no longer completely lateralized during testing. Some listeners (in the prelingual group with ITDs, in particular) had difficulty determining left vs right even with ITDs on the order of several milliseconds, but these values were used nonetheless when the experiments were carried out. ITD cue sizes typically ranged from ±25 to ±1000 μs. For subjects that could clearly perform the task at values lower than 800, the typical set of values was 25, 50, 100, 200, 400, and 800. If subjects’ performance was not above chance at ITD=1000 μs we used values greater than 1000 μs. ILD values generally varied from ±1 to ±20 CLUs, translating to ±0.17 to ±3.5 dB in stimulation current, noting that a change of 1 CLU corresponds to 20 log 10(175)∕255=0.176 dB in stimulation current. In some cases, very large values were used. In one case (subject IAI, apical pair), data were not collected because the subject was unable to return for further testing due to personal circumstances.
The data consist of individual listeners’ lateral position judgments. The “raw” data are presented as the average (±SD) perceived lateral position for specific values of binaural cues. In addition, these data were subjected to statistical analyses in which comparisons were made for each condition (combinations of pairs of ITD values, pair of electrodes, for every participant). In order to conduct these analyses, responses were first normalized by applying an arcsin transformation; then for each left-right stimulus value pair (e.g., +400 and −400 μs) the distance between the two distributions of responses for the left and right stimulus values was calculated. At each left-right stimulus value the statistic d′ was computed as the difference between the two means divided by a pooled estimate of their standard deviations. For each condition, a best-fit line was calculated for the d′ values for that condition calculated as described above, with the fit constrained to pass through zero. At high values d′ becomes more variable; thus only d′ values less than or equal to 4 were included in the calculation of the best-fit line. Threshold was defined as the point where the line from the best fit intersected with the value d′=1. It is important to note that the values plotted here refer to two times the magnitude of ITD and ILD relative to values for which an auditory image that was perceived to be centered. In the case of ITDs the relative value was 0 μs and known to be perceived in the center of the head from initial testing and stimulus parameter determination (see above). In the case of ILDs the value of 0 refers to the current levels in the two ears that produced a perceptually centered image. A threshold of ITD=400 μs on one of our plots indicates that the value d′=1 was reached when the left and right ITDs each had a value of 200 μs. An ILD threshold of 4 CLUs on our plots indicates that the value d′=1 was reached when the left and right ILDs each had a value of 2 CLUs.
RESULTS
ITD lateralization data are shown for two listeners in Fig. 2. What differentiates these listeners from one another is the age of onset of deafness, with subject IAD (top) in the childhood-onset group and subject IAG (bottom) in the prelingual-onset group. For subject IAD there is a clear indication that varying the ITD values within the physiologically relevant range of ±700 μs [see shaded gray area, panel (A)] results in perceived locations that spread throughout the range of intracranial positions, from far left to far right. Performance is markedly different for subject IAG; although this listener reported that when the pair of electrodes used here (14L and 14R) was activated a fused auditory image was perceived, the location of the image does not vary systematically along an intracranial dimension as a function of ITD. Panels (C) and (F) plot the d′ values obtained at each ITD size tested. Subject IAD’s performance hovers near d′=1 at small ITD values and reaches this value consistently when the absolute difference in right vs left ITD is 400 μs, with threshold estimated as 213 μs based on a best-fit line calculation. In contrast, the fact that subject IAG is unable to perform the task regardless of the ITD cue size is reflected in d′ values smaller than 1 at all ITDs tested, consistent with there being no discriminable difference between the ITDs favoring the right vs left ears. This was true even at “super” ITD values on the order of a few milliseconds. For this listener, whose onset of deafness was thought to have been present at birth, we observed that ITDs do not evoke a lateralized image nor one with a perceived location. Anecdotal reports from this subject suggest that stimuli with ITDs generated images that were broadly distributed within the head and thus not consistently lateralized in either direction.
Figure 2.
Results from ITD experiments are shown for two subjects, one with adult-onset of deafness [(A)–(C)] and one with pre-lingual onset of deafness [(D)–(F)]. Panels (A) and (D) show the average perceived position (±SD) for each interaural timing difference value tested on the lateralization pointer task. Panels (B) and (E) show the proportion of trials on which each perceived location (lateralization response) was selected for each value of ITD. In (A), (B), (D), and (E) the convention is to use positive and negative values on the abscissa to represent left and right leading stimuli, respectively; the shaded area represents the range of stimulation values that are physiologically relevant and are known to produce auditory images that are fully lateralized for normal-hearing listeners (±700 μs). On the ordinate, lateralization response refers to the value representing the perceived intracranial location of the auditory image as indicated during the pointer task during testing. Panels (C) and (F) show the d′ values obtained at each ITD magnitude, that is, absolute difference in right vs left ITD. The dashed line is drawn at d′=1 which is the value used for threshold criterion throughout the manuscript.
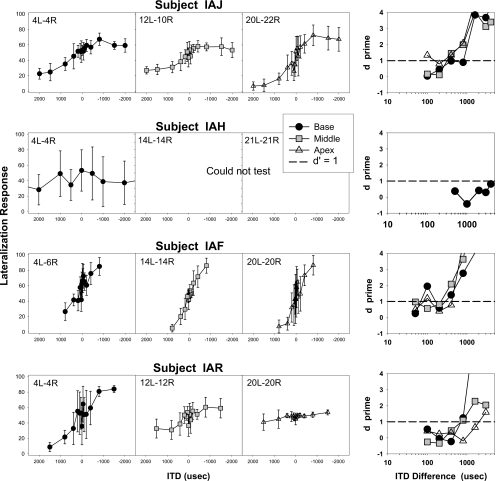
The variability in performance on the ITD lateralization task occurs both amongst and within people who use bilateral cochlear implants, as is illustrated in greater detail in Figs. 345, where lateralization data are plotted for people with onset of hearing loss during adulthood (Fig. 3), mid-childhood (Fig. 4), or prelingually (Fig. 5). In each figure lateralization data are plotted for basal, middle, and apical places of stimulation. The right-most plots in each figure also show d′ calculated for each value of the absolute difference in right vs left ITD (see captions for detail). All four people with bilateral CIs whose onset of deafness occurred during adulthood are able to use ITDs to perceive changes in the lateral position of the intracranial source image. Except for subject IAD’s performance with apical stimulation, lateralization spanned nearly the entire range of responses, suggesting that subjects were able to use ITDs effectively to distinguish intracranial source positions.2 Subject IAD also had trouble with the middle electrode pair but had excellent performance with basal stimulation. It is worth noting that of the four subjects with adult-onset of deafness, this was the only person with long-term asymmetric hearing loss (see Table 1). With regard to the effect of place of stimulation, it appears that performance on this task is not systematically best or worst at any of the places. Unlike IAD whose performance deteriorated as stimulation progressed from base to apex, for other listeners there were assorted best∕worst trends; these can be viewed most clearly in the right-most panels where d′ values are plotted for all three conditions for each listener. For instance, listener IAP’s performance with apical stimulation is notably better than that with basal stimulation. These findings suggest that ITD lateralization is not restricted to regions along the cochlea that respond best to low-frequency signals.
Figure 3.
Results from the ITD task are shown for the four subjects with adult-onset deafness. For each subject the average perceived position (±SD) for each ITD value tested is plotted separately for the three electrode pairs tested, approximately in the base, middle, and apical regions of the arrays; the paired numbers within each panel indicate the left-right electrode numbers for each pair tested. The right-most panels for each subject show d′ values as a function of the left-right ITD tested for the three electrode pairs.
Figure 4.
Same as Fig. 3 for subjects with mid-childhood-onset of deafness.
Figure 5.
Same as Fig. 3 for subjects with prelingual-onset of deafness.
Regarding subjects whose onset of deafness occurred in mid-childhood, it is clear that they are generally able to use ITDs for lateralization. Inter-subject variability is a bit greater than those with adult-onset; whereas subject IAF (Figs. 24) was able to perform as well as people with adult-onset of deafness, IAH performed poorly at the only electrode pair for which testing was possible.3 IAJ and IAR were able to perform the task but the range over which the auditory images were perceived to be lateralized was more restricted. Finally and most notably, subjects whose onset of deafness occurred at birth or prior to age 3 (Fig. 5) were virtually unable to use ITDs on this task, even with “super size” ITD values on the order of several milliseconds. As described above in the statistical analyses section, lateralization thresholds defined as d′=1 were computed for each electrode pair. These values are plotted by subject group in Fig. 6, where the effect of age at onset of deafness is clear, and the lack of trend for place of stimulation is evident as well. Individual thresholds from Fig. 6 are listed in Table 2, along with ILD thresholds (see below). ITD data from the prelingual group fall into one of two categories. As described in Sec. II, some subjects under some conditions were able to perform the task; however, thresholds were >3000 and were thus not accurately determined. These data points are identified in Fig. 6 as “could not determine” (CND). A good example is the apical data point for subject IAI; Fig. 5 contains a curve from measured data, but the ITD at which d′ reached 1 was above 3000 μs. In other cases, testing was attempted but no data were obtained due to extreme difficulty of the task; hence these data are identified as “not measured” (NM). From a statistical perspective CND and NM are essentially the same as in both cases the data are censored, i.e., values of these observations fell outside the range of values that could be determined. Nonetheless, we consider censored data as being meaningful; while they do not provide information regarding thresholds they are informative regarding the subject’s ability to utilize the cues that were manipulated in the experiment.
Figure 6.
Summary of results from ITD sensitivity measures is shown grouped by age at onset of deafness from left to right: prelingual, mid-childhood, and adult. Each data point represents a single subject’s ITD threshold defined as the sum of the microsecond values for the right and left stimuli at which performance reached d′=1; in other words, the ITD value favoring either right or left, multiplied two times. Within each group, results are shown for the three places of stimulation along the cochleae tested (base, middle, and apex). The dashed horizontal line at the top demarcates between data points for which exact threshold was obtained and data points that could not be determined (CND), and data points that were not measured (NM) due to task difficulty (see text).
Table 2.
Thresholds obtained for each subject at each condition tested. Values denoted as CND refer to conditions under which testing was conducted but thresholds were extremely high and could not be determined. Values denoted as NM refer to conditions under which testing was initiated, but thresholds were not obtainable due to extreme difficulty of the task (see text).
| ITD threshold (2×μs) | ILD threshold (2×CLU) | |||||
|---|---|---|---|---|---|---|
| Basal | Middle | Apical | Basal | Middle | Apical | |
| Adult onset | ||||||
| IAD | 180 | 759 | 2662 | 1.41 | 0.53 | 0.64 |
| IAK | 1464 | 753 | 448 | 1.43 | 1.98 | 1.42 |
| IAN | 458 | 400 | 474 | 0.92 | 0.97 | 1.00 |
| IAP | 237 | 321 | 94 | 0.29 | 0.61 | 0.48 |
| Childhood onset | ||||||
| IAF | 276 | 213 | 399 | 1.07 | 0.71 | 1.11 |
| IAH | NM | NM | CND | 0.81 | 1.86 | 1.32 |
| IAJ | 675 | 384 | 340 | 1.42 | 1.12 | 0.51 |
| IAR | 900 | 1154 | 2106 | 1.77 | 0.87 | 0.69 |
| Prelingual | ||||||
| IAE | NM | NM | NM | 0.29 | 0.50 | 0.66 |
| IAG | CND | CND | CND | 5.61 | 3.02 | 4.05 |
| IAI | NM | NM | CND | 1.36 | 1.53 | Not measured |
Statistical analyses consisted of non-parametric tests because there are known limitations to parametric statistics when the N size is small as is the case here and when non-measured or undetermined data points are considered as missing. Subjects were categorized into a 3×2 table of group membership by censorship. Fisher’s exact test for dependence was significant [two-tailed p-value=0.030], thus establishing a relationship between group and censorship, whereby data points from patients with early-onset of deafness were censored more than the other patients. In addition, a test of linear-by-linear association, conducted to evaluate whether there is a linear trend across groups, revealed a significant effect [Χ2(1)=6.433, p=0.015], implying increased likelihood of censorship as subject group progressed from adult-onset to childhood-onset to prelingual-onset of deafness. To evaluate possible condition effects, Friedman’s test was applied to the data collected for uncensored subjects, revealing non-significant findings [Χ2(2)=0.286, p=0.867]. In summary, the statistical analyses conducted here reveal significant effects of age at onset of deafness on ITD sensitivity, but no effects of place of stimulation.
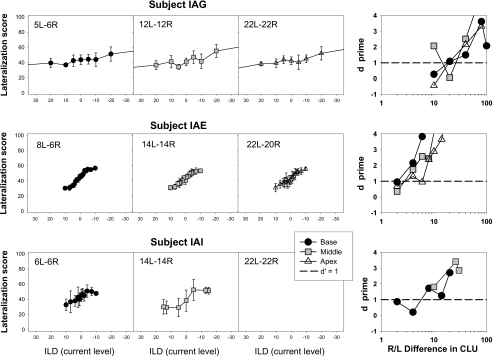
Parallel data from the ILD experiments are shown in Figs. 7891011, where lateralization scores are plotted as a function of CLU offsets to the right or left; 1 CLU corresponds to 0.176 dB in stimulation current (see also footnote n1). Examples from the same two individuals for which ITD data were shown in Fig. 2 are seen in Fig. 7 for ILDs. Subject IAD performed remarkably well on this task, as can be clearly seen in Fig. 7. Worth noting is that error bars for this listener were smaller with ILDs than ITDs, suggesting that perhaps ILDs provided a stronger cue for lateralization. For this listener, ILDs at which d′=1 could barely be measured due to limitations of stimulus presentation at very small CLUs. In contrast, subject IAG showed poorer ability to utilize ILDs for lateralization. On the one hand, although this listener reported hearing a fused auditory image that was perceived to be generally lateralized in the presence of ILDs, her ability to pinpoint specific intracranial positions was poor. In addition, even at large ILDs the auditory event was not greatly shifted in either direction. Nonetheless, it is worth noting that subject IAG was at least able to perform the task with ILD cues, albeit not well, whereas she had been unable to perform the task with ITD cues.
Figure 7.
Results from ILD experiments are shown for two subjects, one with adult-onset of deafness [(A)–(C)] and one with pre-lingual onset of deafness [(D)–(F)]. The ordinate refers to CLUs, noting that a change of 1 CLU corresponds to 20 log 10(175)∕255=0.176 dB in stimulation current (see also footnote n1). Panels (A) and (D) show the average perceived position (±SD) for each interaural level difference value tested on the lateralization pointer task. These are the same subjects for whom ITD data are shown in Fig. 2. Panels (B) and (E) show the proportion of trials on which each perceived location (lateralization response) was selected for each value of ILD. In (A)–(E) the convention is to use positive and negative values on the abscissa to represent left and right leading stimuli, respectively. Panels (C) and (F) show the d′ values obtained at each ILD magnitude, that is, absolute difference in right vs left ILD. The dashed is drawn at d′=1 which is the value used for threshold criterion throughout the manuscript.
Figure 8.
Results from the ILD task are shown for the four subjects with adult-onset deafness. For each subject the average perceived position (±SD) for each ILD value in right-left CLU interaural difference is shown, noting that change of 1 CLU corresponds to 0.176 dB in stimulation current. Each ILD tested is plotted separately for the three electrode pairs tested, approximately in the base, middle, and apical regions of the arrays; the paired numbers within each panel indicate the left-right electrode numbers for each pair tested. The right-most panels for each subject show d′ values as a function of the left-right ILDs tested for the three electrode pairs.
Figure 9.
Same as Fig. 8 for subjects with mid-childhood-onset of deafness.
Figure 10.
Same as Fig. 8 for subjects with prelingual-onset of deafness.
Figure 11.
Summary of results from ILD sensitivity measures are shown, grouped by age at onset of deafness from right to left: adult, mid-childhood, and prelingual. Each data point represents a single subject’s ILD threshold defined as the difference in CLUs between the right and left ILDs at which performance reached d′=1; in other words, the ILD value toward either right or left multiplied two times. Within each group, results are shown for the three places of stimulation along the cochleae tested (base, middle, and apex). There are no data points above the dashed horizontal line at the top because there were not data points that could not be measured or that could not be determined (CND), as was the case in the ITD results.
While subject IAG was selected as an example of a listener with prelingual-onset of deafness whose use of ILDs was poor, there are cases of subjects in this study with prelingual onset of deafness who demonstrate very good lateralization ability using ILDs. Results from the three subject groups are shown in Figs. 8910 in the same order of appearance as the ITD data: adult-, childhood-, and prelingual-onsets of deafness. Unlike the ITD task which depended on the age of onset of deafness, all subjects were able to perform the ILD task. Because the CLU values required to achieve lateralization were somewhat large for some of the listeners, we chose to plot the results with a large range of values on that axis, which compresses the data points for one of the subjects. Nonetheless the equal-range approach allows for direct comparison of performance across all listeners. One notable difference between ITD and ILD lateralization abilities is the greater variation in performance across electrodes with ITDs. For instance, in the adult-onset group, subject IAD who struggled to lateralize the auditory images with middle and apical stimulation using ITDs (see Fig. 3) is able to hear the sound images at the full range of intracranial positions for all places of stimulation with ILDs (see Fig. 8). Similarly, in the childhood-onset group subject IAH is a good example of someone for whom ITDs were not easily accessible on this task (see Fig. 4), whereas ILDs provided an excellent lateralization cue (see Fig. 9). For IAJ and IAF ITDs and ILDs are similarly useful, and IAR seems to improve with ILDs at least with apical stimulation. The change in performance with the ILD task is most markedly seen in the subject group with prelingual onset of deafness. Results in Fig. 10 suggest that all three subjects were able to utilize ILD cues on the lateralization task (note that apical data are missing for subject IAI). In contrast with the other two groups of subjects, however, listeners in the prelingual group never seemed to lateralize the auditory image all the way toward either ear, even at very large interaural CLU differences. ILD thresholds are summarized in Fig. 11 to parallel the summary presented for ITDs in Fig. 6. Individual thresholds from Figs. 611 are listed in Table 2. As with the ITD data, analyses were conducted using non-parametric tests. Effect of condition was analyzed using the Friedman test. All 11 subjects were included with the one missing data point excluded, revealing no significant effect [X2(2)=0.545, p=0.761]. A Kruskal–Wallis test to establish whether there exists a relationship between group and rank mean ILD score revealed a non-significant effect [X2(2)=1.326, p=0.553]. The results suggest that ILD thresholds measured here did not appear to depend on the age at onset of deafness nor on the place of stimulation along the cochlear array. One subject (IAG) however, had very high ILD thresholds compare to the rest of the subjects; thus the statistical analyses will be interpreted with caution.
DISCUSSION
In this study we investigated sensitivity to binaural cues using an experimental approach that enabled us to control ITD and ILD cues presented to selected pairs of electrodes along the cochlear array of bilaterally implanted CI users. Two primary observations are worth noting. First, participants in whom the onset of deafness occurred at a very young age were generally able to use ILD cues, but their ability to use ITD was severely compromised. In contrast, there was a trend for listeners with onset of deafness during childhood or adulthood to be able to utilize both ITD and ILD cues. Second, while there appeared not to be a systematic effect of place of stimulation on lateralization of ITD or ILD cues, individual subjects had better performance with some electrode pairs than others.
Binaural sensitivity and effects of auditory deprivation
In general, binaural sensitivity measures from the lateralization data are in agreement with published results using the discrimination paradigm (e.g., Long et al., 2006; van Hoesel and Tyler, 2003; van Hoesel, 2007; Laback et al., 2007) in which ITD thresholds in bilaterally implanted CI users range from tens of microseconds to a few hundred microseconds. Here we examined this variability in the context of the age at the onset of deafness. Sensitivity to ITDs seemed to be more affected by long-term deprivation of auditory input than was sensitivity to ILD. The three pre-lingually deafened individuals were unable to utilize binaural timing cues to subjectively judge the perceived intracranial position of stimuli containing ITDs; sensitivity to ITDs was also problematic for one individual with childhood-onset of deafness. However, all patients with adult-onset deafness were able to lateralize sounds with right-left differences in ITDs ranging from ∼90 to 400 μs for at least one place of stimulation. When considering ILD sensitivity, subjects who had experienced auditory deprivation early in life did not show overall more susceptibility to disruption compared to subjects with onset of deafness later in life. This trend is in agreement with a previous report with five subjects who had adult-onset deafness in which subjects demonstrated good sensitivity to ILD but variable sensitivity to ITD (van Hoesel and Tyler, 2003). While it is tempting to conclude that the mechanisms involved in processing ITD cues could be more susceptible to hearing loss than are the mechanisms associated with ILDs, these findings must be interpreted with caution, and some caveats are worth noting. First, while the total N size of subjects in this study is relatively large (N=11) compared with prior studies (N size 2–5), the number of subjects in each onset-of-deafness sub-group is too small to draw overarching conclusions. The trends observed here advocate for further research on this issue. Second, whereas the ITD is well defined in both acoustic and electrical stimulations, the electrical ILD is arbitrarily related to acoustic decibels in a processor through a loudness mapping function, and in the present experiment, we do not know what the ILDs used would correspond to in acoustic hearing. Thus, while performance across groups was not different, the ability of patients in our sub-groups to use ILDs in real-world situations may differ. Third, it is possible that in the ILD task subjects had access to some overall monaural level cues. That is, they might have been able to extract information regarding the levels in each ear separately and use those cues when making their judgment regarding intracranial position. Informal observations under monaural conditions suggest otherwise, but proper testing under these conditions is required prior to determining whether monaural cues were used, and if so, by which subjects under what conditions. Nonetheless, we specifically avoided another manipulation in which overall level is roved, as is done in many studies in free field, because it could potentially introduce uncontrolled ILD shifts due to unequal changes in loudness in the two ears, even when the same CLU increments∕decrements are applied.
Results from this study are consistent with much of the prior work in the area of cochlear implantation in which individual variability is a clear hallmark. Here, the notable factor that might have contributed to variability is the age at onset of deafness. It is worth considering whether plasticity following altered sensory experience is particular to the binaural system or represents a more general predisposition of auditory system functionality following deprivation and subsequent activation. There is ample evidence from research with cochlear implant users to suggest that in other areas, including speech and language abilities, adults whose deafness occurred during adult life experience better outcomes than adults whose onset of deafness was early in life (Skinner et al., 1994; Freisen et al., 2001; Busby et al., 1993). This evidence, supporting the general predisposition of neural systems to function best with early exposure, has long-standing roots in other sensory systems (Blakemore and Cooper, 1970; Rakic and Goldman-Rakic, 1982; Kaas et al., 1983), and there is little reason to suspect that the findings with regard to effect of age at onset of deafness on performance are unique to audition.
Several of the subjects experienced deprivation of a different nature. Thus, our results also speak to the long-term potency of functional connections in the binaural auditory pathway, which are particularly notable in the individuals who became deaf as young adults, spent numerous years, even decades, being deprived of hearing, and subsequently had their auditory pathways reactivated with electric hearing. Subject IAK in our study is a good example of someone who was profoundly deaf for 30 years, and within 1 year of having received bilateral cochlear implants shows binaural lateralization thresholds of ∼450 μs for ITDs and 1.2 CLUs for ILDs. Subject IAD is another example of someone who lost hearing in the right ear at age 18 and in the left ear at age 46. Thus, he was deprived of binaural hearing for 28 years. Upon activation with bilateral CIs, he had an ITD threshold of 180 μs in the basal electrode pair and ILD thresholds of 1.4 CLUs or less at all stimulation places. Relative to binaural sensitivity of normal-hearing people, where ITD thresholds can be as low as 10–20 μs, thresholds seen here are more than an order of magnitude worse. Nonetheless, the ITD values at threshold are still potentially usable in real life. For instance, if ILD cues are absent then segregation of sources that are widely separated across the right and left hemifields or source location identification could be achieved with such ITDs.
There may be some insights to be gained from the auditory deprivation literature in which animals underwent periods of monaural occlusion during various stages in development. It appears that neural circuits involved in binaural hearing can be recalibrated throughout life in reaction to altered inputs (Kacelnik et al., 2006). What is not clear from that literature is the extent to which the remapping involves alterations in sensitivity to specific binaural cues. Hence, the extent to which the circuits that mediate ITD and ILD, respectively, are affected by experience remains to be studied with greater precision. When plasticity and recalibration of sensitivity to auditory cues are considered, in particular, at the level of the auditory cortex, there appears to be a protracted period of plasticity in the adult animal, which might help to explain the retention or re-establishment of binaural sensitivity in adults tested in the present study. A factor that is most clearly potent in driving plasticity of neural circuits involved in spatial hearing is the shaping that takes place by training and experience (Kacelnik et al., 2006; Keuroghlian and Knudsen, 2007). These findings from the animal literature can be viewed as potentially encouraging with regard to the role of training and rehabilitation of cochlear implant and hearing aid users and the possibility that, with experience, their spatial maps may be altered in ways that will lead to functional improvement in performance. Also noteworthy is the potential role of cues provided in clinical sound processors. If ITDs are not well coded by sound processors, but ILDs are, then listeners are being trained in their everyday situations with ILDs, but not ITDs.
Finally, recent studies in children who are born deaf and receive bilateral CIs as their first mode of auditory stimulation suggest the important role of early activation in the emergence of binaural interaction components at the level of the brainstem (Gordon et al., 2007) and cortex (Bauer et al., 2006). Behavioral results on this topic come from a population of children who are born deaf and who receive bilateral CIs either at a very young age or at older ages. The former group is more likely to reach age-appropriate spatial hearing resolution (Grieco-Calub et al., 2008) than children who are stimulated bilaterally at a later age (Litovsky et al., 2006b, 2006c). An interesting comparison can be made in future studies between the prelingually deaf children and adults in the prelingual group of the present study. While they share the common trait of prelingual deafness, early stimulation of these children may lead to better performance when they reach adulthood. What remains to be seen is whether these children will have better access to binaural cues, in particular, ITDs, given that the type of stimulation they are receiving provides no obligatory coordination of inputs to the two ears.
Effect of place of stimulation along the cochlear array
Data from the investigation of the effect of place of stimulation reported here suggest that there can be good sensitivity to timing differences with stimulation in the basal region of the cochlea, where innervating auditory nerve fibers are tuned to high-frequencies. This finding lends support to the view that ITDs can be processed by neurons with best sensitivity to high-frequency signals (Joris and Yin, 1995; Joris et al., 2004). ITDs may be categorized as differences in timing of the onset, fine-structure (carrier), and envelope. Generally, in normal-hearing listeners, ITD-based lateralization is dominated by onset and fine-structure cues (Neutzel and Hafter, 1976; Bernstein and Trahiotis, 1985). With high-frequency carriers, where basal stimulation predominates, the ITDs in envelopes can also provide reliable binaural cues for lateralization (Bernstein, 2001; Joris et al., 2004). Our data provide further support for the ability of the auditory system to use ITDs under conditions in which high-frequency auditory nerve fibers are stimulated at the basal region of the cochlea.
Remaining gaps and issues in bilateral CI users
Results from 11 subjects whose data are presented here show a broad range of performance and reflect the type of variability that is a hallmark of performance with CIs. Approaches for improving performance can include a number of avenues. Here, direct tests of sensitivity to binaural cues suggest that ILDs are available to all subjects, and that right-left discrimination thresholds can be as low as the smallest ILD (∼0.1–0.2 dB) allowed by clinical implant systems (Lawson et al., 1998; Long et al., 2003; van Hoesel and Tyler, 2003). In contrast, ITD thresholds tested here and by others are highly variable, ranging from ∼50 μs (rare) to several milliseconds depending on the subject, pulse rate, and electrode pair tested (Lawson et al., 1998; van Hoesel and Tyler, 2003; van Hoesel, 2007). This reflects a clear deviation from performance in normal-hearing listeners, in whom sensitivity to ITD for acoustic stimuli can be as good as thresholds of 10–20 μs, which is clearly better, is also more consistent across subjects, and less dependent on stimulus parameters (Klumpp and Eady, 1956; Yost et al., 1971). In addition, ITD sensitivity in bilateral CI users becomes much worse as the rate of stimulation increases from 100 to 1000 pps (e.g., van Hoesel et al., 2009), which contrasts with pure-tone sensitivity in normal-hearing listeners, which actually improves over the same range of rate increase (Bernstein, 2001; Bernstein and Trahiotis, 2002). Thus, it may be the case that ITD coding is where there may be room for improvement in current bilateral implants. There are caveats, however, in the design of bilateral implants that must be overcome prior to this improvement being possible, which include preservation of binaural information by the clinical sound processors. One area that should be investigated is the possibility that stimulation across the two ears can be more selective so as to ensure that both auditory nerves receive information that is frequency-matched in addition to being well-timed.
ACKNOWLEDGMENTS
The authors are grateful to the bilateral cochlear implant users for their participation. This work was supported by the NIH-NIDCD (Contract No. R01 DC 003083 to R.Y.L.). Support for R.v.H. was provided by The Hearing CRC, Australia. Portions of this work were presented at Association for Research in Otolaryngology Mid-winter Meeting, 2005.
Footnotes
The current produced by the device is I=K×10×175(CL∕255), where I is the current in microamperes and K is a calibration factor that can be disregarded in this description. Taking 20 log 10(x) of each side, the product is 20 log 10(I)=20 log 10(10K)+20(CL∕255)log 10(175). Thus, for a change of 1 CLU, Δ[20 log 10(I)]=20 log 10(175)∕255=0.176 dB in terms of current. Note that the definition of clinical level units is independent of acoustic SPL. The number of CLUs corresponding to a given SPL change is determined by a separate mapping function in the processor (the loudness growth map). The same approach has been used in previous studies (e.g., van Hoesel, 2004, 2008; van Hoesel et al., 1993, 2009).
Extensive discussions with subjects convinced the authors of this paper that the ITD cues were indeed being mapped to specific perceived source locations, rather than some other arbitrary percept.
The term “could not test” refers to extensive attempts during testing to find combinations of stimuli that produced binaural fused auditory images that the listeners would also report to be lateralized with large ITD values.
References
- Bauer, P. W., Sharma, A., Martin, K., and Dorman, M. (2006). “Central auditory development in children with bilateral cochlear implants,” Arch. Otolaryngol. Head Neck Surg. 132, 1133–1136. 10.1001/archotol.132.10.1133 [DOI] [PubMed] [Google Scholar]
- Bernstein, L. R. (2001). “Auditory processing of interaural timing information: New insights,” J. Neurosci. Res. 66, 1035–1046. 10.1002/jnr.10103 [DOI] [PubMed] [Google Scholar]
- Bernstein, L. R., and Trahiotis, C. (1985). “Lateralization of low-frequency, complex waveforms: The use of envelope-based temporal disparities,” J. Acoust. Soc. Am. 77, 1868–1880. 10.1121/1.391938 [DOI] [PubMed] [Google Scholar]
- Bernstein, L. R., and Trahiotis, C. (2002). “Enhancing sensitivity to interaural delays at high frequencies by using ‘transposed stimuli’,” J. Acoust. Soc. Am. 112, 1026–1036. 10.1121/1.1497620 [DOI] [PubMed] [Google Scholar]
- Blakemore, C., and Cooper, G. F. (1970). “Development of the brain depends on the visual environment,” Nature (London) 228, 477–478. 10.1038/228477a0 [DOI] [PubMed] [Google Scholar]
- Blauert, J. (1997). Spatial Hearing (The MIT Press, Cambridge, MA: ). [Google Scholar]
- Busby, P. A., Tong, Y. C., and Clark, G. M. (1993). “Electrode position, repetition rate, and speech perception by early- and late-deafened cochlear implant patients,” J. Acoust. Soc. Am. 93, 1058–1067. 10.1121/1.405554 [DOI] [PubMed] [Google Scholar]
- Coco, A., Epp, S. B., Fallon, J. B., Xu, J., Millard, R. E., and Shepherd, R. K. (2007). “Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons?,” Hear. Res. 225, 60–70. 10.1016/j.heares.2006.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen, L. M., Shannon, R. V., Baskent, D., and Wang, X. (2001). “Speech recognition in noise as a function of the number of spectral channels: Comparison of acoustic hearing and cochlear implants,” J. Acoust. Soc. Am. 110, 1150–1163. 10.1121/1.1381538 [DOI] [PubMed] [Google Scholar]
- Gordon, K. A., Valero, J., and Papsin, B. C. (2007). “Auditory brainstem activity in children with 9–30 months of bilateral cochlear implant use,” Hear. Res. 233, 97–107. 10.1016/j.heares.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Grieco-Calub, T. M., Litovsky, R. Y., and Werner, L. A. (2008). “Using the observer-based psychophysical procedure to assess localization acuity in toddlers who use bilateral cochlear implants,” Otol. Neurotol. 29, 235–239. 10.1097/mao.0b013e31816250fe [DOI] [PubMed] [Google Scholar]
- Joris, P. X., and Yin, T. C. (1995). “Envelope coding in the lateral superior olive. I. Sensitivity to interaural time differences,” J. Neurophysiol. 73, 1043–1062. [DOI] [PubMed] [Google Scholar]
- Joris, P. X., Schreiner, C. E., and Rees, A. (2004). “Neural processing of amplitude-modulated sounds,” Physiol. Rev. 84, 541–577. 10.1152/physrev.00029.2003 [DOI] [PubMed] [Google Scholar]
- Kaas, J. H., Merzenich, M. M., and Killackey, H. P. (1983). “The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals,” Annu. Rev. Neurosci. 6, 325–356. 10.1146/annurev.ne.06.030183.001545 [DOI] [PubMed] [Google Scholar]
- Kacelnik, O., Nodal, F. R., Parsons, C. H., and King, A. J. (2006). “Training-induced plasticity of auditory localization in adult mammals,” PLoS Biol. 4, e71. 10.1371/journal.pbio.0040071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuroghlian, A. S., and Knudsen, E. I. (2007). “Adaptive auditory plasticity in developing and adult animals,” Prog. Neurobiol. 82, 109–1021. 10.1016/j.pneurobio.2007.03.005 [DOI] [PubMed] [Google Scholar]
- Klumpp, R. G., and Eady, H. R. (1956). “Some measurements of interaural time difference thresholds,” J. Acoust. Soc. Am. 28, 859–860. 10.1121/1.1908493 [DOI] [Google Scholar]
- Laback, B., Majdak, P., and Baumgartner, W. D. (2007). “Lateralization discrimination of interaural time delays in four-pulse sequences in electric and acoustic hearing,” J. Acoust. Soc. Am. 121, 2182–2192. 10.1121/1.2642280 [DOI] [PubMed] [Google Scholar]
- Lawson, D. T., Wilson, B. S., Zerbi, M., van den Honert, C., Finley, C. C., Farmer, J. C., Jr., McElveen, J. T., Jr., and Roush, P. A. (1998). “Bilateral cochlear implants controlled by a single speech processor,” Am. J. Otol. 19, 758–761. [PubMed] [Google Scholar]
- Leake, P. A., Hradek, G. T., and Snyder, R. L. (1999). “Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness,” J. Comp. Neurol. 412, 543–562. [DOI] [PubMed] [Google Scholar]
- Litovsky, R. Y., Parkinson, A., and Arcaroli, J. (2009). “Spatial hearing and speech intelligibility in bilateral cochlear implant users,” Ear Hear. 30, 419–431. 10.1097/AUD.0b013e3181a165be [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R. Y., Parkinson, A., Arcaroli, J., Peters, R., Lake, J., Johnstone, P., and Yu, G. (2004). “Bilateral cochlear implants in adults and children,” Arch. Otolaryngol. Head Neck Surg. 130, 648–655. 10.1001/archotol.130.5.648 [DOI] [PubMed] [Google Scholar]
- Litovsky, R. Y., Parkinson, A., Arcaroli, J., and Sammeth, C. (2006a). “Simultaneous bilateral cochlear implantation in adults: A multicenter clinical study,” Ear Hear. 27, 714–731. 10.1097/01.aud.0000246816.50820.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R. Y., Johnstone, P. M., and Godar, S. P. (2006b). “Benefits of bilateral cochlear implants and/or hearing aids in children,” Int. J. Audiol. 45, S78–S91. 10.1080/14992020600782956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R. Y., Johnstone, P. M., Godar, S., Agrawal, S., Parkinson, A., Peters, R., and Lake, J. (2006c). “Bilateral cochlear implants in children: Localization acuity measured with minimum audible angle,” Ear Hear. 27, 43–59. 10.1097/01.aud.0000194515.28023.4b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, C. J., Carlyon, R. P., Litovsky, R. Y., and Downs, D. H. (2006). “Binaural unmasking with bilateral cochlear implants,” J. Assoc. Res. Otolaryngol. 7, 352–360. 10.1007/s10162-006-0049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, C. J., Eddington, D. K., Colburn, H. S., and Rabinowitz, W. M. (2003). “Binaural sensitivity as a function of interaural electrode position with a bilateral cochlear implant user,” J. Acoust. Soc. Am. 114, 1565–1574. 10.1121/1.1603765 [DOI] [PubMed] [Google Scholar]
- Majdak, P., Laback, B., and Baumgartner, W. D. (2006). “Effects of interaural time differences in fine structure and envelope on lateral discrimination in electric hearing,” J. Acoust. Soc. Am. 120, 2190–2201. 10.1121/1.2258390 [DOI] [PubMed] [Google Scholar]
- McDermott, H., McKay, C., and Vandali, A. (1992). “A new portable sound processor for the University of Melbourne/Nucleus Limited multi electrode cochlear implant,” J. Acoust. Soc. Am. 91, 3367–3371. 10.1121/1.402826 [DOI] [PubMed] [Google Scholar]
- Nuetzel, J. M., and Hafter, E. R. (1976). “Lateralization of complex waveforms: Effects of fine structure, amplitude, and duration,” J. Acoust. Soc. Am. 60, 1339–1346. 10.1121/1.381227 [DOI] [PubMed] [Google Scholar]
- Nopp, P., Schleich, P., and D’Haese, P. (2004). “Sound localization in bilateral users of MED-EL COMBI 40∕40+ cochlear implants,” Ear Hear. 25, 205–214. 10.1097/01.AUD.0000130793.20444.50 [DOI] [PubMed] [Google Scholar]
- Oxenham, A. J., Bernstein, J. G., and Penagos, H. (2004). “Correct tonotopic representation is necessary for complex pitch perception,” Proc. Natl. Acad. Sci. U.S.A. 101, 1421–1425. 10.1073/pnas.0306958101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic, P., and Goldman-Rakic, P. S. (1982). “The development and modifiability of the cerebral cortex,” Neurosci. Res. Program Bull. 20, 433–438. [PubMed] [Google Scholar]
- Schleich, P., Nopp, P., and D’Haese, P. (2004). “Head shadow, squelch, and summation effects in bilateral users of the MED-EL COMBI 40∕40+ cochlear implant,” Ear Hear. 25, 197–204. 10.1097/01.AUD.0000130792.43315.97 [DOI] [PubMed] [Google Scholar]
- Seligman, P. M., Patrick, J. F., Tong, Y. C., Clark, G. M., Dowell, R. C., and Crosby, P. A. (1984). “A signal processor for a multiple-electrode hearing prosthesis,” Acta Oto-Laryngol., Suppl. 411, 135–139. [PubMed] [Google Scholar]
- Skinner, M. W., Clark, G. M., Whitford, L. A., Seligman, P. M., Staller, S. J., Shipp, D. B., Shallop, J. K., Everingham, C., Menapace, C. M., Arndt, P. L. (1994). “Evaluation of a new spectral peak coding strategy for the Nucleus 22 channel cochlear implant system,” Am. J. Otol. 15, 15–27. [PubMed] [Google Scholar]
- van Hoesel, R. J. (2004). “Exploring the benefits of bilateral cochlear implants,” Audiol. Neuro-Otol. 9, 234–246. 10.1159/000078393 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. (2007). “Sensitivity to binaural timing in bilateral cochlear implant users,” J. Acoust. Soc. Am. 121, 2192–2206. 10.1121/1.2537300 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. M. (2008). “Observer weighting of level and timing cues in bilateral cochlear implant users,” J. Acoust. Soc. Am. 124, 3861–3872. 10.1121/1.2998974 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J., and Tyler, R. S. (2003). “Speech perception, localization, and lateralization with bilateral cochlear implants,” J. Acoust. Soc. Am. 113, 1617–1630. 10.1121/1.1539520 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. M., Jones, G. L., and Litovky, R. Y. (2009). “Interaural time-delay sensitivity in bilateral cochlear implant users: Effects of pulse rate, modulation rate, and place of stimulation,” J. Assoc. Res. Otolaryngol. 10, 557–567. 10.1007/s10162-009-0175-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoesel, R. J., Tong, Y. C., Hollow, R. D., and Clark, G. M. (1993). “Psychophysical and speech perception studies: A case report on a binaural cochlear implant subject,” J. Acoust. Soc. Am. 94, 3178–3189. 10.1121/1.407223 [DOI] [PubMed] [Google Scholar]
- Wilson, B. S., and Dorman, M. F. (2008a). “Cochlear implants: A remarkable past and a brilliant future,” Hear. Res. 242, 3–21. 10.1016/j.heares.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, B. S., and Dorman, M. F. (2008b). “Cochlear implants: Current designs and future possibilities,” J. Rehabil. Res. Dev. 45, 695–730. 10.1682/JRRD.2007.10.0173 [DOI] [PubMed] [Google Scholar]
- Wilson, B. S., Finley, C. C., Lawson, D. T., Wolford, R. D., Eddington, D. K., and Rabinowitz, W. M. (1991). “Better speech recognition with cochlear implants,” Nature (London) 352, 236–238. 10.1038/352236a0 [DOI] [PubMed] [Google Scholar]
- Wright, B. A., and Zhang, Y. (2006). “A review of learning with normal and altered sound-localization cues in human adults,” Int. J. Audiol. 45, S92–S98. 10.1080/14992020600783004 [DOI] [PubMed] [Google Scholar]
- Yost, W. A., Wightman, F. L., and Green, D. M. (1971). “Lateralization of filtered clicks,” J. Acoust. Soc. Am. 50, 1526–1531. 10.1121/1.1912806 [DOI] [PubMed] [Google Scholar]