Abstract
Neuroprosthetic devices have made a major impact in the treatment of a variety of disorders such as paralysis and stroke. However, a major impediment in the advancement of this technology is the challenge of maintaining device performance during chronic implantation (months to years) due to complex intrinsic host responses such as gliosis or glial scarring. The objective of this review is to bring together research communities in neurobiology, tissue engineering, and neuroprosthetics to address the major obstacles encountered in the translation of neuroprosthetics technology into long-term clinical use. This article draws connections between specific challenges faced by current neuroprosthetics technology and recent advances in the areas of nerve tissue engineering and neurobiology. Within the context of the device–nervous system interface and central nervous system implants, areas of synergistic opportunity are discussed, including platforms to present cells with multiple cues, controlled delivery of bioactive factors, three-dimensional constructs and in vitro models of gliosis and brain injury, nerve regeneration strategies, and neural stem/progenitor cell biology. Finally, recent insights gained from the fields of developmental neurobiology and cancer biology are discussed as examples of exciting new biological knowledge that may provide fresh inspiration toward novel technologies to address the complexities associated with long-term neuroprosthetic device performance.
Keywords: neuroprosthetic, gliosis, nerve regeneration, signal degradation, Utah electrode, drug delivery, neural progenitor, neural probes
Introduction
Neuroprosthetic device technology has seen major advances in recent years but the full potential of these devices remains unrealized due to outstanding challenges, such as the ability to record consistently over long periods of time. Existing data relates this signal reliability problem to an intrinsic host tissue response upon neuroelectrode implantation, namely glial scarring or gliosis, which involves a complex series of events that occur following implantation and whose effects influence device performance over long periods of time. The fabrication, implantation, and operation of neuroprosthetic devices are all highly complex areas in their own right and the major advances made to date in neuroprosthetics, such as the BrainGate® system developed by Cyberkinetics Corp. (Figure 1) and the Boston Retinal Implant Project (Winter et al., 2007a), are a testament to the success of numerous interdisciplinary collaborations. However, the complex biological interface between neuroprosthetic devices and the nervous system is still not completely understood, presenting both challenges as well as opportunities. Historical divisions have existed between research communities in neurobiology, tissue engineering, and neuroprosthetics but each discipline stands to benefit from the contributions of the other. For example, the neurobiology community has developed several in vivo and in vitro models to elucidate mechanistic aspects of central nervous system (CNS) wound healing. The tissue engineering community has devised tools to regenerate tissue using novel three-dimensional (3-D) constructs, scaffolds, and bioreactors. The neuroprosthetics community has developed a wide range of highly sophisticated stimulating and recording devices and demonstrated their efficacy with primate and human trials.
Figure 1.
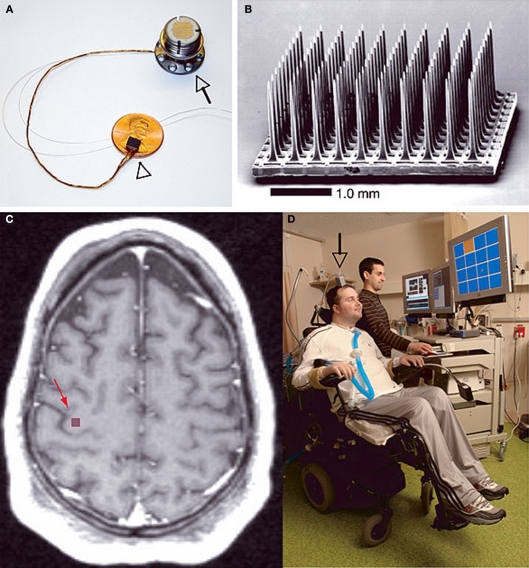
The BrainGate® neural interface system created by Cyberkinetics Corp described by Hochberg et al.(2006). (A) Shows the device assembly consisting of the sensor resting on a U.S. penny, a 13-cm ribbon cable, and a percutaneous titanium pedestal which is secured to the skull. (B) Scanning electron micrograph of the probe, which is a 100-electrode Utah Array. (C) T1-weighted brain MRI of a tetraplegic patient showing the approximate location of the sensor implant site. (D) The first participant in the device trial showing complete external instrumentation of the BrainGate® system which allows him to move a computer mouse pointer on a screen toward the orange square directed solely by intent. Reprinted by permission from Macmillan Publishers Ltd: Nature 442, 164–171, copyright 2006.
This article presents a brief review of challenges faced by current neuroprosthetics technology within the context of the device–nervous system interface and CNS implants. The complexity of the neural tissue response to implantation is described from the perspective of neuroprosthetics as well as that of neurobiology. These descriptions provide a framework within which specific areas of synergy between neuroprosthetics, tissue engineering, and neurobiology are discussed. These areas of synergy include in vitro models of gliosis and brain injury, nerve regeneration strategies, and neural stem/progenitor cell (NPC) biology.
Electrical Interface Challenges in Neural Implants
The primary concern in translating neuroprosthetic technology from laboratory settings to the clinic is the degradation of electrode performance over time. A recent review by Schwartz has suggested that on an average, a chronic electrode implanted in monkey cortex has only about 40–60% probability of recording activity with the exception of the most resilient animal or the electrode that sustains several months to years of good recording (Schwartz, 2004). The signal attenuation of implanted neuroelectrodes in chronic settings occurs primarily due to the biological response of host brain tissue to implanted foreign material, i.e., reactive gliosis (Rousche and Normann, 1998; Liu et al., 1999; Nicolelis et al., 2003; see Polikov et al., 2005 for a comprehensive review). Several groups have reported gradual attenuation of electrical signals over a period of a few days to months after implantation (Rousche and Normann, 1998; Liu et al., 1999, 2006; Williams et al., 1999; Nicolelis et al., 2003; Hochberg et al., 2006).
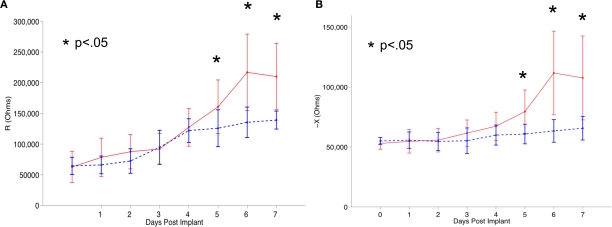
To address this problem, future generations of neuroelectrodes are being designed with the aim of reducing tissue encapsulation and improving long-term device utilization. Although such engineered probes/models show better success rates in vitro (Massia and Hubbell, 1990; Ignatius et al., 1998; Saneinejad and Shoichet, 1998; Tong and Shoichet, 1998, 2001; Cui et al., 2001; Webb et al., 2001; Kapur and Shoichet, 2003; Moore et al., 2006; Gomez and Schmidt, 2007; Gomez et al., 2007; Achyuta et al., 2009), animal studies have shown that engineered probes elicit similar host tissue response chronically, compared to their un-modified cohorts. The eventual result is signal degradation over time (Cui et al., 2003; Ludwig et al., 2006). For example, in vivo studies in rats conducted on polypyrrole/peptide coated neural probes failed to record signals following 2 weeks of implantation (Cui et al., 2003). In another study by Ludwig et al. (2006), chronic recordings in rats with electrodes coated with poly(3,4-ethylenedioxythiophene) (PEDOT; a conducting polymer) (Groenendaal et al., 2000) showed lower impedance initially, but gradually matched that of the uncoated probe, yet again indicating signal attenuation with time (Ludwig et al., 2006). Figure 2 shows a quantitative illustration of the increase in impedance over time observed by Williams et al. (2007) using microwire electrode arrays implanted in rat cortices.
Figure 2.
Tissue reaction against implanted neural electrodes eliciting minor gliosis (shown as dotted blue line) and exacerbated gliosis (shown as solid red line) in a rat in vivo system quantified (via impedance spectroscopy) by Williams et al. (2007). In the figure, (A) and (B) represent the real (R) and imaginary (X) components of complex impedance. The asterisks denote the days where impedance values were significantly different between the groups (p < 0.05). Reproduced from Williams et al. (2007) with permission from IOP Publishing.
Immune rejection and reactive gliosis are not the only reasons for the electrode performance drop over time. For instance, Hochberg et al. (2006) (Figure 1) reported failure of recording in a human subject after 6.5 months of implantation due to physical short circuiting of the electrodes, cable and/or the connector to ground. In the same report, following 10 months of successful recording in a 55-year-old second human subject, an abrupt signal loss owing to an unknown technical problem resulted in the termination of the trial. In another report by Williams et al. (1999) loosening of skull cap (that keeps the electrode in place) was observed along with medical complications leading to failure of recording capability within 15–25 weeks of implantation. In addition, since the position of most neuroelectrodes is fixed with respect to the cortical surface, device micro-motion may lead to movement of the device to a different cortical area or into the white matter leading to signal loss over time (Williams et al., 1999; Schwartz, 2004; Zhong and Bellamkonda, 2008). These failures serve as potent motivators to rethink and re-examine the signal recording reliability problem because successful implantation in patients would require probes to record consistently over decades (Nicolelis and Ribeiro, 2002).
Biological Response to Neuroprosthetic Devices
The conventional view of host response to implanted neuroprosthetics centers on an acute response, characterized by injury, inflammation and microglial activation, followed by a chronic response, which terminates in the formation of an impenetrable glial/fibrotic scar around the implant. A schematic overview of the glial scar formation process is shown in Figure 3. These responses are highly complex because the activity and organization of key biological mediators of the host response are dynamic (see examples in Table 1) and depend on the type and location of implant. This section provides an overview of these responses (Sections “Acute Response to Neuroprosthetic Implants,” “Chronic Response to Neuroprosthetic Implants,” and “Effects of Implantation Procedure on Biological Response”) as well as a discussion of how recent findings from the field of neurobiology provide additional insights into the dynamics and heterogeneity of the CNS scar environment (Sections “CNS Injury Invokes a Complex Cellular and Molecular Response” and “Heterogeneity and Dynamics of the Glial Scar Environment”).
Figure 3.
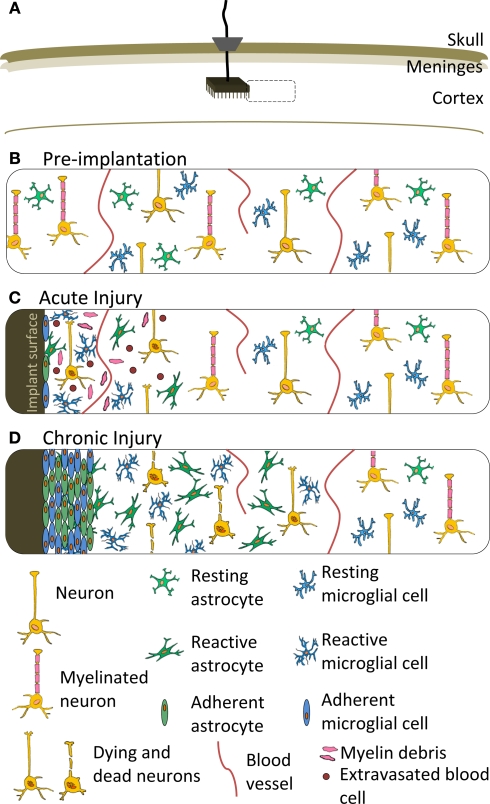
Schematic representation of the cellular response to a neuroprosthetic implant. (A) Location of implant featuring a Utah Array, ribbon cable and percutaneous pedestal secured to the skull. Dashed box represents the location of the close-up views in (B–D). (B) Cellular organization prior to device implantation. (C) Acute injury response featuring microglia and astrocyte adhesion to the implant surface, as well as a variety of cellular responses local to the implant, including acute inflammation, extravasated red and white blood cells, myelin debris, edema and damaged neurons. (D) Chronic injury response to a neuroprosthetic implant featuring isomorphic glial scar and neuronal death local to the implant and surrounded by anisomorphic glial scar at a distance from the implant. The acute inflammation has subsided and edema and cellular debris is cleared. Meningeal fibroblasts are not shown, but may contribute a fibrotic component to the scar depending on characteristics of the device and implantation procedure. Features are not drawn to scale.
Table 1.
Representative biomolecules secreted in response to CNS injury*.
| CNS cell types | Secreted molecules | ECM components secreted | |
|---|---|---|---|
| Inhibitory | Neurotrophic | ||
| Oligodendrocytes and their precursors | NI250, MAG, tenascin-R (Fawcett and Asher, 1999) | GDNF (Wilkins et al., 2003) | NG2, chondroitin sulfate proteoglycans (Rhodes et al., 2006) |
| Microglia/macrophages | MCP-1, IL-1, IL-6, TNF-α, reactive oxygen species, glutamate, IL-1β, IL-3, VEGF (Liberto et al., 2004; Polikov et al., 2005) | NGF, BDNF, NT-3 (Polikov, 2009) | Fibronectins (Kao et al., 2001), thrombospondin (Chamak et al., 1994) |
| Astrocytes | Vimentin, tenascin, semaphorin 3, ephrin-B2, TGF-β1, β2 (Fitch and Silver, 2008) | Laminins, N-cadherin, N-CAM, IGF, NGF, BDNF, NT-3, ADNF, HGF (Fawcett and Asher, 1999; Liberto et al., 2004; Rolls et al., 2009) | Chondroitin sulfate proteoglycans, basal lamina components (Fawcett and Asher, 1999; Polikov, 2009) |
| Meningeal cells | NG2 proteoglycan (Fawcett and Asher, 1999) | Laminins (Fawcett and Asher, 1999) | Collagens, fibronectins, chondroitin sulfate proteoglycans (Manwaring et al., 2001) |
Acute response to neuroprosthetic implants
The acute response is characterized by formation of a wound by the implantation process and the inflammatory and attempted wound healing responses that follow. The electrode implantation procedure ruptures the blood brain barrier (Zhong and Bellamkonda, 2008) and disrupts the neurovasculature (Bjornsson et al., 2006), causing hypoxia and resulting in the death of glial and neuronal cells (Polikov, 2009). Inflammation (a normal defense mechanism that helps to clear infected, dead, and damaged tissue and return it to a normal state) ensues and results in the activation of microglia, astrocytes and infiltrated peripheral macrophages. Once activated, these cells secrete a number of beneficial and harmful factors, including cytokines, chemokines, neurotransmitters and reactive oxygen species (for a comprehensive review, see Whitney et al., 2009). As a result of the initial mechanical trauma and inflammation, swelling of the tissue may also occur (Black, 1999; Ludwig et al., 2006) resulting in the displacement of neighboring neurons away from the electrode surface (Biran et al., 2005). In addition, the blood brain barrier rupture causes blood components such as leukocytes and platelets (Polikov et al., 2005) to infiltrate into the CNS along with serum components (Nadal et al., 1997) and cytokines (Balasingam et al., 1994; Raivich et al., 1999) that actively participate in inflammatory and wound healing processes (Fitch and Silver, 1997; Fawcett and Asher, 1999). These initial events result in altered basal synaptic transmission and plasticity (Dityatev et al., 2008; Kawasaki et al., 2008) and are exacerbated by the large mismatch in the elastic modulus of the silicon (widely-used probe material; ∼100 GPa) and brain tissue (∼100 kPa) (Miller and Chinzei, 2002). Furthermore, due to the presence of necrotic tissue following insertion, the hydrostatic pressure around the implanted electrodes is increased, causing edema and adding to the damaging effect resulting from the presence of electrodes (Schmidt et al., 1993). After about 6–8 days of implantation, the microglia have cleared a substantial amount of cellular debris by phagocytosis (Giordana et al., 1994; Fujita et al., 1998) and the excess fluid due to edema is reabsorbed (Stensaas and Stensaas, 1976). These events are seen as an acute reaction of the brain tissue to the implanted foreign object and can be externally detected in the form of a spike in impedance values during the first few days of recording (Vetter et al., 2004; Ludwig et al., 2006).
Chronic response to neuroprosthetic implants
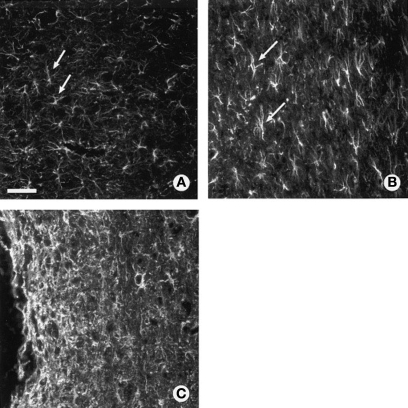
Once the acute reaction subsides, the chronic reaction is initiated due to the persistent presence of an insoluble foreign material (Landis, 1994; Fawcett and Asher, 1999; Turner et al., 1999; Szarowski et al., 2003; Biran et al., 2005; Polikov et al., 2005; Polikov, 2009). This reaction is a complex cascade of events characterized by continued inflammation, adhesion of activated microglia on the probe surface, astrocyte activation, and the formation of glial/fibrotic scar tissue that surrounds and insulates the probe (Turner et al., 1999; Szarowski et al., 2003; Polikov et al., 2005; Brazda and Muller, 2009). Figure 4 shows the characteristic glial scarring response that follows after spinal cord injury in a rat.
Figure 4.
Glial scar ultrastructure within adult rat spinal cord as revealed by GFAP staining. (A) Astrocytes in normal uninjured thoracic spinal cord. (B) Isomorphic gliosis showing tissue surrounding GFAP positive astrocytes (arrows) is much less disturbed following a lacerating injury (out of frame). (C) Anisomorphic gliosis showing a dense scar tissue composed of activated astrocytes with interlocking processes encapsulating the damaged region of CNS (to the left of the frame is a spinal lesion). Scale = 20 μm. Figure adapted from McGraw et al. (2001). Copyright 2001 Wiley & Co. Reproduced with permission.
The first event that occurs in the chronic reaction, and one that probably persists throughout the duration of the presence of implant, is the attachment and clustering of microglia on the implant surface (Stensaas and Stensaas, 1976; Winn et al., 1989; Mofid et al., 1997; Kao et al., 1999), as shown in Figure 5. This attachment is thought to be mediated by the adsorption of serum on the implant surface or due to the release of chemo-attractants by serum factors such as monocytes chemotactic protein-1 (MCP 1) and macrophage inflammatory protein (MIP-1) at injury sites (Saadoun et al., 2005). Following colonization, these cells try to degrade and remove the implant by secreting lytic enzymes and reactive oxygen species (Kyrkanides et al., 2001; Takeuchi et al., 2001). The action of these cells is analogous to that of peripherally derived macrophages that fuse into giant multi-nucleated cells to degrade foreign objects (Stensaas and Stensaas, 1976). In addition, microglia are also known to produce cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) that may result in astrocyte activation (Merrill and Benveniste, 1996; John et al., 2005). Furthermore, microglia have been postulated to regulate the production of the basal lamina, a thin sheet comprising extracellular matrix (ECM) proteins, that aids in organizing the glial scar (Polikov, 2009). The proteins in this basal lamina can act synergistically to present a substrate for cellular attachment via laminin and collagen (Alberts et al., 2001).
Figure 5.
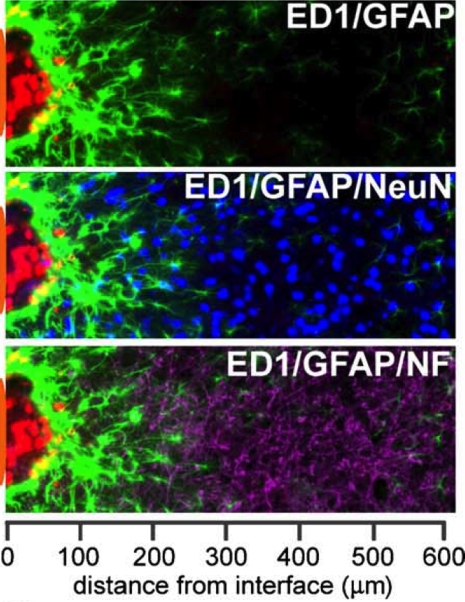
Immunoreactive cellular stratification at the brain-neuroelectrode interface examined using cell-specific markers in a histological section of a rodent brain following a 4-week microlectrode implantation. Microglial (ED1+; red) proximity to the immediate vicinity of the probe indicates inflammation. Scar-like astrocytes (GFAP+; green) with interwoven processes indicate anisomorphic gliosis. The area of inflammation and intense astrocytic reactivity has a reduced number of neurons, indicated by NeuN+ (blue) and neurofilament+ (purple) cells indicating loss of connectivity between the probe and the neurons following 4 weeks of implantation. The neuroelectrode position is illustrated by an orange patch on the left side of the image. Reprinted from Biran et al. (2005). Copyright 2005, with permission from Elsevier.
In addition to the influence of microglia, astrocyte activation is also thought to be mediated by blood-borne factors such as thrombin and albumin that infiltrate the brain during the neuroelectrode insertion procedure (Logan and Berry, 2002). Following activation, the astrocytes begin to proliferate and secrete inhibitory molecules such as chondroitin sulfate proteoglycans, ultimately leading to an organized, dense sheath around the implant (Fawcett and Asher, 1999; Turner et al., 1999; Szarowski et al., 2003; Polikov et al., 2005; Polikov, 2009). The final structure of the glial scar is so robust that it prevents any regenerative axon penetration by mechanical (Fawcett and Asher, 1999) and chemical (Roitbak and Sykova, 1999) means. In fact, a novel hypothesis indicates that the formation of glial scar (Liu et al., 1999; Turner et al., 1999) and microglial activation (Biran et al., 2005) together result in pushing the neuronal bodies away from the surface of the electrodes (as indicated in Figure 5) thereby increasing the impedance over time and leading to signal loss in chronic recordings. More recently, McConnell et al. (2009) have proposed that the this loss of connectivity between the neurons and the electrode substrate can also result from the progressive degeneration of neurons caused due to local chronic inflammation similar to the degeneration observed in Alzheimer's disease.
While this review focuses on the injury response of neuronal and glial cells to neuroprosthetic implants, it is important to note the possible role of fibroblasts that may infiltrate from injured meninges and damaged blood vessels into the device environment (Berry et al., 1983; Carbonell and Boya, 1988). These fibroblasts can further contribute to the encapsulation of the device by cellular and ECM materials and exacerbate the insulative and barrier properties of this scar tissue that compromise device performance and neuronal function. The extent of fibroblast infiltration depends on the proximity of the device to the meninges as well as the extent of injury caused by the device geometry, implantation procedure and implant micro-motion (as described in the next section).
Effects of implantation procedure on biological response
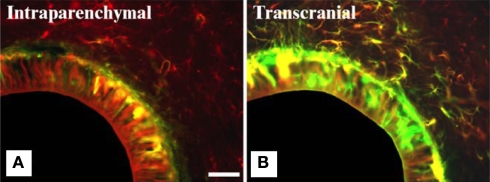
The aforementioned events are restricted to the acute and chronic response of the brain tissue upon implanting a foreign object. However, other factors such as device insertion technique [manual (Stensaas and Stensaas, 1976; Liu et al., 1999) or robotic (Maynard et al., 2000; Csicsvari et al., 2003; Nicolelis et al., 2003; Szarowski et al., 2003)], speed of insertion (Edell et al., 1992; Nicolelis et al., 2003; Bjornsson et al., 2006), and most importantly, implantation approach (Biran et al., 2007) all have been reported to affect the biological response significantly. For instance, Kim et al. (2004) elegantly compared the biological response elicited by two implantation schemes in rat cortices (Figure 6). The first scheme utilized was transcranial implantation (a practical model with respect to neuroelectrodes) of hollow fiber membranes (HFMs) and the second scheme was intracranial implantation. A crucial finding of this study was that transcranially implanted HFMs elicited exacerbated inflammation and gliosis (as indicated by enhanced positive immunofluorescence reactivity for ED1 and GFAP). Furthermore, elevated ECM deposition and fibroblastic encapsulation were observed around the transcranially implanted HFMs. In contrast, intracranial implants (which were completely surrounded by brain tissue) elicited mitigated inflammation and gliosis. Since the transcranially implanted HFMs were in chronic contact with the meninges, the authors concluded that fibroblasts or NPCs had infiltrated into the grey matter resulting in an intense reaction.
Figure 6.
Intensity of glial scarring in rats based on implantation procedure. (A,B) show co-expression analysis of GFAP (red) and vimentin (green) of implanted hollow fiber membranes (HFMs) in rats via two implantation schemes. Vimentin is expressed by astrocytes, microglia and fibroblasts derived from the meninges and other connective tissues. (A) Intraparenchymal implant (where the entire implant is surrounded by brain tissue) show less vimentin immunoreactivity along with GFAP reactivity. (B) Transcranial HFMs show thick layers of GFAP+/vimentin+ cells suggesting meningeal fibroblast infiltration due to skull-tethering of HFMs in such implants. The same study also showed higher ED1 reactivity in transcranial implants compared to intraparenchymal implants highlighting the exacerbated gliosis in the former implantation scheme. Scale = 100 μm. Reprinted from Kim et al. (2004). Copyright 2003, with permission from Elsevier.
An alternative interpretation to the authors’ observations, however, could be that skull-tethered transcranial implants may increase the risk of micro-motion thereby intensifying the scar. Indeed, recent work by Biran et al. (2007) showed that tethered implants showed higher tissue reactivity compared to un-tethered cohorts, highlighting the effect of elevated micro-motion forces in conventionally implanted neural probes. Moreover, such micro-motion forces increase the probability of serum release into the brain, which has been shown to increase the extent of glial scarring in vitro (Polikov et al., 2009) whereas their absence has been shown to elicit diminished glial scarring in vivo (Nadal et al., 1995, 1997; Raivich et al., 1999). The above findings from several research groups motivate the development of wireless neuroelectrodes or strategies to reduce the mechanical mismatch between the neural probe and the brain parenchyma. Both of the aforementioned strategies are currently being pursued (Wise et al., 2004; Capadona et al., 2008), as discussed in Section “Recent Efforts to Address Biological Response in Neural Probe Engineering.”
CNS injury invokes a complex cellular and molecular response
As stated above, the host response to implants is a complex, yet well-recognized cascade of events characterized by the initiation of inflammation and microglial activation, and terminating with astrocyte activation and formation of an impenetrable glial scar. However, the basic knowledge underlying our understanding of host response to implants is being challenged by recent findings centered on the cellular and molecular biology of CNS response to injury. These findings question our basic assumptions about the types of cells involved, including reactive astrocytes, inflammatory cells, and stem cells.
The glial scar, rich in reactive astrocytes, is generally considered an inhibitory physical and biochemical barrier to axonal growth. However, emerging studies indicate that reactive astrocytes can play protective roles during the acute injury phase by aiding wound healing, protecting and supporting neurons, and limiting secondary damage due to uncontrolled inflammation, demyelination and tissue damage (Bush et al., 1999; Faulkner et al., 2004; Hertz and Zielke, 2004; Okada et al., 2006; Renault-Mihara et al., 2008). Moreover, depending on the structure of the scar tissue and the molecules expressed by the reactive astrocytes, under some circumstances, reactive astrocytes may support axonal outgrowth (Li and Raisman, 1995; Sivron and Schwartz, 1995; Ridet et al., 1997). This response may be tied to the nature of the injury (Davies et al., 1996), the region in the CNS where injury occurred (Alonso and Privat, 1993; Li and Raisman, 1995; Malhotra and Shnitka, 2002) and factors present in the injury microenvironment (e.g., soluble molecules including cytokines, growth factors and serum components as well as insoluble components such as ECM and cell surface molecules) (Kawaja and Gage, 1991; Li and Raisman, 1995; Ridet et al., 1997). As discussed in Section “Heterogeneity and Dynamics of the Glial Scar Environment,” the heterogeneous organization and temporal dynamics of these components are also key considerations for addressing the CNS response to neuroprosthetic implants.
Like glial scar formation, inflammation is another response to CNS injury that unfortunately is not well understood. In addition to clearing damaged tissue, inflammation can also cause damage to surrounding healthy tissue (Blight, 1994; Fitch and Silver, 1997) and inhibit axonal regeneration by promoting the synthesis of inhibitory ECM (Fitch and Silver, 2008); thus inflammation is considered to be both beneficial and detrimental toward the healing of damaged CNS tissue. Indeed, studies that manipulated the population or activity of immune cells in sites of CNS injury have drawn conflicting conclusions as to whether inflammation on the whole is favorable toward CNS repair (Rapalino et al., 1998; Popovich et al., 1999). Moreover, the impact of inflammation on CNS repair may depend on the activation state of the immune cells (Lotan and Schwartz, 1994; Rapalino et al., 1998; Fitch and Silver, 2008) and the extent to which activated astrocytes (Ridet et al., 1997) and native neural stem cells are involved (Butovsky et al., 2006; Das and Basu, 2008; Whitney et al., 2009). As our understanding of the beneficial roles reactive astrocytes and microglia continue to be elucidated, the potential power of driving these cell responses toward supporting improved neuroprosthetic device performance becomes more realistic.
Another area of perhaps even greater versatility is the role of NPCs in CNS response to injury and the potential of transplanted NPCs to treat areas of damaged or lost neuronal function (for comprehensive reviews see Jessell and Sanes, 2000; Goh et al., 2003; Zhao et al., 2008). NPCs are capable of differentiating into neurons, astrocytes, and oligodendrocytes. Neurogenic NPCs are found in the adult mammalian brain in the subventricular zone of the lateral ventricles and the subgranular zone of the hippocampus and dentate gyrus. When damage or inflammation occurs within the brain, NPCs are signaled to proliferate and migrate toward the injury site (Nait-Oumesmar et al., 1999; Arvidsson et al., 2002; Nakatomi et al., 2002; Jin et al., 2003; Goings et al., 2004), and in the case of neuroprosthetic implants, NPCs accompany microglia in attaching to the device surfaces (Stensaas and Stensaas, 1976; Winn et al., 1989; Mofid et al., 1997). These processes are influenced by soluble factors as well as cues within the glial scar (Rolls et al., 2009). Along these lines, studies involving NPC transplants have indicated that priming the cells toward the neuronal fate is required because NPCs tend to remain undifferentiated or become glia in non-neurogenic implant sites (Fricker et al., 1999; Sheen et al., 1999; Cao et al., 2001; Han et al., 2002; Gao et al., 2006; Lepore et al., 2006) and this effect is exacerbated in glial scar microenvironments (Cao et al., 2001, 2002; Faijerson et al., 2006). Inflammation is also known to influence NPC response: mild acute inflammation can induce neurogenesis while uncontrolled and longer-term inflammation mediated by large numbers of activated microglia can greatly reduce the number of newborn neurons (Ekdahl et al., 2003; Butovsky et al., 2006). As with the potential beneficial roles of astrocytic and microglial cells, our ever increasing understanding of how host and transplanted NPCs respond in the implant environment has great promise to yield potentially powerful mechanisms for improving long-term device performance.
In summary, these studies of general CNS response to injury underscore the importance of using caution while interpreting the cellular and molecular responses that occur in the specific case of neuroprosthetic implants in the CNS. Yet, the unraveling of these complex cellular responses may provide potential mechanisms to drive favorable biological outcomes and result in improved long-term neuroprosthetic device performance. However, as discussed in the next section, the spatial heterogeneity and temporal dynamics under which these components interact must also be considered in order to better understand the complex CNS tissue response to neuroprosthetic implants.
Heterogeneity and dynamics of the glial scar environment
Further complicating the current understanding of CNS response to injury is the fact that the cellular and molecular response within the damaged tissue varies both with time post-injury as well as proximity to the wound itself. In terms of temporal dynamics, the progression of acute response to implants toward formation of a glial scar is discussed in Section “Biological Response to Neuroprosthetic Devices”; however, the chronic stage response to even relatively simple surgical trauma may not terminate with the formation of glial scar, but instead proceed 6 months or longer after surgery toward further tissue damage via cell lysis and tissue disassembly (Frontczak-Baniewicz and Walski, 2006). An example of a phenomenon relating to the spatial heterogeneity is the diffusion of secreted cytokines and inflammatory factors away from the implant site. In fact, cells located at least 0.5 mm away from the edge of an implant can be impacted by the diffusion of these molecules (Biran et al., 2005), and moreover, the concentration profile of these factors will change spatially and temporally after device implantation.
Perhaps not surprisingly, therefore, two classifications of glial scar ultrastructure have been noted to describe the tissue surrounding the injury site (Ridet et al., 1997; McGraw et al., 2001) (Figure 4) and the type of ultrastructure that predominates depends in part on the location and time period relative to tissue injury. Anisomorphic gliosis may occur within the immediate area of a traumatic injury or chronic implant and is defined by a dense scar tissue composed of activated astrocytes with interlocking processes. Anisomorphic gliosis results in a permanent disruption to normal tissue architecture. Isomorphic gliosis occurs in tissues that are much less disturbed, such as in simpler injuries or at locations some distance away from the implant site. This less extreme response is characterized by astrocyte hypertrophy and may resolve without formation of a permanent scar or represent a transition stage to anisomorphic gliosis. In other words, the extent of injury influences the dynamics, structure and composition of the glial scar, and as discussed in Section “CNS Injury Invokes a Complex Cellular and Molecular Response,” in turn also impacts the level of astrocyte and microglial activation (Fernaud-Espinosa et al., 1993). Thus, it is becoming increasingly apparent that a thorough analysis of CNS response to neuroprosthetic device implantation must include multiple tissue locations (adjacent to, local to and distant from the device surface) as well as multiple acute and chronic time-points.
Studies focused on testing therapeutic treatments for CNS injury have begun verify the importance of considering the time-scale of biological processes that occur during the acute and chronic stages of glial scar formation (McGraw et al., 2001; Rolls et al., 2009). For example, therapies targeted toward degrading or preventing the synthesis of inhibitory components of the scar ECM are only effective if administration is delayed relative to the initial injury (Rolls et al., 2008). Comparable effects have been found with timing treatments to ablate activated astrocytes (Okada et al., 2006), as astrocytes may play a beneficial role in the acute stage of tissue repair, but become detrimental in the chronic injury state. Similarly, the timing of therapeutics aimed toward promoting axonal regeneration and restoration of function should be carefully considered: studies that delayed treatment 2–4 weeks after injury (Coumans et al., 2001; Iarikov et al., 2007) have yielded benefits, as the environment posed by very early stages of injury response are associated with necrosis and cytotoxic inflammatory processes such as edema and the production of reactive oxygen species. However, treatment cannot be delayed indefinitely because once the acute tissue response subsides, the formation of dense scar tissue prevents axonal penetration into the injury site. Despite these findings, the timing of therapeutics has not yet been adequately considered (McGraw et al., 2001; Gervasi et al., 2008) and poses particular challenges for the neuroprosthetics community because the timing of the acute and chronic phases depends on the type, shape and location of implant. Fundamental studies, such as the recent report by Harris et al. (2009) that define temporal and spatial changes in the mRNA levels of inhibitory molecules in the cerebral cortex following traumatic brain injury as well as time-course studies of neurotrophin and ECM expression (Catapano et al., 2001; Lagord et al., 2002; Wang et al., 2007), will greatly benefit our understanding of the dynamics of these complex processes.
Host response to retinal and cochlear implants
Although this review focuses primarily on the host response to neural prostheses implanted in brain tissue, it is important to consider the response elicited by other CNS implant systems such as epiretinal, subretinal, and cochlear implants. Because of the heterogeneity in the microenvironments, inherent differences in microanatomy in the vicinity of the implant, and the presence of cell types that are specific to the implantation site, specific types of retinal and cochlear devices differ in the extent of elicited glial scarring and fibrotic tissue formation in vivo (Bertschinger et al., 2008; Swan et al., 2008). With respect to subretinal and epiretinal implants, although chronic astrogliosis is observed after a few months of implantation (Montezuma et al., 2006), the extent of scarring is not as intense when compared to that of brain tissue mostly due to the absence of microglia and the relatively lower density of nerve fibers (implying lesser neurotrauma post-insertion) (Weiland et al., 2005). In addition, previous work has also shown that a special type of NPCs known as “Müller Glia” can differentiate into photoreceptor cells following retinal injury (Bernardos et al., 2007). Consequently, delivering the appropriate growth factors following injury might augment NPC differentiation and neural regeneration following retinal prosthesis implantation.
With respect to cochlear implants, the microenvironment not only favors the production of a robust fibrotic scar along with the death of spiral ganglion neurons (SGN), but also supports formation of calcified tissue near the scala tympani in humans (Clark, 2003). This new bone formation is a part of the healing phase in the chronic inflammatory response governed by the synergistic activation of cytokines such as fibroblast growth factor (FGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and transforming growth factor (TGF-β1) which is specific to the cochlear tissue. Calcification, glial scarring and SGN death can increase the impedance of the cochlear electrodes analogous to the impedance increase encountered by neural prostheses implanted in the brain. However, recent work by Richardson et al. (2009) has shown that delivering the trophic support of brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) along with electrical stimulation would augment the SGN survival and chronic activity of cochlear implants, in vivo; this approach of trophic factor delivery is analogous to approaches being pursued with neural implants in the brain, as discussed below.
Recent Efforts to Address Biological Response in Neural Probe Engineering
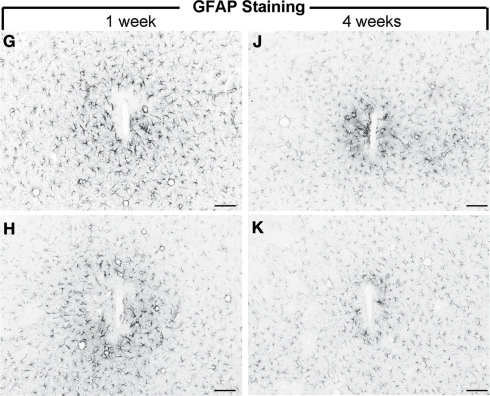
Future-generation neural prosthetic devices are being designed with a greater emphasis on reducing the tissue encapsulation problem to ensure consistent recordings in clinical settings. Some of the most promising work conducted in alleviating the glial scar problem has included bioactive coatings, reducing mechanical mismatch between the probe-brain interfaces and developing wireless implantable neuroelectrodes (see Table 2). For instance, Webb et al. (2001) immobilized a neural cell adhesion molecule (L1-NCAM) on glass substrates and showed in vitro that these bioactive coatings attract primary CNS neurons and support neurite outgrowth while repelling primary astrocytes, meningeal cells and fibroblasts. However, one of the limitations of this work was that the study did not include microglia, the frontline responder cells, to completely validate their model. Another bioactive coating strategy was developed by He et al. (2007) with an immobilized anti-inflammatory tridecapeptide, α-MSH, on silicon probes, demonstrating diminished inflammation and gliosis (Figure 7). Although this study illustrated exceptionally promising results with respect to in vitro as well as in vivo substantiation of the bioactivity of the peptide tethered electrodes, the group did not evaluate or show results pertaining to neuronal loss around the electrodes, which has been a major impediment to obtaining chronic consistent recordings (Biran et al., 2005). Cell-based bioactive coatings on neural probes have also been examined; Schlosshauer et al. (2001) encapsulated rat Schwann cells within a fibrin gel and placed the gel in contact with a slice of rat spinal cord adhered to a neural probe. To simulate a glial scar, fibroblasts were pre-adhered onto the probe, which had sieves in the size range of 40–70 μm. With this arrangement, the authors observed marked neurite penetration of the sieves relative to controls with no Schwann cells.
Table 2.
Highlights of efforts to address biological response in neural probe engineering.
| Goal | Approach | Reference |
|---|---|---|
| Enhance neuronal adhesion while inhibiting astrocyte and meningeal cell adhesion | Immobilized neural cell adhesion molecule L1-NCAM on SiO2 surfaces | Webb et al. (2001) |
| Mitigate astrocyte and microglial reaction | Immobilized anti-inflammatory tridecapeptide (α-MSH) on single shank planar electrodes | He et al. (2007) |
| Achieve solvent-induced change in elastic modulus | Water-based stimuli responsive cellulose nanofibers embedded in a matrix formed from ethylene oxide epichlorohydrin or poly (vinylacetate) | Capadona et al. (2008) |
| Fabricate multi-functional neural electrodes with low impedance, reduced mechanical mismatch between electrode-tissue interface and drug releasing properties | Silicon probe coated with biodegradable nanofibers of PLGA loaded with dexamethasone followed by a coat of alginate gel to induce slow drug release. PEDOT electrochemically polymerized onto electrode sites around PLGA within the alginate gel to reduce impedance | Abidian and Martin (2009) |
| Fabricate neuroprosthetic device with microfluidic channels for biomolecule release to reduce tissue reaction | 3-D probe structures (micron-sized channels) fabricated using surface micromachining and DRIE techniques to facilitate diffusion-mediated delivery of transferrin in vivo | Retterer et al. (2004) |
Figure 7.
GFAP reactivity of uncoated silicon microelectrodes (G, J) and an anti-inflammatory peptide (α-MSH) tethered electrode (H, K) following 1 week and 4 weeks of implantation in rats. The α-MSH coated electrodes elicited mitigated astrocytic and microglial reactivity (not shown here) indicating bioactivity of the coated implant. Scale Bar = 100 μm. Images reprinted from He et al. (2007). Copyright Wiley-VCH Verlag GmbH & Co. KGaA 2007. Reproduced with permission.
With respect to minimizing micro-motion, a recent review by Wise et al. (2004) discusses a wide range of wireless implantable devices that have been developed in recent years; hence a transition from transcranial designs to intracranial device designs is feasible if the latter can be shown to mitigate the tissue response, as suggested by the work of Tresco and colleagues (Kim et al., 2004; Biran et al., 2007). On the other hand, with regard to stimulus-responsive materials, recent work by Capadona et al. (2008) describes a coating material for probes that can exhibit a solvent-induced change in elastic modulus, analogous to tissues found in sea cucumber animals. Although this work demonstrated great promise, a recent report by Bellamkonda (2008) indicates that, the total range of elastic modulus is modest and the softest state was actually far from the required modulus for camouflaging a probe implanted in brain tissue.
The aforementioned studies have showed tremendous potential to improve the reliability of chronic recordings in neural probes. Nevertheless, very few studies to date have effectively incorporated and implemented designs in a functional neuroprosthetic device that completely overcomes the effects of tissue reaction, in vivo.
Bridging the Divide: Tissue Engineering Technologies to Advance Neuroprosthetics
As discussed above, critical challenges face the neuroprosthetics field, and thus far, these challenges have been addressed with materials science innovations or single-factor means to augment biocompatibility. Meanwhile, neurobiologists have made significant strides in understanding the complexity of how CNS tissue responds to injury. Great promise therefore exists in the possibility of bridging the divide between the neuroprosthetics and neurobiological communities, but how can this be best accomplished? Even if we knew the complete neurobiological response to injury and chronic implantation, how could we use this knowledge to make improved neuroprosthetic devices? How do we reconcile the fact that understanding the biological response is paramount, yet the CNS response to injury is so intricate that we may not ever have adequate technology and control over the biological response to seamlessly integrate a neuroprosthetic device into undisturbed CNS tissue?
We argue that tissue engineering technologies are well-poised to address these challenges by bridging the technological and biological intricacies of this complex problem. Tools recently developed in the tissue engineering community are helping to define how multifactorial cues determine cell response and providing mechanisms to harness the body's inherent regenerative potential. As discussed below, these tools include (1) platforms that present two or more simultaneous substrate cues via patterning of co-cultured cells, substrate topography and adhesive biomolecules; (2) controlled delivery of soluble bioactive molecules; and (3) constructs and biomimetic tissue models that are created via advances in hydrogel chemistry, 3-D patterning, and 3-D culture. This section concludes with the highlight of several issues on the frontier of neural and cancer cell biology and suggestions for how tissue engineering principles could apply these new insights toward new experimental and therapeutic neuroprosthetic technologies.
Platforms to present cells with multiple substrate cues
Modifying the implant surface is one simple means to determine how cells respond to the foreign surfaces presented by neuroprosthetic devices in the implant site. Indeed, a variety of surfaces have been explored, as described in Sections “Electrical Interface Challenges in Neural Implants” and “Recent Efforts to Address Biological Response in Neural probe Engineering.” However, these cues are typically presented individually to cells and it is likely that once the cue is masked by serum deposition or scar tissue, it will be rendered ineffective. Therefore, a multifunctional approach may provide advantages toward directing specific cellular responses. For example, separate works by the Hoffman-Kim and Tresco groups have demonstrated co-culture techniques for initiating patterns of glial cells that subsequently prove effective for guiding neuronal adhesion and neurite outgrowth (Biran et al., 2003; Bruder et al., 2007). While originally reported as tools to augment spinal cord and peripheral nerve regeneration, these approaches may prove to be useful for direct application to neuroprosthetic devices as well as in vitro studies of the molecular mechanisms by which glial cells affect neuronal response.
Another multifunctional surface modification approach that may have direct implications for neuroprosthetic devices is one that presents cells with topographical cues as well as patterned biomolecules that support cell adhesion. The essential goal of this approach is to mimic both the physical and the biochemical aspects of ECM that are favorable for neurite outgrowth. One example comprises electrospun polyamide nanofibrillar surfaces that are covalently bound with peptides derived from tenascin-C (a neuroactive component of the ECM); these surfaces significantly enhanced neuronal adhesion and neurite outgrowth in vitro as compared to glass coverslips or nanofibers modified only with poly-l-lysine (a common and relatively simple biomolecule that supports neuronal adhesion) (Ahmed et al., 2006). Similarly, to develop a model to investigate neuronal development, Li and Folch (2005) generated substrates that vary with microtopography and ability to support neurite outgrowth. This model was composed of microfabricated ridges of polydimethylsiloxane that were coated with poly-d-lysine, seeded with neurons and then covered with Matrigel, a gel matrix that supports cell and neurite infiltration. This study showed that axons decide where to grow by integrating permissiveness and topographical cues that are presented in the neuron's 3-D microenvironment. Such strategies to understand how cells respond to multiple substrate factors may prove useful in determining which kinds of device properties are most important in promoting neuronal viability and neurite growth toward neuroprosthetic devices while ameliorating inhibitory responses from glia and immune cells in the implant site.
Although the above mentioned routes of administering multiple cues to cells have enhanced our fundamental understanding of neuroglial interactions and embryonic development, translating these technologies toward chronic and consistent recordings of neuroprosthetic devices would still prove to be an arduous challenge. Such a limitation stems from the fact that all the aforementioned technologies utilize embryonic neurons for their studies; however, the adult nervous system comprises of post-mitotic neurons (McMillian et al., 1994; Wu and Schwartz, 1998). Therefore, it is not apparent if presenting multiple cues on neural probe surfaces will produce neurite outgrowth in adult neurons and result in extensive neuronal networks with greater proximity to implanted electrodes and chronic activity.
Delivery of soluble bioactive molecules and cells
In addition to substrate cues, soluble bioactive molecules are a powerful means to influence cell response in the implant environment. To be successful, this approach must apply the appropriate individual or cocktail of factors with suitable dosing (i.e., concentration, timing, and location). Neuroprosthetic engineers have begun to explore this approach by incorporating microfluidic channels into devices (Chen et al., 1997, 2008; Lee et al., 2004; Retterer et al., 2004; Takeuchi et al., 2005; Papageorgiou et al., 2006; Mercanzini et al., 2008) or implementing surfaces modified with a controlled-release matrix loaded with drug (Klaver and Caplan, 2007; Winter et al., 2007b; Jun et al., 2008; Abidian and Martin, 2009; Jhaveri et al., 2009). Recent studies from the research group of David Martin (Abidian and Martin, 2009; Abidian et al., 2009) have elegantly illustrated the potential of multifunctional surface coatings on neural probes (Figure 8). In this work, a silicon probe was first coated with biodegradable nanofibers of poly(lactic-co-glycolic acid) (PLGA) loaded with dexamethasone, a neuroprotective molecule. This layer was encapsulated within an alginate hydrogel. In the final step of coating assembly, a conducting polymer, PEDOT, was electrochemically polymerized onto the electrode sites on the probe and around the PLGA nanofibers; this polymerization occurred within the alginate hydrogel. The PEDOT coating effectively increases the surface area available to neurons for electrical connectivity while the PLGA provides controlled release of the neuroprotective drug. Implant studies in rat barrel cortex using a form of this device manufactured with PEDOT nanotubes demonstrated improved signal-to-noise ratios, lower electrode impedance, and higher charge capacity density, as well as the abilities to function in chronic (7 weeks) recording applications and sense the transition from acute inflammation to chronic response (Abidian et al., 2009). Though the controlled release aspect of these devices was not yet tested in vivo, these promising results highlight the potential of such multifunctional approaches toward achieving improved neuroprosthetic device performance in chronic applications.
Figure 8.
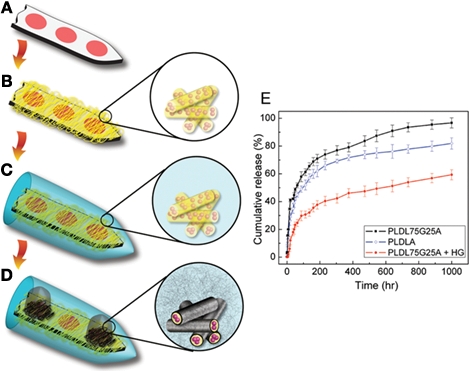
(A–D) Design of a multi-functional neural probe with low impedance and controlled drug-release capability. A planar Michigan probe is first coated with biodegradable nanofibers that are pre-loaded loaded with dexamethasone, an anti-inflammatory drug. An alginate gel overcoat ensures slow drug release. Within the gel, a conducting material PEDOT is electrochemically polymerized from the electrode sites. (E) Two different compositions of nanofibers are examined, poly(lactic acid) and poly(lactic-co-glycolic acid) and the inclusion of the alginate gel overcoat (HG) is shown to significantly slow down the release of dexamethasone. All images reprinted from Abidian and Martin (2009). Copyright Wiley-VCH Verlag GmbH & Co. KGaA 2009. Reproduced with permission.
However, challenges remain in ensuring that the molecules are released at the appropriate time and place to treat the complex cascade of cellular responses following implantation and maintain long-term release of bioactive molecules in chronic implants. Fortunately, similar requirements have been identified in other tissue engineering applications and several potential strategies exist to meet these challenges. Examples include materials that are “cell-responsive” by degrading upon exposure to cell-secreted enzymes (Lutolf et al., 2003) and materials that control the release of bioactive factors via similar mechanisms used by the ECM sequester and release growth factors (Tanihara et al., 2001; Ohta et al., 2004). Materials chemistry and processing can also be manipulated to preprogram separate release rates of multiple bioactive factors (Richardson et al., 2001; Holland et al., 2005; Park et al., 2007). Finally, another means to provide biological sophistication to the delivery of bioactive molecules is to use a cell-based approach. Cells respond to external stimuli, can secrete molecules for long time periods, and can manufacture molecules that would be impractical or impossible to produce and purify ex vivo. Cell-based drug delivery approaches are often implemented by encapsulating cells in a hydrogel depot to protect the cells and further control the biomolecule release. (For recent comprehensive reviews on controlled release of biomolecules and cell-based drug delivery, see Schmidt et al., 2008; Lin and Anseth, 2009; Silva et al., 2009.) Thus a multifunctional device design that utilizes our growing understanding of astrocyte, microglial and NPC cell response to drive cell-based delivery of bioactive factors could be a potentially powerful means to control CNS response to long-term neuroprosthetic implants.
Delivery of NPC cells along with neuroprosthetic devices may provide trophic support to local neurons, or perhaps even replace dead neurons and replace host circuitry. One particularly daunting challenge that may be addressed by this approach is the progressive neuronal degeneration following chronic implantation of neural prosthetic devices. Recent work by Englund et al. (2002) has shown that delivering a multipotent progenitor cell line known as RN33B into postnatal day 1–2 rat neural tissue can produce functional cortical networks even after host neurogenesis is complete and in brain areas outside the classical neurogenic regions. However, it is also known that the extent of neurogenesis of progenitor cell types such as RN33B differs greatly between different brain regions (Lundberg et al., 2002) even though this cell type has natural propensity to differentiate into functional neurons in vitro as well as in vivo (Onifer et al., 1993; Whittemore and White, 1993; Shihabuddin et al., 1996). Hence it is not clear whether foreign cells would have sufficient survival or even invoke aberrant synaptic activity after incorporation into the host circuitry.
3-D constructs and biomimetic tissue models
It is well recognized that a static monolayer culture has a limited capability to recapitulate the in vivo microenvironment; for example, in a 1999 review, Fawcett and Asher (1999) conclude that much of the astrocyte literature, and in particular, the studies relating to whether astrocytes aid or inhibit axonal growth, is confusing largely because current in vitro model systems are limited in their ability to mimic the in vivo scenario. Indeed, in the last decade, cell biologists and tissue engineers have made rapid progress in defining the cellular and molecular basis for how cells respond to culture dimensionality (2-D vs 3-D) (Cukierman et al., 2001; Pampaloni et al., 2007; Tibbitt and Anseth, 2009) and mechanical stimuli (Chen, 2008; Wang et al., 2009a) and are developing in vitro model systems that more faithfully represent in vivo conditions. Key criteria for in vitro models of CNS are discussed below and include, (1) biologically-relevant scaffolding materials; (2) most or all of the relevant cell types, namely neurons, astrocytes, microglia, endothelial cells and meningeal fibroblasts; and (3) appropriate spatial arrangement of the scaffolding materials and cells.
Because the ECM plays a role in cell response to injury, it is important to carefully consider the scaffolding material selected for in vitro models of CNS. Three-dimensional culture studies have demonstrated that neurons can survive and grow neurites within scaffolds composed of agarose, type I collagen, fibrin, and Matrigel to name a few (O'Connor et al., 2001; Gingras et al., 2003; Suuronen et al., 2004; LaPlaca et al., 2005; Lin et al., 2005). The ECM of the CNS is composed primarily of laminin, type IV collagen, and various proteoglycans and glycosaminoglycans such as hyaluronan, tenascins, and heparan sulfate proteoglycans (Venstrom and Reichardt, 1993; Fitch and Silver, 2008; Hubert et al., 2009). The distribution of these molecules changes during periods of axonal growth, such as in development, as compared to physiological levels in adults. The ECM of injured CNS tissue and glial scar, however, contains increased amounts of proteoglycans (e.g., chondroitin sulfate) as well as components resulting from damage to the vasculature and dura mater, such as fibrin, fibronectin and type I collagen. Because many of these molecules directly or indirectly impact neuronal behavior, it is very important select a scaffolding material with greatest relevance to the biological or pathological scenario of interest.
Of particular relevance to neuroprosthetic devices are studies carried out by three groups with the intent of developing in vitro co-culture models of neuronal response to glial scar resulting from microelectrode implantation and mechanical injury. First, studies by Polikov and Reichert adapted an established in vitro model of neuroinflammatory response toward the investigation of glial response to microelectrode implantation (Polikov et al., 2006, 2009) (Figure 9). This model system consists of a confluent layer of neurons, astrocytes and microglia that are derived from embryonic rat midbrain. An injury was induced to the culture by scraping away a section of the cells and/or placing a foreign body (50 μm diameter stainless steel wire) on top of the cells. The cultures were assessed at 6 h and 10 days after injury by immunocytochemical staining for markers for neurons and reactive forms of astrocytes and microglia. Their initial report found that the developed in vitro model compared favorably to in vivo response to injury in the brain (Polikov et al., 2006), whereas their follow-up report focused on improving the reproducibility of the model system via optimized culture conditions and established a quantitative method for interpreting the results (Polikov et al., 2009). Because this model system was carefully designed to account for multiple cell types and provides a mechanism to rigorously compare different types of injuries and implant materials, this approach has great potential for unveiling fundamental mechanisms of glial scar formation and neuroprosthetic performance.
Figure 9.
An in vitro model of glial scarring developed by Polikov and colleagues (Polikov et al. , 2006, 2009) using an optimized cell culture monolayer of primary astrocytes and microglia. (A) Upon implantation of a 50-μm metallic wire into this culture, distinct traits of glial scarring are observed, such as microglial (red) activation and attachment to the implanted microwires, astrocyte (green) activation beyond the microglial layer in the form of GFAP up-regulation and encapsulation of wires by reactive astrocytes. (B) Shows the scar phenomena at higher magnification. Reprinted from Polikov et al. (2006). Copyright 2006, with permission from Elsevier.
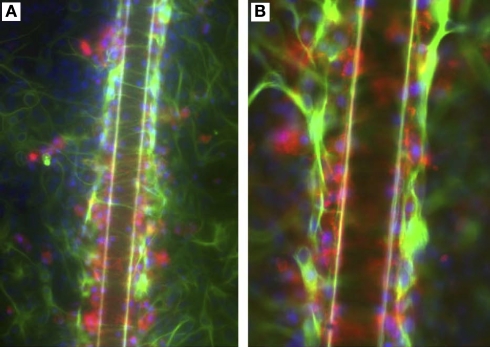
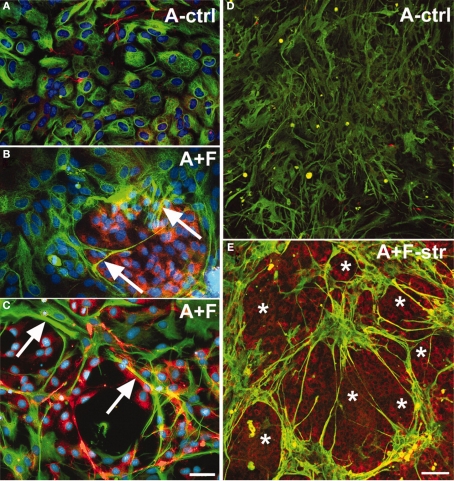
Second, Wanner et al. (2008) developed a model of mechanical injury to investigate the roles of astrocyte activation and meningeal fibroblasts in neuronal response (Figure 10). Newborn rat astrocytes with and without meningeal fibroblasts were grown on deformable silastic membrane and were exposed to two short pulses of stretch to mimic mechanical injury. Neurons derived from the cortex, spinal cord and dorsal root ganglia of postnatal and embryonic rat were seeded on the astrocyte cultures. The investigators found that stretch injury activated the astrocytes, resulting in significantly reduced neurite outgrowth compared to unstretched cultures. Interestingly, the extents of neurite outgrowth varied with age (greater inhibition in postnatal vs embryonic neurons) and type of neuronal population. This model highlights the importance of considering cell type and age when designing in vitro and animal model systems. For example, cell activation and the extent of glial scar formation is greatly enhanced in adult compared to neonatal animals, but unfortunately, few studies have focused on the heterogeneity of cell response to injury as a function of cell source or age (Ridet et al., 1997).
Figure 10.
Co-culturing meningeal fibroblasts along with astrocytes in vitro can cause astrocyte reactivity and increased GFAP expression similar to in vivo glial scarring [157]. (A) Astrocytes (A-ctrl) in 2-D culture show perinuclear GFAP reactivity and are flat, round, or oval-shaped cells. (B) Long-term astrocyte–fibroblast co-cultures (A + F) show spindle-shaped astrocytes with elongated processes surrounded by meningeal fibroblasts. Also observed are astrocytic processes entering fibroblast territory (arrows) and brighter GFAP staining of astrocytes contacting fibroblasts. (C) Shows a 2-day mixed culture of astrocytes and fibroblasts showing strongly GFAP+ astrocytes (arrows) on and around patches of fibroblasts. (D) Shows differentiated astrocytes on collagen-coated silastic membranes without the presence of fibronectin+ cells. (E) Shows astrocyte–fibroblast co-culture 3 days following fibroblast addition and 24 h stretching (A + F-str) show disruption of processes, with clusters of star shaped astrocytes forming “bridges” of bundled processes across spaces shown as asterisks. Increased GFAP signals are found accumulated in stellate processes that are fibronectin+ (yellow). Fibronectin-positive fibroblasts (asterisks) remain evenly distributed after stretching. Bar = 20 μm. In (A–E), GFAP: green; fibronectin: red; nuclei: blue). Reprinted from Wanner et al. (2008). Copyright 2008 Wiley-Liss Inc. Reproduced with permission.
Third, LaPlaca's laboratory has developed an in vitro model for traumatic brain injury that is based on rat embryonic astrocytes and rat postnatal cortical neurons that are co-cultured within Matrigel constructs and either exposed to controlled shear (LaPlaca et al., 2005) or injected with NPCs to mimic damage inflicted during cell transplantation (Cullen et al., 2007). These culture platforms were able to support neuronal viability and neurite outgrowth for up to 3 weeks; interestingly, at the beginning of culture, the astrocyte:neuron ratio was 1:1, but at later time periods, this ratio became 9:1 and was suggested to result from astrocyte proliferation (Irons et al., 2008). These diverse approaches toward modeling the CNS injury environment in vitro are very useful models for exploring factors related to neuronal vs glial viability as a function of complex culture conditions and cell activation due to injury.
Finally, as biomaterials and bioMEMs scientists continue to report new and imaginative means to create 3-D cultures that enable manipulation of the concentrations and positions of multiple cell types as well as soluble cues and insoluble matrix molecules (Mapili et al., 2005; Arcaute et al., 2006; Khetani and Bhatia, 2006; Khademhosseini et al., 2007; Papavasiliou et al., 2008; Lee et al., 2009), we anticipate great advances in the area of advanced in vitro model systems. When combined with a rich background of technologies centered on hydrogels and bio-inspired materials for cell transplantation and the delivery drugs, proteins and genes (Giordano et al., 2008; Murua et al., 2008; Quaglia, 2008; Schmidt et al., 2008; Lin and Anseth, 2009; Silva et al., 2009) the time is right for applying new knowledge gained from controlled in vitro studies toward animal and human neuroprosthetic implants.
Issues on the frontier: gleaning from developmental and cancer biology
Because effective therapies for repairing CNS injuries have remained elusive despite decades of extensive research, researchers are examining this problem from other angles. For example, insights from developmental neurobiology on fundamental mechanisms of neuronal cell migration, axonal outgrowth and pathfinding (guided growth to synaptic targets) have yielded knowledge about cues that are favorable or inhibitory for axonal growth. Interestingly, these inhibitory molecules can also be found in sites of injury in the adult CNS (De Winter et al., 2002; Goldshmit et al., 2004; Hashimoto et al., 2004). For example, one family of developmental cues, the Eph receptors and their ligands, the ephrins, are known mediators of tissue organization during development (for a comprehensive review, see Goldshmit et al., 2006). Though the details are not yet understood, there is a link between Eph/ephrin molecules, inflammation and glial scar, and evidence indicates that Eph/ephrin expression and activation play roles in inhibiting neuronal regeneration following injury in the CNS. Therefore, tissue engineering technologies could be used to explore potential strategies such as controlled release of ephrin (via biomaterials or cell-based approaches mentioned above) or overexpression of Eph receptors toward the aim of guiding neurite growth or mediating inflammation and scarring in the implant microenvironment. Such developmental cues that mediate cell-cell contact or act at a distance through diffusible signals provide a wealth of new avenues for exploration using the cell/biomolecule patterning tools, controlled release platforms and advanced culture models mentioned above.
Another possible source of fresh insight into CNS repair following injury comes from the cancer biology field and their particular insight into factors that control cell cycle (Byrnes and Faden, 2007). Entry into cell cycle is commonly recognized as the means by which cells initiate proliferation, which is the case for mitotic cells, such as astrocytes, microglia and other inflammatory cells. Neurons in the CNS, however, are post-mitotic (with the exception of NPCs) and for these cells, entry into the cell cycle results in apoptosis (programmed cell death) (Nguyen et al., 2002; Wartiovaara et al., 2002; Wang et al., 2009b). Thus, prevention of entry into the cell cycle is favorable for both neuronal survival as well as prevention of uncontrolled glial scarring and inflammation.
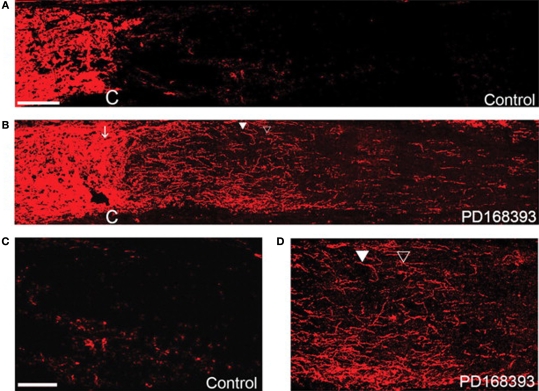
Fortunately, entry into the cell cycle and drug candidates that may control this response are heavily investigated in the cancer biology field, as uncontrolled proliferation is a hallmark of cancer cells. In fact, a recent study (Koprivica et al., 2005) was undertaken to screen at least 400 small molecules for their ability to promote neurite outgrowth on an inhibitory surface of myelin (a component of spinal cord injury scar); the majority of compounds had no effect, but several drugs related to inhibiting the epidermal growth factor receptor (EGFR) were able to block the inhibitory effect of the myelin substrate and resulted in robust neurite outgrowth, as shown in Figure 11. These effects were corroborated in a mouse model of optic nerve injury, resulting in significant nerve regeneration in treated animals compared to control mice without treatment. The cellular mechanism behind the effectiveness of EGFR inhibition relates to intracellular signaling cascades involving known players in cancer biology, including the MAPK, Akt and JNK pathways that impact DNA synthesis and cell proliferation (Oda et al., 2005). Interestingly, EGFR is not the only mediator of these pathways that has been linked to CNS injury (Di Giovanni et al., 2005; Milenkovic et al., 2005; Neary and Kang, 2005; Nicole et al., 2005; Kaneko et al., 2007; Lim et al., 2007) and several molecules that inhibit these pathways are already approved as potential drugs for cancer treatment. Thus, a promising and immediately feasible line of research is to test these drugs in conjunction with neuroprosthetic devices to promote neuronal survival and inhibit scar formation and inflammation. This approach is particularly exciting because of the immediate implications for treatment of CNS injury (Miller, 2005) and like the possible insights gleaned from developmental biology mentioned above, the wealth of knowledge from cancer biology provides potentially powerful new avenues of exploration for addressing CNS injuries including those associated with neuroprosthetic devices.
Figure 11.
Regeneration of the optic nerve in mice using an EGFR blocking molecule, PD168393. Optic nerves stained with antibodies against GAP43 compared to controls (A,C) or PD168393-treated (B,D) mice. Lectin staining and visual inspection were used to identify the injury site (marked by C). The images in (C,D) are magnified views of the post crush area that were treated and untreated by PD168393. In (C), the control nerve shows very few GAP-43 fibers whereas in (D), numerous regenerating fibers are observed, including some that had changed their direction (filled triangle). Scale bar = 100 μm in (A,B) and 50 μm in (C,D). Reprinted from Koprivica et al. (2005). Copyright Science AAAS 2005. Reproduced with permission.
Conclusions
Major advances in neuroprosthetics device design have been achieved, yet great challenges remain in controlling the cellular and molecular interface between the device and the CNS. Despite successes in short-term implant studies, these challenges have barred reliable long-term performance in patient implants. We believe that current areas of device design innovation, including wireless devices, new approaches to minimize device micro-motion, and novel device materials that mimic the mechanics of CNS tissue will further the success of these devices. The most significant advances, however, will arise from collaborations on the intersection between the neuroprosthetics, tissue engineering and neurobiology fields. Each community stands to benefit from such interactions to yield improved model systems, therapeutic strategies, and quantitative metrics for assessing outcomes. We stress the importance of appreciating the complexity of the biological response to CNS injury, and specifically, response to neuroprosthetic devices that rely on implant type, location and other factors. Such responses involve numerous biological components that are organized spatially and temporally in a dynamic fashion. Multifactorial approaches that address multiple biological aims, such as drug delivery and patterning technologies are well-poised to address these issues related to dynamics and heterogeneity. Moreover, tissue-mimetic model systems present test beds with greater physiological relevance and complexity and are likely to work synergistically with animal studies to more rapidly identify promising device technologies. Finally, insights from the developmental and cancer biology fields are providing fresh insights that will yield novel mechanisms to influence CNS response to neuroprosthetic implants. We look toward the future of neuroprosthetic devices with optimism and confidence that such interdisciplinary collaborations will lead us ever closer toward successful long-term device performance.
Conflict of Interest Statement
The authors declare that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by NIH-NINDS (1R01NS065205 to Jennie B. Leach; 1R44NS047952 to GVD Corp. and subcontract to Shashi K. Murthy) and the Henry Luce Foundation (Jennie B. Leach).
References
- Abidian M. R., Ludwig K. A., Marzullo T. C., Martin D. C., Kipke D. R. (2009). Interfacing conducting polymer nanotubes with the central nervous system: chronic neural recording using poly(3,4-ethylenedioxythiophene) nanotubes. Adv. Mater. 21, 3764–3770 10.1002/adma.200900887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abidian M. R., Martin D. C. (2009). Multifunctional nanobiomaterials for neural interfaces. Adv. Funct. Mater. 19, 573–585 10.1002/adfm.200801473 [DOI] [Google Scholar]
- Achyuta A. K. H., Cieri R., Unger K., Murthy S. K. (2009). Synergistic effect of immobilized laminin and nerve growth factor on PC12 neurite outgrowth. Biotechnol. Prog. 25, 227–234 10.1002/btpr.58 [DOI] [PubMed] [Google Scholar]
- Ahmed I., Liu H. Y., Mamiya P. C., Ponery A. S., Babu A. N., Weik T., Schindler M., Meiners S. (2006). Three-dimensional nanofibrillar surfaces covalently modified with tenascin-C-derived peptides enhance neuronal growth in vitro. J. Biomed. Mater. Res. A 76, 851–860 10.1002/jbm.a.30587 [DOI] [PubMed] [Google Scholar]
- Alberts B., Johnson A., Lewis A., Raff M., Roberts K., Walter P. (2001). Molecular Biology of the Cell. New York, NY, Garland [Google Scholar]
- Alonso G., Privat A. (1993). Neuropeptide Y-producing neurons of the arcuate nucleus regenerate axons after surgical deafferentation of the mediobasal hypothalamus. J. Neurosci. Res. 34, 510–522 10.1002/jnr.490340504 [DOI] [PubMed] [Google Scholar]
- Arcaute K., Mann B. K., Wicker R. B. (2006). Stereolithography of three-dimensional bioactive poly(ethylene glycol) constructs with encapsulated cells. Ann. Biomed. Eng. 34, 1429–1441 10.1007/s10439-006-9156-y [DOI] [PubMed] [Google Scholar]
- Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O. (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8, 963–970 10.1038/nm747 [DOI] [PubMed] [Google Scholar]
- Balasingam V., Tejadaberges T., Wright E., Bouckova R., Yong V. W. (1994). Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J. Neurosci. 14, 846–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamkonda R. V. (2008). Biomimetic materials – marine inspiration. Nat. Mater. 7, 347–348 10.1038/nmat2176 [DOI] [PubMed] [Google Scholar]
- Bernardos R. L., Barthel L. K., Meyers J. R., Raymond P. A. (2007). Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J. Neurosci. 27, 7028–7040 10.1523/JNEUROSCI.1624-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M., Maxwell W. L., Logan A., Mathewson A., McConnell P., Ashhurst D. E., Thomas G. H. (1983). Deposition of scar tissue in the central nervous system. Acta Neurochir. Suppl. (Wien) 32, 31–53 [DOI] [PubMed] [Google Scholar]
- Bertschinger D. R., Beknazar E., Simonutti M., Safran A. B., Sahel J. A., Rosolen S. G., Picaud S., Salzmann J. (2008). A review of in vivo animal studies in retinal prosthesis research. Graefes Arch. Clin. Exp. Ophthalmol. 246, 1505–1517 10.1007/s00417-008-0891-7 [DOI] [PubMed] [Google Scholar]
- Biran R., Martin D. C., Tresco P. A. (2005). Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 195, 115–126 10.1016/j.expneurol.2005.04.020 [DOI] [PubMed] [Google Scholar]
- Biran R., Martin D. C., Tresco P. A. (2007). The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J. Biomed. Mater. Res. A 82A, 169–178 10.1002/jbm.a.31138 [DOI] [PubMed] [Google Scholar]
- Biran R., Noble M. D., Tresco P. A. (2003). Directed nerve outgrowth is enhanced by engineered glial substrates. Exp. Neurol. 184, 141–152 10.1016/S0014-4886(03)00253-X [DOI] [PubMed] [Google Scholar]
- Bjornsson C. S., Oh S. J., Al-Kofahi Y. A., Lim Y. J., Smith K. L., Turner J. N., De S., Roysam B., Shain W., Kim S. J. (2006). Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J. Neural Eng. 3, 196–207 10.1088/1741-2560/3/3/002 [DOI] [PubMed] [Google Scholar]
- Black J. (1999). Biological Performance of Materials: Fundamentals of Biocompatibility. New York, Dekker [Google Scholar]
- Blight A. R. (1994). Effects of silica on the outcome from experimental spinal cord injury: implication of macrophages in secondary tissue damage. Neuroscience 60, 263–273 10.1016/0306-4522(94)90220-8 [DOI] [PubMed] [Google Scholar]
- Brazda N., Muller H. W. (2009). Pharmacological modification of the extracellular matrix to promote regeneration of the injured brain and spinal cord. Prog. Brain Res. 175, 269–281 10.1016/S0079-6123(09)17518-0 [DOI] [PubMed] [Google Scholar]
- Bruder J. M., Lee A. P., Hoffman-Kim D. (2007). Biomimetic materials replicating Schwann cell topography enhance neuronal adhesion and neurite alignment in vitro. J. Biomater. Sci. Polym. Ed. 18, 967–982 10.1163/156856207781494412 [DOI] [PubMed] [Google Scholar]
- Bush T. G., Puvanachandra N., Horner C. H., Polito A., Ostenfeld T., Svendsen C. N., Mucke L., Johnson M. H., Sofroniew M. V. (1999). Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23, 297–308 10.1016/S0896-6273(00)80781-3 [DOI] [PubMed] [Google Scholar]
- Butovsky O., Ziv Y., Schwartz A., Landa G., Talpalar A. E., Pluchino S., Martino G., Schwartz M. (2006). Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 31, 149–160 10.1016/j.mcn.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Byrnes K. R., Faden A. I. (2007). Role of cell cycle proteins in CNS injury. Neurochem. Res. 32, 1799–1807 10.1007/s11064-007-9312-2 [DOI] [PubMed] [Google Scholar]
- Cao Q. L., Howard R. M., Dennison J. B., Whittemore S. R. (2002). Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp. Neurol. 177, 349–359 10.1006/exnr.2002.7981 [DOI] [PubMed] [Google Scholar]
- Cao Q. L., Zhang Y. P., Howard R. M., Walters W. M., Tsoulfas P., Whittemore S. R. (2001). Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp. Neurol. 167, 48–58 10.1006/exnr.2000.7536 [DOI] [PubMed] [Google Scholar]
- Capadona J. R., Shanmuganathan K., Tyler D. J., Rowan S. J., Weder C. (2008). Stimuli-responsive polymer nanocomposites inspired by the sea cucumber dermis. Science 319, 1370–1374 10.1126/science.1153307 [DOI] [PubMed] [Google Scholar]
- Carbonell A. L., Boya J. (1988). Ultrastructural study on meningeal regeneration and meningo-glial relationships after cerebral stab wound in the adult rat. Brain Res. 439, 337–344 10.1016/0006-8993(88)91491-6 [DOI] [PubMed] [Google Scholar]
- Catapano L. A., Arnold M. W., Perez F. A., Macklis J. D. (2001). Specific neurotrophic factors support the survival of cortical projection neurons at distinct stages of development. J. Neurosci. 21, 8863–8872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamak B., Morandi V., Mallat M. (1994). Brain macrophages stimulate neurite growth and regeneration by secreting thrombospondin. J. Neurosci. Res. 38, 221–233 10.1002/jnr.490380213 [DOI] [PubMed] [Google Scholar]
- Chen C. S. (2008). Mechanotransduction – a field pulling together? J. Cell. Sci. 121, 3285–3292 10.1242/jcs.023507 [DOI] [PubMed] [Google Scholar]
- Chen D., Du W. B., Liu Y., Liu W. S., Kuznetsov A., Mendez F. E., Philipson L. H., Ismagilov R. F. (2008). The chemistrode: a droplet-based microfluidic device for stimulation and recording with high temporal, spatial, and chemical resolution. Proc. Natl. Acad. Sci. U.S.A. 105, 16843–16848 10.1073/pnas.0807916105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wise K. D., Hetke J. F., Bledsoe S. C. Jr. (1997). A multichannel neural probe for selective chemical delivery at the cellular level. IEEE Trans. Biomed. Eng. 44, 760–769 10.1109/10.605435 [DOI] [PubMed] [Google Scholar]
- Clark G. (2003). Cochlear Implants: Fundamentals and Applications. New York, Springer; 14624429 [Google Scholar]
- Coumans J. V., Lin T. T., Dai H. N., MacArthur L., McAtee M., Nash C., Bregman B. S. (2001). Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J. Neurosci. 21, 9334–9344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J., Henze D. A., Jamieson B., Harris K. D., Sirota A., Bartho P., Wise K. D., Buzsaki G. (2003). Massively parallel recording of unit and local field potentials with silicon-based electrodes. J. Neurophysiol. 90, 1314–1323 10.1152/jn.00116.2003 [DOI] [PubMed] [Google Scholar]
- Cui X. Y., Lee V. A., Raphael Y., Wiler J. A., Hetke J. F., Anderson D. J., Martin D. C. (2001). Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J. Biomed. Mater. Res. 56, 261–272 [DOI] [PubMed] [Google Scholar]
- Cui X. Y., Wiler J., Dzaman M., Altschuler R. A., Martin D. C. (2003). In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials 24, 777–787 10.1016/S0142-9612(02)00415-5 [DOI] [PubMed] [Google Scholar]
- Cukierman E., Pankov R., Stevens D. R., Yamada K. M. (2001). Taking cell-matrix adhesions to the third dimension. Science 294, 1708–1712 10.1126/science.1064829 [DOI] [PubMed] [Google Scholar]
- Cullen D. K., Stabenfeldt S. E., Simon C. M., Tate C. C., LaPlaca M. C. (2007). In vitro neural injury model for optimization of tissue-engineered constructs. J. Neurosci. Res. 85, 3642–3651 10.1002/jnr.21434 [DOI] [PubMed] [Google Scholar]
- Das S., Basu A. (2008). Inflammation: a new candidate in modulating adult neurogenesis. J. Neurosci. Res. 86, 1199–1208 10.1002/jnr.21585 [DOI] [PubMed] [Google Scholar]
- Davies S. J., Field P. M., Raisman G. (1996). Regeneration of cut adult axons fails even in the presence of continuous aligned glial pathways. Exp. Neurol. 142, 203–216 10.1006/exnr.1996.0192 [DOI] [PubMed] [Google Scholar]
- De Winter F., Oudega M., Lankhorst A. J., Hamers F. P., Blits B., Ruitenberg M. J., Pasterkamp R. J., Gispen W. H., Verhaagen J. (2002). Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp. Neurol. 175, 61–75 10.1006/exnr.2002.7884 [DOI] [PubMed] [Google Scholar]
- Di Giovanni S., Movsesyan V., Ahmed F., Cernak I., Schinelli S., Stoica B., Faden A. I. (2005). Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc. Natl. Acad. Sci. U.S.A. 102, 8333–8338 10.1073/pnas.0500989102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A., Bukalo O., Schachner M. (2008). Modulation of synaptic transmission and plasticity by cell adhesion and repulsion molecules. Neuron Glia Biol. 4, 197–209 10.1017/S1740925X09990111 [DOI] [PubMed] [Google Scholar]
- Edell D. J., Toi V. V., McNeil V. M., Clark L. D. (1992). Factors influencing the biocompatibility of insertable silicon microshafts in cerebral-cortex. IEEE Trans. Biomed. Eng. 39, 635–643 10.1109/10.141202 [DOI] [PubMed] [Google Scholar]
- Ekdahl C. T., Claasen J. H., Bonde S., Kokaia Z., Lindvall O. (2003). Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. U.S.A. 100, 13632–13637 10.1073/pnas.2234031100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund U., Bjorklund A., Wictorin K., Lindvall O., Kokaia M. (2002). Grafted neural stem cells develop into functional pyramidal neurons and integrate into host cortical circuitry. Proc. Natl. Acad. Sci. U.S.A. 99, 17089–17094 10.1073/pnas.252589099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faijerson J., Tinsley R. B., Aprico K., Thorsell A., Nodin C., Nilsson M., Blomstrand F., Eriksson P. S. (2006). Reactive astrogliosis induces astrocytic differentiation of adult neural stem/progenitor cells in vitro. J. Neurosci. Res. 84, 1415–1424 10.1002/jnr.21044 [DOI] [PubMed] [Google Scholar]
- Faulkner J. R., Herrmann J. E., Woo M. J., Tansey K. E., Doan N. B., Sofroniew M. V. (2004). Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24, 2143–2155 10.1523/JNEUROSCI.3547-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J. W., Asher R. A. (1999). The glial scar and central nervous system repair. Brain Res. Bull. 49, 377–391 10.1016/S0361-9230(99)00072-6 [DOI] [PubMed] [Google Scholar]
- Fernaud-Espinosa I., Nieto-Sampedro M., Bovolenta P. (1993). Differential activation of microglia and astrocytes in aniso- and isomorphic gliotic tissue. Glia 8, 277–291 10.1002/glia.440080408 [DOI] [PubMed] [Google Scholar]
- Fitch M. T., Silver J. (1997). Activated macrophages and the blood-brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp. Neurol. 148, 587–603 10.1006/exnr.1997.6701 [DOI] [PubMed] [Google Scholar]
- Fitch M. T., Silver J. (2008). CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209, 294–301 10.1016/j.expneurol.2007.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker R. A., Carpenter M. K., Winkler C., Greco C., Gates M. A., Bjorklund A. (1999). Site-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J. Neurosci. 19, 5990–6005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontczak-Baniewicz M., Walski M. (2006). Glial scar instability after brain injury. J. Physiol. Pharmacol. 57(Suppl. 4), 97–102 [PubMed] [Google Scholar]
- Fujita T., Yoshimine T., Maruno M., Hayakawa T. (1998). Cellular dynamics of macrophages and microglial cells in reaction to stab wounds in rat cerebral cortex. Acta Neurochir. 140, 275–279 10.1007/s007010050095 [DOI] [PubMed] [Google Scholar]
- Gao J., Prough D. S., McAdoo D. J., Grady J. J., Parsley M. O., Ma L., Tarensenko Y. I., Wu P. (2006). Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp. Neurol. 201, 281–292 10.1016/j.expneurol.2006.04.039 [DOI] [PubMed] [Google Scholar]
- Gervasi N. M., Kwok J. C., Fawcett J. W. (2008). Role of extracellular factors in axon regeneration in the CNS: implications for therapy. Regen. Med. 3, 907–923 10.2217/17460751.3.6.907 [DOI] [PubMed] [Google Scholar]
- Gingras M., Bergeron J., Dery J., Durham H. D., Berthod F. (2003). In vitro development of a tissue-engineered model of peripheral nerve regeneration to study neurite growth. FASEB J. 17, 2124–2126 [DOI] [PubMed] [Google Scholar]
- Giordana M. T., Attanasio A., Cavalla P., Migheli A., Vigliani M. C., Schiffer D. (1994). Reactive cell proliferation and microglia following injury to the rat brain. Neuropathol. Appl. Neurobiol. 20, 163–174 10.1111/j.1365-2990.1994.tb01175.x [DOI] [PubMed] [Google Scholar]
- Giordano C., Causa F., Bianco F., Perale G., Netti P. A., Ambrosio L., Cigada A. (2008). Gene delivery systems for gene therapy in tissue engineering and central nervous system applications. Int. J. Artif. Organs 31, 1017–1026 [DOI] [PubMed] [Google Scholar]
- Goh E. L., Ma D., Ming G. L., Song H. (2003). Adult neural stem cells and repair of the adult central nervous system. J. Hematother. Stem Cell Res. 12, 671–679 10.1089/15258160360732696 [DOI] [PubMed] [Google Scholar]
- Goings G. E., Sahni V., Szele F. G. (2004). Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 996, 213–226 10.1016/j.brainres.2003.10.034 [DOI] [PubMed] [Google Scholar]
- Goldshmit Y., Galea M. P., Wise G., Bartlett P. F., Turnley A. M. (2004). Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J. Neurosci. 24, 10064–10073 10.1523/JNEUROSCI.2981-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y., McLenachan S., Turnley A. (2006). Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res. Rev. 52, 327–345 10.1016/j.brainresrev.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Gomez N., Lu Y., Chen S. C., Schmidt C. E. (2007). Immobilized nerve growth factor and microtopography have distinct effects on polarization versus axon elongation in hippocampal cells in culture. Biomaterials 28, 271–284 10.1016/j.biomaterials.2006.07.043 [DOI] [PubMed] [Google Scholar]
- Gomez N., Schmidt C. E. (2007). Nerve growth factor-immobilized polypyrrole: bioactive electrically conducting polymer for enhanced neurite extension. J. Biomed. Mater. Res. A 81A, 135–149 10.1002/jbm.a.31047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenendaal B. L., Jonas F., Freitag D., Pielartzik H., Reynolds J. R. (2000). Poly(3,4-ethylenedioxythiophene) and its derivatives: past, present, and future. Adv. Mater. 12, 481–494 [DOI] [Google Scholar]
- Han S. S., Kang D.Y., Mujtaba T., Rao M. S., Fischer I. (2002). Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Exp. Neurol. 177, 360–375 10.1006/exnr.2002.7995 [DOI] [PubMed] [Google Scholar]
- Harris N. G., Carmichael S. T., Hovda D. A., Sutton R. L. (2009). Traumatic brain injury results in disparate regions of chondroitin sulfate proteoglycan expression that are temporally limited. J. Neurosci. Res. 87, 2937–2950 10.1002/jnr.22115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Ino H., Koda M., Murakami M., Yoshinaga K., Yamazaki M., Moriya H. (2004). Regulation of semaphorin 3A expression in neurons of the rat spinal cord and cerebral cortex after transection injury. Acta Neuropathol. 107, 250–256 10.1007/s00401-003-0805-z [DOI] [PubMed] [Google Scholar]
- He W., McConnell G. C., Schneider T. M., Bellamkonda R. V. (2007). A novel anti-inflammatory surface for neural electrodes. Adv. Mater. 19, 3529–3533 10.1002/adma.200700943 [DOI] [Google Scholar]
- Hertz L., Zielke H. R. (2004). Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 27, 735–743 10.1016/j.tins.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Hochberg L. R., Serruya M. D., Friehs G. M., Mukand J. A., Saleh M., Caplan A. H., Branner A., Chen D., Penn R. D., Donoghue J. P. (2006). Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442, 164–171 10.1038/nature04970 [DOI] [PubMed] [Google Scholar]
- Holland T. A., Tabata Y., Mikos A. G. (2005). Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J. Control Release 101, 111–125 10.1016/j.jconrel.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Hubert T., Grimal S., Carroll P., Fichard-Carroll A. (2009). Collagens in the developing and diseased nervous system. Cell. Mol. Life Sci. 66, 1223–1238 10.1007/s00018-008-8561-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iarikov D. E., Kim B. G., Dai H. N., McAtee M., Kuhn P. L., Bregman B. S. (2007). Delayed transplantation with exogenous neurotrophin administration enhances plasticity of corticofugal projections after spinal cord injury. J. Neurotrauma 24, 690–702 10.1089/neu.2006.0172 [DOI] [PubMed] [Google Scholar]
- Ignatius M. J., Sawhney N., Gupta A., Thibadeau B. M., Monteiro O. R., Brown I. G. (1998). Bioactive surface coatings for nanoscale instruments: Effects on CNS neurons. J. Biomed. Mater. Res. 40, 264–274 [DOI] [PubMed] [Google Scholar]
- Irons H. R., Cullen D. K., Shapiro N. P., Lambert N. A., Lee R. H., Laplaca M. C. (2008). Three-dimensional neural constructs: a novel platform for neurophysiological investigation. J. Neural Eng. 5, 333–341 10.1088/1741-2560/5/3/006 [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Sanes J. R. (2000). Development. The decade of the developing brain. Curr. Opin. Neurobiol. 10, 599–611 10.1016/S0959-4388(00)00136-7 [DOI] [PubMed] [Google Scholar]
- Jhaveri S. J., Hynd M. R., Dowell-Mesfin N., Turner J. N., Shain W., Ober C. K. (2009). Release of nerve growth factor from HEMA hydrogel-coated substrates and its effect on the differentiation of neural cells. Biomacromolecules 10, 174–183 10.1021/bm801101e [DOI] [PubMed] [Google Scholar]
- Jin K., Sun Y., Xie L., Peel A., Mao X. O., Batteur S., Greenberg D. A. (2003). Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol. Cell. Neurosci. 24, 171–189 10.1016/S1044-7431(03)00159-3 [DOI] [PubMed] [Google Scholar]
- John G. R., Lee S. C., Song X. Y., Rivieccio M., Brosnan C. F. (2005). IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia 49, 161–176 10.1002/glia.20109 [DOI] [PubMed] [Google Scholar]
- Jun S. B., Hynd M. R., Dowell-Mesfin N. M., Al-Kofahi Y., Roysam B., Shain W., Kim S. J. (2008). Modulation of cultured neural networks using neurotrophin release from hydrogel-coated microelectrode arrays. J. Neural Eng. 5, 203–213 10.1088/1741-2560/5/2/011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M., Kubo T., Hata K., Yamaguchi A., Yamashita T. (2007). Repulsion of cerebellar granule neurons by chondroitin sulfate proteoglycans is mediated by MAPK pathway. Neurosci. Lett. 423, 62–67 10.1016/j.neulet.2007.06.038 [DOI] [PubMed] [Google Scholar]
- Kao W. J., Hubbell J. A., Anderson J. M. (1999). Protein-mediated macrophage adhesion and activation on biomaterials: a model for modulating cell behavior. J. Mater. Sci. Mater. Med. 10, 601–605 10.1023/A:1008971222932 [DOI] [PubMed] [Google Scholar]
- Kao W. J., Lee D., Schense J. C., Hubbell J. A. (2001). Fibronectin modulates macrophage adhesion and FBGC formation: the role of RGD, PHSRN, and PRRARV domains. J. Biomed. Mater. Res. 55, 79–88 [DOI] [PubMed] [Google Scholar]
- Kapur T. A., Shoichet M. S. (2003). Chemically-bound nerve growth factor for neural tissue engineering applications. J. Biomater. Sci. Polym. Ed. 14, 383–394 10.1163/156856203321478883 [DOI] [PubMed] [Google Scholar]
- Kawaja M. D., Gage F. H. (1991). Reactive astrocytes are substrates for the growth of adult CNS axons in the presence of elevated levels of nerve growth factor. Neuron 7, 1019–1030 10.1016/0896-6273(91)90346-2 [DOI] [PubMed] [Google Scholar]
- Kawasaki Y., Zhang L., Cheng J. K., Ji R. R. (2008). Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 28, 5189–5194 10.1523/JNEUROSCI.3338-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademhosseini A., Eng G., Yeh J., Kucharczyk P. A., Langer R., Vunjak-Novakovic G., Radisic M. (2007). Microfluidic patterning for fabrication of contractile cardiac organoids. Biomed. Microdevices 9, 149–157 10.1007/s10544-006-9013-7 [DOI] [PubMed] [Google Scholar]
- Khetani S. R., Bhatia S. N. (2006). Engineering tissues for in vitro applications. Curr. Opin. Biotechnol. 17, 524–531 10.1016/j.copbio.2006.08.009 [DOI] [PubMed] [Google Scholar]
- Kim Y. T., Hitchcock R. W., Bridge M. J., Tresco P. A. (2004). Chronic response of adult rat brain tissue to implants anchored to the skull. Biomaterials 25, 2229–2237 10.1016/j.biomaterials.2003.09.010 [DOI] [PubMed] [Google Scholar]
- Klaver C. L., Caplan M. R. (2007). Bioactive surface for neural electrodes: decreasing astrocyte proliferation via transforming growth factor-beta1. J. Biomed. Mater. Res. A 81, 1011–1016 10.1002/jbm.a.31153 [DOI] [PubMed] [Google Scholar]
- Koprivica V., Cho K. S., Park J. B., Yiu G., Atwal J., Gore B., Kim J. A., Lin E., Tessier-Lavigne M., Chen D. F., He Z. (2005). EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science 310, 106–110 10.1126/science.1115462 [DOI] [PubMed] [Google Scholar]
- Kyrkanides S., O'Banion M. K., Whiteley P. E., Daeschner J. C., Olschowka J. A. (2001). Enhanced glial activation and expression of specific CNS inflammation-related molecules in aged versus young rats following cortical stab injury. J. Neuroimmunol. 119, 269–277 10.1016/S0165-5728(01)00404-0 [DOI] [PubMed] [Google Scholar]
- Lagord C., Berry M., Logan A. (2002). Expression of TGFbeta2 but not TGFbeta1 correlates with the deposition of scar tissue in the lesioned spinal cord. Mol. Cell. Neurosci. 20, 69–92 10.1006/mcne.2002.1121 [DOI] [PubMed] [Google Scholar]
- Landis D. M. D. (1994). The early reactions of nonneuronal cells to brain injury. Annu. Rev. Neurosci. 17, 133–151 10.1146/annurev.ne.17.030194.001025 [DOI] [PubMed] [Google Scholar]
- LaPlaca M. C., Cullen D. K., McLoughlin J. J., Cargill R. S. II. (2005). High rate shear strain of three-dimensional neural cell cultures: a new in vitro traumatic brain injury model. J. Biomech. 38, 1093–1105 10.1016/j.jbiomech.2004.05.032 [DOI] [PubMed] [Google Scholar]
- Lee K., He J., Clement R., Massia S., Kim B. (2004). Biocompatible benzocyclobutene (BCB)-based neural implants with micro-fluidic channel. Biosens. Bioelectron. 20, 404–407 10.1016/j.bios.2004.02.005 [DOI] [PubMed] [Google Scholar]
- Lee W., Pinckney J., Lee V., Lee J. H., Fischer K., Polio S., Park J. K., Yoo S. S. (2009). Three-dimensional bioprinting of rat embryonic neural cells. Neuroreport 20, 798–803 10.1097/WNR.0b013e32832b8be4 [DOI] [PubMed] [Google Scholar]
- Lepore A. C., Neuhuber B., Connors T. M., Han S. S., Liu Y., Daniels M. P., Rao M. S., Fischer I. (2006). Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience 142, 287–304 10.1016/j.neuroscience.2005.12.067 [DOI] [PubMed] [Google Scholar]
- Li N., Folch A. (2005). Integration of topographical and biochemical cues by axons during growth on microfabricated 3-D substrates. Exp. Cell Res. 311, 307–316 10.1016/j.yexcr.2005.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Raisman G. (1995). Sprouts from cut corticospinal axons persist in the presence of astrocytic scarring in long-term lesions of the adult rat spinal cord. Exp. Neurol. 134, 102–111 10.1006/exnr.1995.1041 [DOI] [PubMed] [Google Scholar]
- Liberto C. M., Albrecht P. J., Herx L. M., Yong V. W., Levison S. W. (2004). Pro-regenerative properties of cytokine-activated astrocytes. J. Neurochem. 89, 1092–1100 10.1111/j.1471-4159.2004.02420.x [DOI] [PubMed] [Google Scholar]
- Lim J. H., Gibbons H. M., O'Carroll S. J., Narayan P. J., Faull R. L., Dragunow M. (2007). Extracellular signal-regulated kinase involvement in human astrocyte migration. Brain Res. 1164, 1–13 10.1016/j.brainres.2007.06.020 [DOI] [PubMed] [Google Scholar]
- Lin C. C., Anseth K. S. (2009). PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 26, 631–643 10.1007/s11095-008-9801-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. W., Wu C. C., Chen C. H., Ho H. O., Chen Y. C., Sheu M. T. (2005). Characterization of cortical neuron outgrowth in two- and three-dimensional culture systems. J. Biomed. Mater. Res. B Appl. Biomater. 75, 146–157 10.1002/jbm.b.30276 [DOI] [PubMed] [Google Scholar]
- Liu X., McCreery D. B., Carter R. R., Bullara L. A., Yuen T. G., Agnew W. F. (1999). Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans. Rehabil. Eng. 7, 315–326 10.1109/86.788468 [DOI] [PubMed] [Google Scholar]
- Liu X. D., McCreery D. B., Bullara L. A., Agnew W. F. (2006). Evaluation of the stability of intracortical microelectrode arrays. IEEE Trans. Neural Syst. Rehabil. Eng. 14, 91–100 10.1109/TNSRE.2006.870495 [DOI] [PubMed] [Google Scholar]
- Logan A., Berry M. (2002). Cellular and molecular determinants of glial scar formation. In: Molecular and Cellular Biology of Neuroprotection in the CNS, Alzheimer C., ed. (New York, NY, Kluwer Academic; ), pp. 115–137 [DOI] [PubMed] [Google Scholar]
- Lotan M., Schwartz M. (1994). Cross talk between the immune system and the nervous system in response to injury: implications for regeneration. FASEB J. 8, 1026–1033 [DOI] [PubMed] [Google Scholar]
- Ludwig K. A., Uram J. D., Yang J. Y., Martin D. C., Kipke D. R. (2006). Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J. Neural Eng. 3, 59–70 10.1088/1741-2560/3/1/007 [DOI] [PubMed] [Google Scholar]
- Lundberg C., Englund U., Trono D., Bjorklund A., Wictorin K. (2002). Differentiation of the RN33B cell line into forebrain projection neurons after transplantation into the neonatal rat brain. Exp. Neurol. 175, 370–387 10.1006/exnr.2002.7888 [DOI] [PubMed] [Google Scholar]
- Lutolf M. P., Lauer-Fields J. L., Schmoekel H. G., Metters A. T., Weber F. E., Fields G. B., Hubbell J. A. (2003). Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. U.S.A. 100, 5413–5418 10.1073/pnas.0737381100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S. K., Shnitka T. K. (2002). Diversity in reactive astrocytes. In: Neuroglia in the Aging Brain, de Vellis J., ed. (Totowa, NJ, Humana Press; ), pp. 17–33 [Google Scholar]
- Manwaring M. E., Biran R., Tresco P. A. (2001). Characterization of rat meningeal cultures on materials of differing surface chemistry. Biomaterials 22, 3155–3168 10.1016/S0142-9612(01)00068-0 [DOI] [PubMed] [Google Scholar]
- Mapili G., Lu Y., Chen S., Roy K. (2005). Laser-layered microfabrication of spatially patterned functionalized tissue-engineering scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 75, 414–424 10.1002/jbm.b.30325 [DOI] [PubMed] [Google Scholar]
- Massia S. P., Hubbell J. A. (1990). Covalent surface immobilization of Arg-Gly-Asp and Tyl-Ile-Gly-Arg-containing peptides to obtain well-defined cell-adhesive substrates. Anal. Chem. 187, 292–301 [DOI] [PubMed] [Google Scholar]
- Maynard E. M., Fernandez E., Normann R. A. (2000). A technique to prevent dural adhesions to chronically implanted microelectrode arrays. J. Neurosci. Methods 97, 93–101 10.1016/S0165-0270(00)00159-X [DOI] [PubMed] [Google Scholar]
- McConnell G. C., Rees H. D., Levey A. I., Gutekunst C. A., Gross R. E., Bellamkonda R. V. (2009). Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural Eng. 6 10.1088/1741-2560/6/5/056003 [DOI] [PubMed] [Google Scholar]
- McGraw J., Hiebert G. W., Steeves J. D. (2001). Modulating astrogliosis after neurotrauma. J. Neurosci. Res. 63, 109–115 [DOI] [PubMed] [Google Scholar]
- McMillian M. K., Thai L., Hong J. S., Ocallaghan J. P., Pennypacker K. R. (1994). Brain injury in a dish – a model for reactive gliosis. Trends Neurosci. 17, 138–142 10.1016/0166-2236(94)90086-8 [DOI] [PubMed] [Google Scholar]
- Mercanzini A., Cheung K., Buhl D. L., Boers M., Maillard A., Colin P., Bensadoun J. C., Bertsch A., Renaud P. (2008). Demonstration of cortical recording using novel flexible polymer neural probes. Sens. Actuators A Phys. 143, 90–96 10.1016/j.sna.2007.07.027 [DOI] [Google Scholar]
- Merrill J. E., Benveniste E. N. (1996). Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 19, 331–338 10.1016/0166-2236(96)10047-3 [DOI] [PubMed] [Google Scholar]
- Milenkovic I., Nedeljkovic N., Filipovic R., Pekovic S., Culic M., Rakic L., Stojiljkovic M. (2005). Pattern of glial fibrillary acidic protein expression following kainate-induced cerebellar lesion in rats. Neurochem. Res. 30, 207–213 10.1007/s11064-004-2443-9 [DOI] [PubMed] [Google Scholar]
- Miller G. (2005). Neuroscience. Cancer drugs may help injured nerve cells regrow their axons. Science 310, 31. 10.1126/science.310.5745.31a [DOI] [PubMed] [Google Scholar]
- Miller K., Chinzei K. (2002). Mechanical properties of brain tissue in tension. J. Biomech. 35, 483–490 10.1016/S0021-9290(01)00234-2 [DOI] [PubMed] [Google Scholar]
- Mofid M. M., Thompson R. C., Pardo C. A., Manson P. N., Vander Kolk C. A. (1997). Biocompatibility of fixation materials in the brain. Plast. Reconstr. Surg. 100, 14–20 10.1097/00006534-199707000-00003 [DOI] [PubMed] [Google Scholar]
- Montezuma S. R., Loewenstein J., Scholz C., Rizzo J. F. (2006). Biocompatibility of materials implanted into the subretinal space of Yucatan pigs. Invest. Ophthalmol. Vis. Sci. 47, 3514–3522 10.1167/iovs.06-0106 [DOI] [PubMed] [Google Scholar]
- Moore K., Macsween M., Shoichet M. (2006). Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Eng. 12, 267–278 10.1089/ten.2006.12.267 [DOI] [PubMed] [Google Scholar]
- Murua A., Portero A., Orive G., Hernandez R. M., de Castro M., Pedraz J. L. (2008). Cell microencapsulation technology: towards clinical application. J. Control Release. 132, 76–83 10.1016/j.jconrel.2008.08.010 [DOI] [PubMed] [Google Scholar]
- Nadal A., Fuentes E., Pastor J., McNaughton P. A. (1995). Plasma albumin is a potent trigger of calcium signals and DNA-synthesis in astrocytes. Proc. Natl. Acad. Sci. U.S.A. 92, 1426–1430 10.1073/pnas.92.5.1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A., Fuentes E., Pastor J., McNaughton P. A. (1997). Plasma albumin induces calcium waves in rat cortical astrocytes. Glia 19, 343–351 [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B., Decker L., Lachapelle F., Avellana-Adalid V., Bachelin C., Van Evercooren A. B. (1999). Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 11, 4357–4366 10.1046/j.1460-9568.1999.00873.x [DOI] [PubMed] [Google Scholar]
- Nakatomi H., Kuriu T., Okabe S., Yamamoto S., Hatano O., Kawahara N., Tamura A., Kirino T., Nakafuku M. (2002). Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110, 429–441 10.1016/S0092-8674(02)00862-0 [DOI] [PubMed] [Google Scholar]
- Neary J. T., Kang Y. (2005). Signaling from P2 nucleotide receptors to protein kinase cascades induced by CNS injury: implications for reactive gliosis and neurodegeneration. Mol. Neurobiol. 31, 95–103 10.1385/MN:31:1-3:095 [DOI] [PubMed] [Google Scholar]
- Nguyen M. D., Mushynski W. E., Julien J. P. (2002). Cycling at the interface between neurodevelopment and neurodegeneration. Cell Death Differ. 9, 1294–1306 10.1038/sj.cdd.4401108 [DOI] [PubMed] [Google Scholar]
- Nicole O., Goldshmidt A., Hamill C. E., Sorensen S. D., Sastre A., Lyuboslavsky P., Hepler J. R., McKeon R. J., Traynelis S. F. (2005). Activation of protease-activated receptor-1 triggers astrogliosis after brain injury. J. Neurosci. 25, 4319–4329 10.1523/JNEUROSCI.5200-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis M. A. L., Dimitrov D., Carmena J. M., Crist R., Lehew G., Kralik J. D., Wise S. P. (2003). Chronic, multisite, multielectrode recordings in macaque monkeys. Proc. Natl. Acad. Sci. U.S.A. 100, 11041–11046 10.1073/pnas.1934665100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis M. A. L., Ribeiro S. (2002). Multielectrode recordings: the next steps. Curr. Opin. Neurobiol. 12, 602–606 10.1016/S0959-4388(02)00374-4 [DOI] [PubMed] [Google Scholar]
- O'Connor S. M., Stenger D. A., Shaffer K. M., Ma W. (2001). Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci. Lett. 304, 189–193 10.1016/S0304-3940(01)01769-4 [DOI] [PubMed] [Google Scholar]
- Oda K., Matsuoka Y., Funahashi A., Kitano H. (2005). A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 1, 2005.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M., Suzuki Y., Chou H., Ishikawa N., Suzuki S., Tanihara M., Mizushima Y., Dezawa M., Ide C. (2004). Novel heparin/alginate gel combined with basic fibroblast growth factor promotes nerve regeneration in rat sciatic nerve. J. Biomed. Mater. Res. A. 71, 661–668 10.1002/jbm.a.30194 [DOI] [PubMed] [Google Scholar]
- Okada S., Nakamura M., Katoh H., Miyao T., Shimazaki T., Ishii K., Yamane J., Yoshimura A., Iwamoto Y., Toyama Y., Okano H. (2006). Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 12, 829–834 10.1038/nm1425 [DOI] [PubMed] [Google Scholar]
- Onifer S. M., Whittemore S. R., Holets V. R. (1993). Variable morphological-differentiation of a raphe-derived neuronal cell-line following transplantation into the adult-rat CNS. Exp. Neurol. 122, 130–142 10.1006/exnr.1993.1114 [DOI] [PubMed] [Google Scholar]
- Pampaloni F., Reynaud E. G., Stelzer E. H. (2007). The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 8, 839–845 10.1038/nrm2236 [DOI] [PubMed] [Google Scholar]
- Papageorgiou D. P., Shore S. E., Bledsoe S. C., Wise K. D. (2006). A shuttered neural probe with on-chip flowmeters for chronic in vivo drug delivery. J. Microelectromech. Syst. 15, 1025–1033 10.1109/JMEMS.2005.863733 [DOI] [Google Scholar]
- Papavasiliou G., Songprawat P., Perez-Luna V., Hammes E., Morris M., Chiu Y. C., Brey E. (2008). Three-dimensional pattering of poly(ethylene glycol) hydrogels through surface-initiated photopolymerization. Tissue Eng. C Methods 14, 129–140 [DOI] [PubMed] [Google Scholar]
- Park H., Temenoff J. S., Tabata Y., Caplan A. I., Mikos A. G. (2007). Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials 28, 3217–3227 10.1016/j.biomaterials.2007.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikov V. S. (2009). An In Vitro Model of the Brain Tissue Reaction to Chronically Implanted Recording Electrodes Reveals Essential Roles for Serum and bfgf in Glial Scarring. Doctor of Philosophy Thesis, Department of Biomedical Engineering, Duke University, Durham, NC [Google Scholar]
- Polikov V. S., Block M. L., Fellous J. M., Hong J. S., Reichert W. M. (2006). In vitro model of glial scarring around neuroelectrodes chronically implanted in the CNS. Biomaterials 27, 5368–5376 10.1016/j.biomaterials.2006.06.018 [DOI] [PubMed] [Google Scholar]
- Polikov V. S., Su E. C., Bail M. A., Hong J. S., Reichert W. M. (2009). Control protocol for robust in vitro glial scar formation around microwires: Essential roles of bFGF and serum in gliosis. J. Neurosci. Methods 181, 170–177 10.1016/j.jneumeth.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikov V. S., Tresco P. A., Reichert W. M. (2005). Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 10.1016/j.jneumeth.2005.08.015 [DOI] [PubMed] [Google Scholar]
- Popovich P. G., Guan Z., Wei P., Huitinga I., van Rooijen N., Stokes B. T. (1999). Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 158, 351–365 10.1006/exnr.1999.7118 [DOI] [PubMed] [Google Scholar]
- Quaglia F. (2008). Bioinspired tissue engineering: the great promise of protein delivery technologies. Int. J. Pharm. 364, 281–297 10.1016/j.ijpharm.2008.04.030 [DOI] [PubMed] [Google Scholar]
- Raivich G., Bohatschek M., Kloss C. U. A., Werner A., Jones L. L., Kreutzberg G. W. (1999). Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res. Rev. 30, 77–105 10.1016/S0165-0173(99)00007-7 [DOI] [PubMed] [Google Scholar]
- Rapalino O., Lazarov-Spiegler O., Agranov E., Velan G. J., Yoles E., Fraidakis M., Solomon A., Gepstein R., Katz A., Belkin M., Hadani M., Schwartz M. (1998). Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat. Med. 4, 814–821 10.1038/nm0798-814 [DOI] [PubMed] [Google Scholar]
- Renault-Mihara F., Okada S., Shibata S., Nakamura M., Toyama Y., Okano H. (2008). Spinal cord injury: emerging beneficial role of reactive astrocytes’ migration. Int. J. Biochem. Cell Biol. 40, 1649–1653 10.1016/j.biocel.2008.03.009 [DOI] [PubMed] [Google Scholar]
- Retterer S. T., Smith K. L., Bjornsson C. S., Neeves K. B., Spence A. J., Turner J. N., Shain W., Isaacson M. S. (2004). Model neural prostheses with integrated microfluidics: a potential intervention strategy for controlling reactive cell and tissue responses. IEEE Trans. Biomed. Eng. 51, 2063–2073 10.1109/TBME.2004.834288 [DOI] [PubMed] [Google Scholar]
- Rhodes K. E., Raivich G., Fawcett J. W. (2006). The injury response of oligodendrocyte precursor cells is induced by platelets, macrophages and inflammation-associated cytokines. Neuroscience 140, 87–100 10.1016/j.neuroscience.2006.01.055 [DOI] [PubMed] [Google Scholar]
- Richardson R. T., Wise A. K., Thompson B. C., Flynn B. O., Atkinson P. J., Fretwell N. J., Fallon J. B., Wallace G. G., Shepherd R. K., Clark G. M., O'Leary S. J. (2009). Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials 30, 2614–2624 10.1016/j.biomaterials.2009.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson T. P., Peters M. C., Ennett A. B., Mooney D. J. (2001). Polymeric system for dual growth factor delivery. Nat. Biotechnol. 19, 1029–1034 10.1038/nbt1101-1029 [DOI] [PubMed] [Google Scholar]
- Ridet J. L., Malhotra S. K., Privat A., Gage F. H. (1997). Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 20, 570–577 10.1016/S0166-2236(97)01139-9 [DOI] [PubMed] [Google Scholar]
- Roitbak T., Sykova E. (1999). Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia 28, 40–48 [DOI] [PubMed] [Google Scholar]
- Rolls A., Shechter R., London A., Segev Y., Jacob-Hirsch J., Amariglio N., Rechavi G., Schwartz M. (2008). Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med. 5, e171. 10.1371/journal.pmed.0050171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A., Shechter R., Schwartz M. (2009). The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 10, 235–241 10.1038/nrn2591 [DOI] [PubMed] [Google Scholar]
- Rousche P. J., Normann R. A. (1998). Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J. Neurosci. Methods 82, 1–15 10.1016/S0165-0270(98)00031-4 [DOI] [PubMed] [Google Scholar]
- Saadoun S., Papadopoulos M. C., Watanabe H., Yan D. H., Manley G. T., Verkman A. S. (2005). Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J. Cell Sci. 118, 5691–5698 10.1242/jcs.02680 [DOI] [PubMed] [Google Scholar]
- Saneinejad S., Shoichet M. S. (1998). Patterned glass surfaces direct cell adhesion and process outgrowth of primary neurons of the central nervous system. J. Biomed. Mater. Res. 42, 13–19 [DOI] [PubMed] [Google Scholar]
- Schlosshauer B., Brinker T., Muller H. W., Meyer J. U. (2001). Towards micro electrode implants: in vitro guidance of rat spinal cord neurites through polyimide sieves by Schwann cells. Brain Res. 903, 237–241 10.1016/S0006-8993(01)02391-5 [DOI] [PubMed] [Google Scholar]
- Schmidt J. J., Rowley J., Kong H. J. (2008). Hydrogels used for cell-based drug delivery. J. Biomed. Mater. Res. A. 87, 1113–1122 10.1002/jbm.a.32287 [DOI] [PubMed] [Google Scholar]
- Schmidt S., Horch K., Normann R. (1993). Biocompatibility of silicon-based electrode arrays implanted in feline cortical tissue. J. Biomed. Mater. Res. 27, 1393–1399 10.1002/jbm.820271106 [DOI] [PubMed] [Google Scholar]
- Schwartz A. B. (2004). Cortical neural prosthetics. Annu. Rev. Neurosci. 27, 487–507 10.1146/annurev.neuro.27.070203.144233 [DOI] [PubMed] [Google Scholar]
- Sheen V. L., Arnold M. W., Wang Y., Macklis J. D. (1999). Neural precursor differentiation following transplantation into neocortex is dependent on intrinsic developmental state and receptor competence. Exp. Neurol. 158, 47–62 10.1006/exnr.1999.7104 [DOI] [PubMed] [Google Scholar]
- Shihabuddin L. S., Brunschwig J. P., Holets V. R., Bunge M. B., Whittemore S. R. (1996). Induction of mature neuronal properties in immortalized neuronal precursor cells following grafting into the neonatal CNS. J. Neurocytol. 25, 101–111 10.1007/BF02284789 [DOI] [PubMed] [Google Scholar]
- Silva A. K., Richard C., Bessodes M., Scherman D., Merten O. W. (2009). Growth factor delivery approaches in hydrogels. Biomacromolecules 10, 9–18 10.1021/bm801103c [DOI] [PubMed] [Google Scholar]
- Sivron T., Schwartz M. (1995). Glial cell types, lineages, and response to injury in rat and fish: implications for regeneration. Glia 13, 157–165 10.1002/glia.440130302 [DOI] [PubMed] [Google Scholar]
- Stensaas S. S., Stensaas L. J. (1976). Reaction of cerebral cortex to chronically implanted plastic needles. Acta Neuropathol. 35, 187–203 [PubMed] [Google Scholar]
- Suuronen E. J., McLaughlin C. R., Stys P. K., Nakamura M., Munger R., Griffith M. (2004). Functional innervation in tissue engineered models for in vitro study and testing purposes. Toxicol. Sci. 82, 525–533 10.1093/toxsci/kfh270 [DOI] [PubMed] [Google Scholar]
- Swan E. E. L., Mescher M. J., Sewell W. F., Tao S. L., Borenstein J. I. (2008). Inner ear drug delivery for auditory applications. Adv. Drug Deliv. Rev. 60, 1583–1599 10.1016/j.addr.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarowski D. H., Andersen M. D., Retterer S., Spence A. J., Isaacson M., Craighead H. G., Turner J. N., Shain W. (2003). Brain responses to micro-machined silicon devices. Brain Res. 983, 23–35 10.1016/S0006-8993(03)03023-3 [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Miyaishi O., Kiuchi K., Isobe K. (2001). Macrophage colony-stimulating factor is expressed in neuron and microglia after focal brain injury. J. Neurosci. Res. 65, 38–44 10.1002/jnr.1125 [DOI] [PubMed] [Google Scholar]
- Takeuchi S., Ziegler D., Yoshida Y., Mabuchi K., Suzuki T. (2005). Parylene flexible neural probes integrated with microfluidic channels. Lab. Chip 5, 519–523 10.1039/b417497f [DOI] [PubMed] [Google Scholar]
- Tanihara M., Suzuki Y., Yamamoto E., Noguchi A., Mizushima Y. (2001). Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J. Biomed. Mater. Res. 56, 216–221 [DOI] [PubMed] [Google Scholar]
- Tibbitt M. W., Anseth K. S. (2009). Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 103, 655–663 10.1002/bit.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y. W., Shoichet M. S. (1998). Peptide surface modification of poly(tetrafluoroethylene-co-hexafluoropropylene) enhances its interaction with central nervous system neurons. J. Biomed. Mater. Res. 42, 85–95 [DOI] [PubMed] [Google Scholar]
- Tong Y. W., Shoichet M. S. (2001). Enhancing the neuronal interaction on fluoropolymer surfaces with mixed peptides or spacer group linkers. Biomaterials 22, 1029–1034 10.1016/S0142-9612(00)00338-0 [DOI] [PubMed] [Google Scholar]
- Turner J. N., Shain W., Szarowski D. H., Andersen M., Martins S., Isaacson M., Craighead H. (1999). Cerebral astrocyte response to micromachined silicon implants. Exp. Neurol. 156, 33–49 10.1006/exnr.1998.6983 [DOI] [PubMed] [Google Scholar]
- Venstrom K. A., Reichardt L. F. (1993). Extracellular matrix. 2: role of extracellular matrix molecules and their receptors in the nervous system. FASEB J. 7, 996–1003 [DOI] [PubMed] [Google Scholar]
- Vetter R. J., Williams J. C., Hetke J. F., Nunamaker E. A., Kipke D. R. (2004). Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans. Biomed. Eng. 51, 896–904 10.1109/TBME.2004.826680 [DOI] [PubMed] [Google Scholar]
- Wang N., Tytell J. D., Ingber D. E. (2009a). Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75–82 10.1038/nrm2594 [DOI] [PubMed] [Google Scholar]
- Wang W., Bu B., Xie M., Zhang M., Yu Z., Tao D. (2009b). Neural cell cycle dysregulation and central nervous system diseases. Prog. Neurobiol. 89, 1–17 10.1016/j.pneurobio.2009.01.007 [DOI] [PubMed] [Google Scholar]
- Wang Y., Moges H., Bharucha Y., Symes A. (2007). Smad3 null mice display more rapid wound closure and reduced scar formation after a stab wound to the cerebral cortex. Exp. Neurol. 203, 168–184 10.1016/j.expneurol.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Wanner I. B., Deik A., Torres M., Rosendahl A., Neary J. T., Lemmon V. P., Bixby J. L. (2008). A new in vitro model of the glial scar inhibits axon growth. Glia 56, 1691–1709 10.1002/glia.20721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartiovaara K., Barnabe-Heider F., Miller F. D., Kaplan D. R. (2002). N-myc promotes survival and induces S-phase entry of postmitotic sympathetic neurons. J. Neurosci. 22, 815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb K., Budko E., Neuberger T. J., Chen S. Z., Schachner M., Tresco P. A. (2001). Substrate-bound human recombinant L1 selectively promotes neuronal attachment and outgrowth in the presence of astrocytes and fibroblasts. Biomaterials 22, 1017–1028 10.1016/S0142-9612(00)00353-7 [DOI] [PubMed] [Google Scholar]
- Weiland J. D., Liu W. T., Humayun M. S. (2005). Retinal prosthesis. Annu. Rev. Biomed. Eng. 7, 361–401 10.1146/annurev.bioeng.7.060804.100435 [DOI] [PubMed] [Google Scholar]
- Whitney N. P., Eidem T. M., Peng H., Huang Y., Zheng J. C. (2009). Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J. Neurochem. 108, 1343–1359 10.1111/j.1471-4159.2009.05886.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore S. R., White L. A. (1993). Target regulation of neuronal differentiation in a temperature-sensitive cell-line derived from medullary raphe. Brain Res. 615, 27–40 10.1016/0006-8993(93)91111-5 [DOI] [PubMed] [Google Scholar]
- Wilkins A., Majed H., Layfield R., Compston A., Chandran S. (2003). Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. J. Neurosci. 23, 4967–4974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Hippensteel J. A., Dilgen J., Shain W., Kipke D. R. (2007). Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J. Neural Eng. 4, 410–423 10.1088/1741-2560/4/4/007 [DOI] [PubMed] [Google Scholar]
- Williams J. C., Rennaker R. L., Kipke D. R. (1999). Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res. Protoc. 4, 303–313 10.1016/S1385-299X(99)00034-3 [DOI] [PubMed] [Google Scholar]
- Winn S. R., Aebischer P., Galletti P. M. (1989). Brain tissue reaction to permselective polymer capsules. J. Biomed. Mater. Res. 23, 31–44 10.1002/jbm.820230104 [DOI] [PubMed] [Google Scholar]
- Winter J. O., Cogan S. F., Rizzo J. F. III. (2007a). Retinal prostheses: current challenges and future outlook. J. Biomater. Sci. Polym. Ed. 18, 1031–1055 10.1163/156856207781494403 [DOI] [PubMed] [Google Scholar]
- Winter J. O., Cogan S. F., Rizzo J. F. III. (2007b). Neurotrophin-eluting hydrogel coatings for neural stimulating electrodes. J. Biomed. Mater. Res. B Appl. Biomater. 81, 551–563 10.1002/jbm.b.30696 [DOI] [PubMed] [Google Scholar]
- Wise K. D., Anderson D. J., Hetke J. F., Kipke D. R., Najafi K. (2004). Wireless implantable microsystems: high-density electronic interfaces to the nervous system. Proc. IEEE 92, 76–97 10.1109/JPROC.2003.820544 [DOI] [Google Scholar]
- Wu V. W., Schwartz J. P. (1998). Cell culture models for reactive gliosis: new perspectives. J. Neurosci. Res. 51, 675–681 [DOI] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 10.1016/j.cell.2008.01.033 [DOI] [PubMed] [Google Scholar]
- Zhong Y. H., Bellamkonda R. V. (2008). Biomaterials for the central nervous system. J. R. Soc. Interface 5, 957–975 10.1098/rsif.2008.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]