Abstract
The development of in vivo EPR has made it feasible to perform tooth dosimetry measurements in situ, greatly expanding the potential for using this approach for immediate screening after radiation exposures. The ability of in vivo tooth dosimetry to provide estimates of absorbed dose has been established through a series of experiments using unirradiated volunteers with specifically irradiated molar teeth placed in situ within gaps in their dentition and in natural canine teeth of patients who have completed courses of radiation therapy for head and neck cancers. Multiple measurements in patients who have received radiation therapy demonstrate the expected heterogeneous dose distributions. Dose response curves have been generated using both populations and, using the current methodology and instrument, the standard error of prediction based on single 4.5 minute measurements is approximately 1.5 Gy for inserted molar teeth and between 2.0 and 2.5 Gy in the more irregularly shaped canine teeth. Averaging of independent measurements can reduce this error significantly to values near 1 Gy. Developments to reduce these errors are underway, focusing on geometric optimization of the resonators, detector positioning techniques, and optimal data averaging approaches. In summary, it seems plausible that the EPR dosimetry techniques will have an important role in retrospective dosimetry for exposures involving large numbers of individuals.
INTRODUCTION
Electron paramagnetic resonance (EPR) spectroscopy has been applied to perform retrospective radiation biodosimetry using extracted samples of calcified tissues, especially tooth enamel, following radiation accidents and exposures resulting from weapon use, testing, and production (Ikeya et al. 1984, Ikeya et al. 1986, Chumak et al. 1998, Tolstykh et al. 2000, Romanyukha et al. 2006). Ionizing radiation generates large numbers of unpaired electron species in irradiated materials, including biologic tissues. While radicals generated in soft tissue react very quickly to form non-paramagnetic, EPR-silent species, radicals generated in tooth enamel are very stable, persisting indefinitely at levels that are directly proportional to dose (Desrosiers and Schauer 2001). Conventional EPR dosimetry involves the isolation of tooth enamel for measurements made at X-band (~10GHz) in a remote laboratory, either using a universal calibration curve or using a calibration based on the addition of several known doses (Onori et al. 2000, Wieser et al. 2005, Güttler and Wieser 2008). While this technique is well suited for use in limited populations following certain exposures, its use as a tool to perform screening after an event where a large number of people have potentially been exposed to clinically relevant doses is limited by the need to extract the tissue and remote processing. In order to meet the need for large-scale biodosimetry, likely in concert with complementary biologically based techniques, the instruments and procedures to perform EPR dosimetry in vivo with intact teeth, in the field, are being developed (Iwasaki et al. 2005, Swartz et al. 2005, Swartz et al. 2006, Demidenko et al. 2007, Swartz et al. 2007, Williams et al. 2007).
In vivo EPR tooth dosimetry has several very desirable characteristics for screening and providing guidance for triage following a mass-exposure incident. The EPR technique is applicable on individual subjects and can provide an accurate estimate of the absorbed dose within the oral cavity. Because the information is from a specific site, it can be used in combination with other information and dosimetric assays to estimate the whole body dose or to characterize a heterogeneous exposure. As a physical assay based on inert tissue, rather than biological assay of dynamic tissues and markers, in vivo EPR dosimetry is independent from confounding biologic factors, such as stress or simultaneous tissue damage, including wounds and burns, which are likely to occur with irradiation and potentially confound assays based on biological responses. The measurement process is completely non-invasive and painless. Owing to the stability of the irradiation signature in the enamel, the dosimetry measurements can be performed immediately after the event and repeated at any time to confirm dose estimates or increase their precision. Because of the stability of the dosimetric signal, the possibility of prior irradiation of subjects during radiotherapy must be addressed, likely through a simple questionnaire at the time of measurement. Instruments capable of operation in the field, by non-expert operators, are being developed and prototypes are currently being evaluated (Swartz et al. 2007). No local or remote processing of samples is needed. Dose estimates can therefore be provided immediately after measurement, while the subjects are present, so that the information can be immediately factored into the subjects’ care.
Development of In Vivo EPR Dosimetry
There is a long history of EPR of mineralized biologic tissue being used as radiation biodosimeters, dating back the seminal measurements of Gordy et al. (Gordy et al. 1955) and the proposal by Brady et al. (Brady et al. 1968) that such measurements could be used for assessment of accidental exposures in biologic tissues. Over the following decades EPR dosimetry has been applied extensively to assess such exposures using isolated tooth enamel and bone samples with X-band (~10GHz) EPR. The present work and the following literature summary focus on the use of low frequency EPR (1.2GHz) for in vivo estimation of absorbed dose in whole teeth.
Miyake et al. investigated the feasibility of low frequency in vivo EPR dosimetry for use in human subjects through a set of measurements of isolated human teeth, estimating that precision of 20cGy or lower could be achieved (Miyake et al. 2000). Additional investigations using whole isolated human teeth were performed by Zdravkova et al., which described the neutron dose response and further defined spectroscopic characteristics of the EPR signals of the irradiated teeth at L-band (Zdravkova et al. 2002, Zdravkova et al. 2002, Zdravkova et al. 2003, Zdravkova et al. 2003). In 2005 Iwasaki and Swartz reported the first in vivo EPR dosimetry measurements of whole human teeth within the mouths of volunteers (Iwasaki et al. 2005). Additional in vitro measurements were performed that demonstrated a linear dose dependence for human incisor teeth, indicated that signal-to-noise ratios for in vivo measurements would be comparable to those achieved in vitro, and demonstrated that the presence of amalgam in teeth being measured or in neighboring teeth did not corrupt the EPR measurements (other than through a reduction in the amount of enamel) or cause local heating. In another manuscript, Iwasaki et al. described the analysis of measurements of whole isolated teeth, distinguishing between four components in the observed signals, including the radiation induced EPR signal, the native radiation-independent EPR signal, and coherent and random noise. It described differences in the g-values, lineshapes, and saturation characteristics of the EPR signals that could be used to distinguish these components (Iwasaki et al. 2005). A size correction was proposed for isolated molar teeth to normalize the EPR signal amplitudes and provide a common dose response (Iwasaki et al. 2005). Several reviews of progress in the development of in vivo EPR dosimetry and the field-deployment of the technology have been provided by Swartz et al. (Swartz et al. 2005, Swartz et al. 2006) , including results of the first measurements of intact irradiated teeth in the mouths of volunteers who had completed courses of radiation therapy (Swartz et al. 2007). Williams et al. discussed potential sources of variability in the dosimetric in vivo EPR signals and described the procedures used for in vivo EPR dosimetry, including instrumental settings, patient positioning and immobilization practices, and a technique for accurate and reproducible positioning of the resonator detector on the tooth surface (Williams et al. 2007). Spectral analysis procedures for estimation of absorbed dose from the recorded EPR signals, based on non-linear least squares fitting with several proposed spectral models, were described by Demidenko et al. (Demidenko et al. 2007). Data are presented for repeated measurements of irradiated teeth which had been placed within a gap in the dentition of a volunteer, as they would exist naturally, that demonstrate standard errors of dose prediction (SEP) of 1.8Gy for individual measurements and 0.5Gy after averaging three independent measurement acquired on different days. This result demonstrates the ability of averaging to decrease variability in the dose estimates, through effective reduction of random noise and compensation for variability in resonator positioning. In preparation for the intended use during triage following a major radiation accident or terrorist event Flood et al. review government policy considerations, regulatory clearance, and device production for implementation of EPR dosimetry (Flood et al. 2007).
Spectral Modeling and Data Analysis
Characterization of the dosimetric EPR signals of tooth enamel measured at X-band and Q-band have been published (e.g. (Vanhaelewyn et al. 2001, Vanhaelewyn et al. 2002)). At L-band the EPR signals become less complex and the spectral model can be simplified to 2 independent components; a radiation induced signal (RIS) and an intrinsic native (NAT) signal that is independent of irradiation. The physical basis of these signals is presented in detail elsewhere (e.g. (Desrosiers 1991, Vanhaelewyn et al. 2001, Vanhaelewyn et al. 2002)). Slightly differing characterizations of the L-band signals were published by Zdravkova et al. (Zdravkova et al. 2003) and Iwasaki et al. (Iwasaki et al. 2005). Zdravkova reports an experimental RIS peak-to-peak linewidth of 0.26 mT at g = 2.0009 and supports Vanhaelewyn’s (Steinberg et al.) X-band characterization of an isotropic NAT signal with linewidth of 0.78 mT at g = 2.0045. Iwasaki describes a RIS component at g = 2.0005±0.002 and a NAT signal with a peak-to-peak linewidth of 0.39 mT at g = 2.0024±0.002. These values for the RIS g-value are consistent with 2.0003±0.0037 reported by Miyake et al (Miyake et al. 2000).
In vivo EPR Tooth Dose Response
In this manuscript new data are presented which characterize the ability of in vivo tooth dosimetry to provide estimates of absorbed dose in different tooth types and teeth that have been irradiated and measured in situ. This is done through a series of experiments using irradiated and unirradiated whole isolated teeth, irradiated teeth placed in situ in volunteers with gaps in the their natural dentition, and teeth of volunteer patients who have received radiation therapy for the treatment of head and neck cancers. These initial measurements have occurred in a laboratory setting to enable rapid and systematic development of instrumentation and measurement procedures, in preparation for later refinements for robust use in the field.
In vivo measurements have been performed using molar, premolar, and canine teeth. A study of the occurrence of dental restorations§ in molars indicated that while in a random cross-section of individuals there would be some subjects that did not have one or more fully intact molars, the number of individuals in this state would be limited and acceptable for the needs of screening in a general population. However, in order to enable data collection in the field with the greatest level of flexibility we have also investigated the potential for in vivo EPR tooth dosimetry using the anterior teeth; premolars, canine teeth, and incisors. A strong stimulus for such studies arose during measurements in patients who had received radiation to their teeth in the course of radiation therapy for head and neck cancers. The often compromised conditions in the oral cavity are further exacerbated by radiation, and therefore preventive removal of teeth may be carried out prior to radiation therapy. Consequently, it is not surprising that we found that most of these patients did not have suitable molars.
Another approach under investigation is the measurement of the enamel on the lingual surfaces of upper incisors using specifically developed resonators. Interest in such measurements is supported dental health data§ which indicated that most people have intact incisors even when many of their other teeth are missing or restored and, equally importantly, the lingual surfaces of these incisors is considerably more smooth than the biting surfaces of other teeth. Additionally, measurement at this site avoids complications reported in the literature due to EPR signals induced in the buccal surfaces of the incisors by sunlight (Ivannikov et al. 1997). The ease of access to the incisor teeth is an additional potential advantage, especially when considering that measurements in the field are likely to be made by very minimally trained individuals.
MATERIALS AND METHODS
EPR Instrumentation
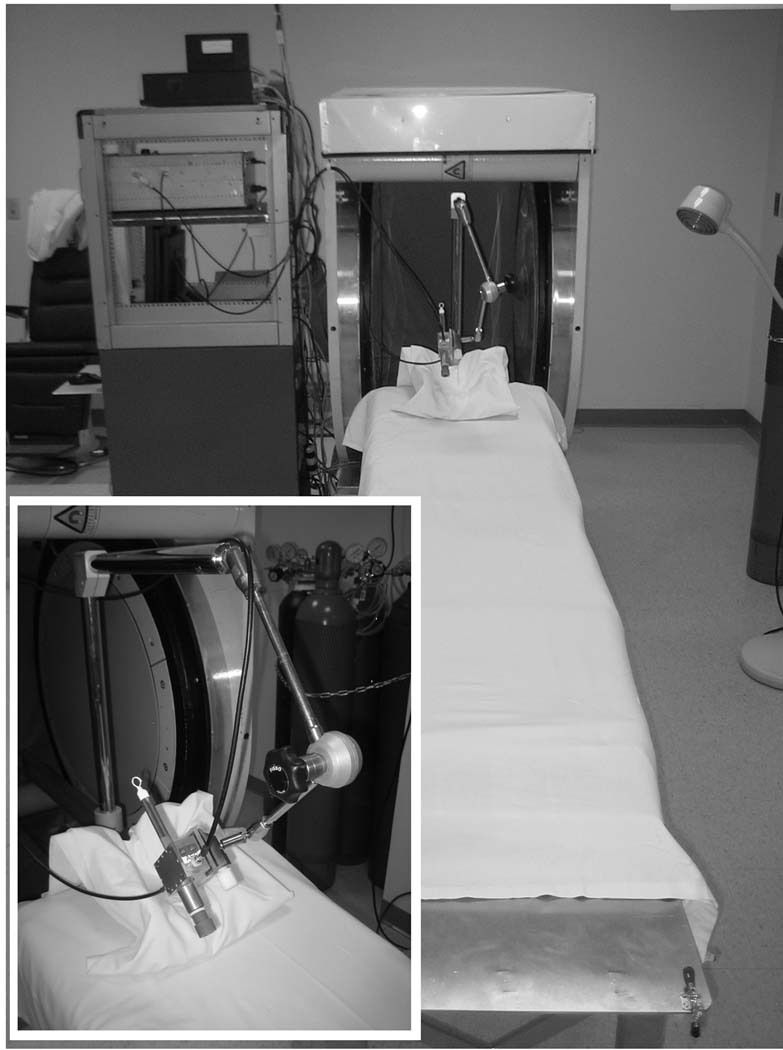
These measurements and developments were made using a clinical L-band EPR spectrometer designed specifically to enable measurements in human subjects, shown in Fig. 1 and described earlier by Salikhov et al. (Salikhov et al. 2005). This spectrometer operates in continuous wave (CW) mode with homodyne detection at an excitation frequency near 1.2 GHz (L-band) using a 41 mT permanent magnet with 50 cm pole separation. All measurements are performed using surface loop resonators that have been specifically designed for tooth dosimetry and intra-oral application (Salikhov et al. 2003, Swartz et al. 2005). The base of the magnet attaches to a retractable bed that allows facile positioning of the subject in the magnet. The resonator is held on the distal end of a lockable articulating arm that is mounted to the bed (Williams et al. 2007).
Fig. 1.
The low-frequency clinical EPR spectrometer is shown with adaptations for tooth dosimetry, including the retractable subject bed, head support, articulating arm for resonator positioning, and surface-loop resonator for intra-oral measurements.
For measurements of anterior teeth it was necessary to design and fabricate new resonators that have smaller detection loops, as the use of the larger resonators intended for molars leads to suboptimal EPR detection sensitivity due to the poor filling factor. The trade offs in EPR sensitivity depending on loop size are complex; for example, the use of the smaller loop samples less enamel, but can lead to a better filling factor, a reduced influence of lossy tissue, and a larger proportion of the enamel that is close to the loop where the field sensitivity is higher. These small loop resonators have detection loops with diameters of ~8 mm, rather than ~12 mm, and resonators with loops as small as 4 mm are under development. While sufficient EPR sensitivity is achieved using these resonators, dosimetric limitations due to variability in resonator positioning and the sizes and shapes of these teeth remain and are a focus of ongoing research.
EPR Data Collection
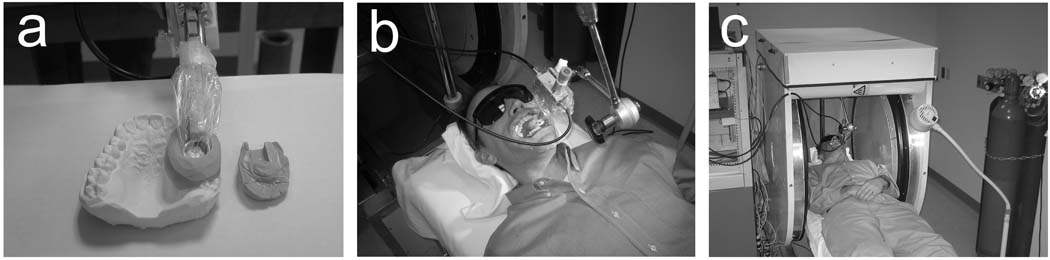
The experimental procedures for in vivo measurements including subject immobilization, resonator positioning, and instrumental settings have been published (Williams et al. 2007). Spectra are acquired with a nominal modulation amplitude of 0.4mT and an incident microwave power of 50mW which maximize the signal-to-noise ratio with the current instrument. In practice, due to inhomogeneity of the modulation field and variations in the positions of the teeth in the spectrometer, the achieved modulation amplitude deviates from exactly 0.4mT. However, as described below, a reference standard placed in close proximity to the tooth is used to estimate the modulation amplitude and allow for a correction to be applied. These procedures are summarized in the photographs included in Fig. 2. The resonator is held in position over the teeth using a custom-made mold which includes impressions of the involved teeth and the detection loop of the resonator. The molds are formed prior to measurement, using either a multi-step procedure or formed in situ immediately prior to measurement. In situ formation of the mold can be completed in < 5 minutes (Nicolalde et al. 2009 (In Press)). In vivo and in vitro data were collected using the same instrumental settings, requiring 4.5 minutes of data acquisition time. From 90 individual scans collected in this period, mean or median values at each field-point are calculated and analyzed to estimate the amplitude of the RIS component. This amplitude, which is a voltage, is expressed here in arbitrary units (AU) to acknowledge variable spectrometer gains and instrumental conditions during the course of developments. The complete measurement procedure, using previously prepared molds, can be accomplished in approximately 10 minutes (Nicolalde et al. 2009 (In Press)). Note that for a queue of subjects mold production and EPR measurements can be performed in parallel to maximize throughput.
Fig. 2.
During in vivo tooth dosimetry measurements: (a) the resonator is held in proper position using a custom-made mold, (b) the mold and resonator are placed in the oral cavity over the teeth of interest, along with absorptive pads to sequester saliva, and (c) the patient bed is inserted into the magnet for measurement with the teeth centered with respect to the magnet and modulation coils.
In all recent measurements an external reference standard has been measured simultaneously with the tooth. In order to avoid significant overlap in the spectral features of the tooth and standard, 15N-substituted perdeuterated 2,2,6,6-tetramethyl-4-oxopiperidine-1-oxyl (15N-PDT) has been used as it has 2 off-center EPR peaks separated by approximately 2.25 mT. The 15N-PDT is sealed in a glass vial at a concentration of 8 mM in D2O and fixed to the transmission line of the resonator, such that its distal tip enters the sensitive volume of the resonator and the amplitude of the standard signal is comparable to that of a 30Gy tooth. We estimate that the high field resonance is approximately 1.03 mT above the center of the RIS resonance and that the intrinsic linewidth is near 0.03 mT. This reference standard is currently used to confirm proper performance of the spectrometer during dosimetry measurements, as a magnetic field marker and a monitor of the modulation amplitude, and as an indicator of the RF phase. Developments to enable its reliable use as an amplitude standard are underway.
EPR Data Analysis
Estimates of spectral parameters of the tooth signals and the PDT reference standard were used to develop a spectral model that is employed in non-linear least squares fitting analysis of the spectra to estimate the peak-to-peak amplitude of the RIS signal (Demidenko et al. 2007). Under in vivo measurement conditions, especially when data are collected with appropriately short acquisition times on the order of 5 minutes, the modest EPR sensitivity and noise on the spectral baseline make the less intense NAT components difficult to identify accurately. Including both RIS and NAT components in the model used for spectral fitting, which necessitates an increase in the number of degrees of freedom, often results in an increased uncertainty in the estimate of the RIS amplitude. Therefore our current spectral model explicitly includes only the RIS component and the effect of the NAT component gives rise to a non-zero intercept in the dose response relationship. In the current model, both the RIS and PDT lineshapes are approximated using a Lorentzian lineshape subject to distortion from Zeeman modulation (Robinson et al. 1999). In this analysis, the linewidths and center separation of the RIS and PDT signals are fixed, and the modulation amplitude (Bmod) and peak-to-peak amplitudes are allowed to vary. Following estimation of the observed parameters, the RIS amplitude is normalized to a modulation amplitude of 0.4 mT by dividing the estimate by (1.48 mT−1×Bmod + 0.410). An additional correction for the size of the tooth is applied (Miyake et al. 2000, Iwasaki et al. 2005, Demidenko et al. 2007). Data analysis can be performed immediately online within the data acquisition software and also via post-processing of the recorded spectral data.
For dose calibration experiments, where the estimates of the true doses are available, the precision of the EPR dose estimates are assessed using the standard error of prediction (SEP), defined as
| (1) |
where N is the number of dose estimates, Di are the individual estimates from EPR, and D̂i are the known doses.
Dose Calibration and Estimation
Systematic studies have been performed under three basic sets of conditions which have enabled developments ranging from focused interrogation of experimental variables to controlled measurements of irradiated teeth in human subjects. Initial tooth measurements and studies to support ongoing development have been made using isolated teeth, and are referred to as in vitro studies (Iwasaki et al. 2005, Iwasaki et al. 2005). These studies allow for the greatest level of flexibility and avoid many of the complexities inherent to the in vivo measurements, such as subject motion, saliva, and more complex resonator positioning procedures. Irradiations were performed using a 6 MV X-ray beam produced by a clinical linear accelerator. Teeth were placed in full water equivilant buildup material and were placed at the depth of maximum dose as measured in water. Dose is stated in Gy, i.e. J/kg of absorbed energy. Calculations were preformed ignoring heterogeneities produced by the tooth perturbation of the water equivalent medium. Individual teeth were mounted in non-lossy dental putty which had been formed with an impression of the resonator loop to allow for reproducible positioning of the resonator with respect to the tooth. In vitro measurements employed the same clinical spectrometer that was used for measurements in human subjects. In vivo measurements have been made in two distinct populations of human subjects: non-irradiated volunteers with irradiated teeth inserted into gaps in their dentition and volunteers who have received doses between 1 Gy and 30 Gy to their teeth in the course of radiation therapy. The studies with inserted irradiated teeth facilitate the repeatable collection of EPR dosimetry data in particular subjects with a wide range of well characterized doses, but are limited by the fact that only individual teeth have been irradiated, rather than entire sets of teeth as expected in real-world application. The studies involving volunteers who have either completed courses of radiation therapy or were receiving therapy closely parallels the intended application of the technology, where intact teeth have been irradiated and are measured in situ. Patients were treated for head and neck cancers using megavoltage photon beams. The dose delivered to teeth of interest was calculated using the Philips Pinnacle Monte Carlo photon kernel based convolution / superposition algorithm. This photon dose calculation algorithm has been shown to be accurate to approximately 3% in heterogeneous phantom measurements. A significant residual difference in these subjects is that irradiation during therapy is highly focused on the tumor treatment volume, which can lead to large gradients in absorbed dose across the teeth and other tissues, while irradiation during an accident or attack may be expected to produce a more uniform dose distribution.
It should be noted in these calibration experiments that the observed dose responses are dependent on the tooth type, specific resonator geometry and placement, and instrumental conditions which have varied during the development of the technology. Therefore, the results of the dose calibrations performed under these varying conditions cannot readily be interpreted en masse, but rather in groups of data that were acquired under comparable conditions, as noted in the results section.
RESULTS
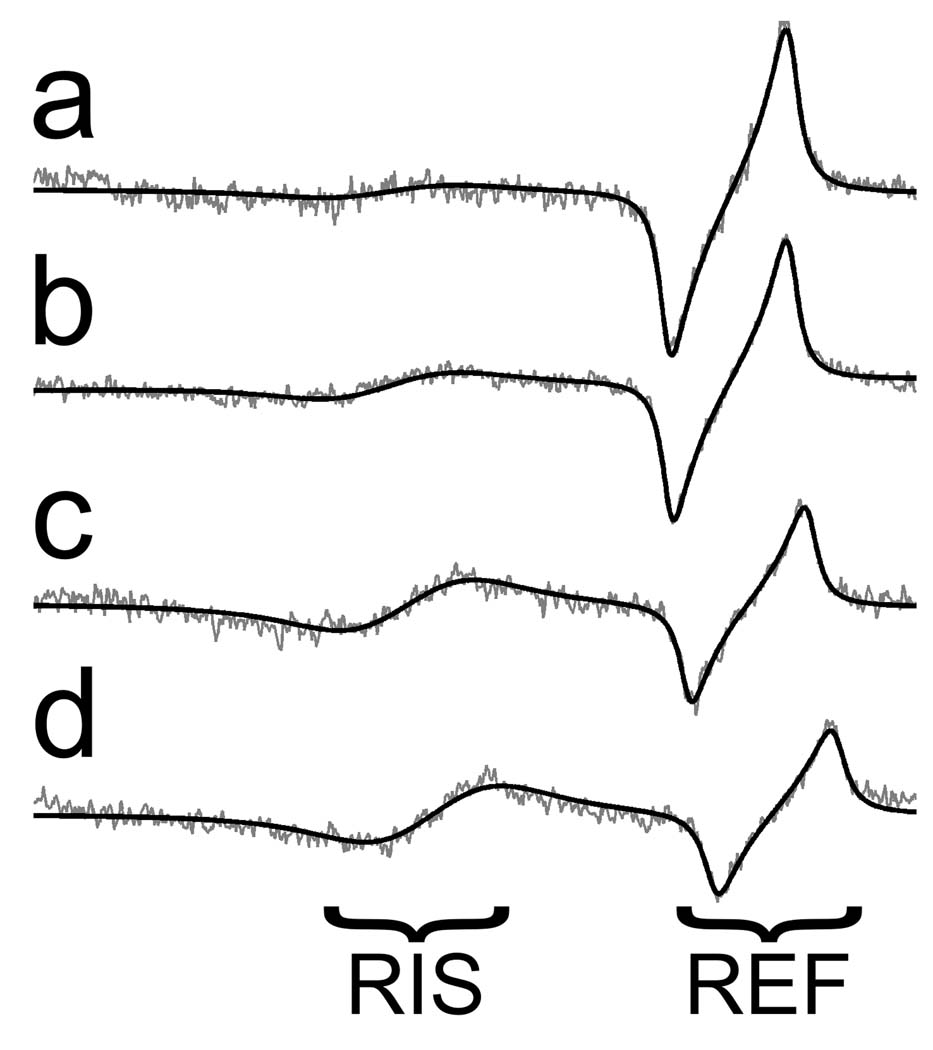
As a simple demonstration of the shape of the EPR spectrum and the adequacy of the spectral model, a set of spectra were collected in vivo for inserted molar teeth with doses of 0 Gy, 2 Gy, 5 Gy, and 7.5 Gy. The averaged EPR spectra and the non-linear least squares fits to the data are shown in Fig. 3. These spectra demonstrate a largely consistent spectral shape for the tooth signal across doses and lack of regular structure in the residuals, which support the use of the developed spectral model. Distortion of the baseline signal, which is independent of the tooth and reference samples and related to modulation of the electrical properties of the resonator during data collection, gives rise to spectral features that do not match the shape defined by the spectral model. In spectra with considerable baseline distortion, spectral fitting with a restrictive model provides an effective, but imperfect, means to distinguish between the desired EPR signals and the variable baseline. Through spectral fitting the effects of features of the baseline distortion which do not match those defined within the model are minimized, however, baseline features which do resemble those in the modeled spectrum directly effect the estimated spectral parameters.
Fig. 3.
Spectral fitting of (a) 0 Gy, (b) 2 Gy, (c) 5 Gy and (d) 7.5 Gy in vivo data. The black smooth line is a fit to the underlying experimental spectrum.
Measurements in Molar Teeth
In vivo measurements were made using non-irradiated volunteers with inserted molar teeth that were irradiated to doses up to 30 Gy in order to facilitate the development of the EPR techniques and instrumentation. When grouped by date, to account for developmental variations in the spectrometer and the resonators, consistent dose responses have been observed across subjects. In a study involving 2 subjects with unique sets of 6 molar teeth irradiated to doses between 0 Gy and 30 Gy, measurements averaged across 3 separate days provided statistically indistinguishable linear dose responses (F-test, p=0.34) with a joint slope of 0.028 AU/Gy and an intercept of 0.152 AU. The SEP values were estimated to be 1.2 Gy and 1.52 Gy based on individual calibrations, and 1.72 Gy using the jointly derived calibration.
In addition to calibration of the dose response over this wide range, experiments have been performed that focus on lower doses, in the range for which decisions regarding treatment of acute radiation syndrome are expected to focus. The measurements with lower doses summarized here were made with inserted irradiated teeth in two volunteer subjects, on three different days for each subject, using separate sets of teeth that had received absorbed doses of 0, 2, 5, 7.5 and 10 Gy. The results are summarized in (Table 1) and Fig. 4. The results show that the in vivo dose responses measured under consistent experimental conditions, but with different subjects and different inserted teeth, give rise to consistent dose calibrations that can be applied across individuals. The dose response relationships had linear regressions that were not statistically different according to the F-test (p-value >0.6). Included in (Table 1) are SEP estimates for both individual measurements of each subject and after the 3 measurements for each subject had been averaged together. Consistent with previously published results (Demidenko et al. 2007), based on averages for both the in vitro and in vivo results, the SEP for individual measurements is approximately 1.5 Gy and the SEP for averaged measurements is reduced to near 1 Gy. Based on the SEP values for the joint calibrations, for which the greatest number of measurements are included in the analysis, errors in the estimated doses are similar for in vivo and in vitro studies, though there is presently an approximate factor of 2 decrease in the EPR signal amplitude for in vivo measurements relative to those done in vitro. The similarity in SEP estimates reflects a concomitant decrease in baseline noise when measurements are made in vivo . These results indicate that doses could be resolved with sufficient accuracy and precision to perform screening and assist during triage to separate the population into groups identified for additional interrogation or broad treatment categories.
Table 1.
In Vivo Molar Dose Response Parameters and SEP Values
| Conditions | Subject | Calibration Slope (AU/Gy) |
Calibration Intercept (AU) |
SEP (Gy) (Averaged) |
SEP (Gy) (Individual) |
|---|---|---|---|---|---|
| In vitro | A | 0.078 (0.005) | 0.28 (0.03) | 0.38 | 0.91 |
| B | 0.067 (0.009) | 0.29 (0.06) | 1.60 | 1.82 | |
| A+B | 0.072 (0.005) | 0.29 (0.03) | 1.10 | 1.42 | |
| In vivo | A | 0.033 (0.005) | 0.14 (0.03) | 1.15 | 2.22 |
| B | 0.039 (0.004) | 0.10 (0.02) | 0.82 | 1.19 | |
| A+B | 0.036 (0.003) | 0.12 (0.02) | 0.97 | 1.71 | |
Fig. 4.
In vivo and in vitro dose response relationships for molar teeth for two volunteers subjects. Error bars denote the SD of the data at each dose. The calibration lines for the individual volunteers and the calibration for the combined data were not statistically different according to the F-test (p-value=0.15).
Measurements in Premolar Teeth
Dose responses were measured for a common set of premolar teeth, acquired both in vitro and inserted in vivo for a single volunteer. The SEP values based on data averaged across 3 independent measurements was 0.9 Gy in vitro and 2.7 Gy in vivo. The dose response in vitro had a slope of 0.07 AU/Gy and intercept of 0.24 AU, and the in vivo measurements had slope of 0.03 AU/Gy and intercept of 0.19 AU. While the SEP value observed in vivo is larger than expected, these results are consistent with other data, demonstrating that linear dose responses are observed and a factor of ~2 difference in the slopes of the dose responses observed in vivo and in vitro. These measurements were performed with a different resonator than that used for molar teeth, so identical dose responses are not expected across these measurements. Eight additional sets of in vivo measurements with premolar teeth were made with a different volunteer and a similar dose response was observed (slope = 0.04 ± 0.01, intercept = 0.1 ± 0.2), consistent with there being a universal calibration for similarly executed measurements in premolar teeth.
Measurements in Incisor Teeth
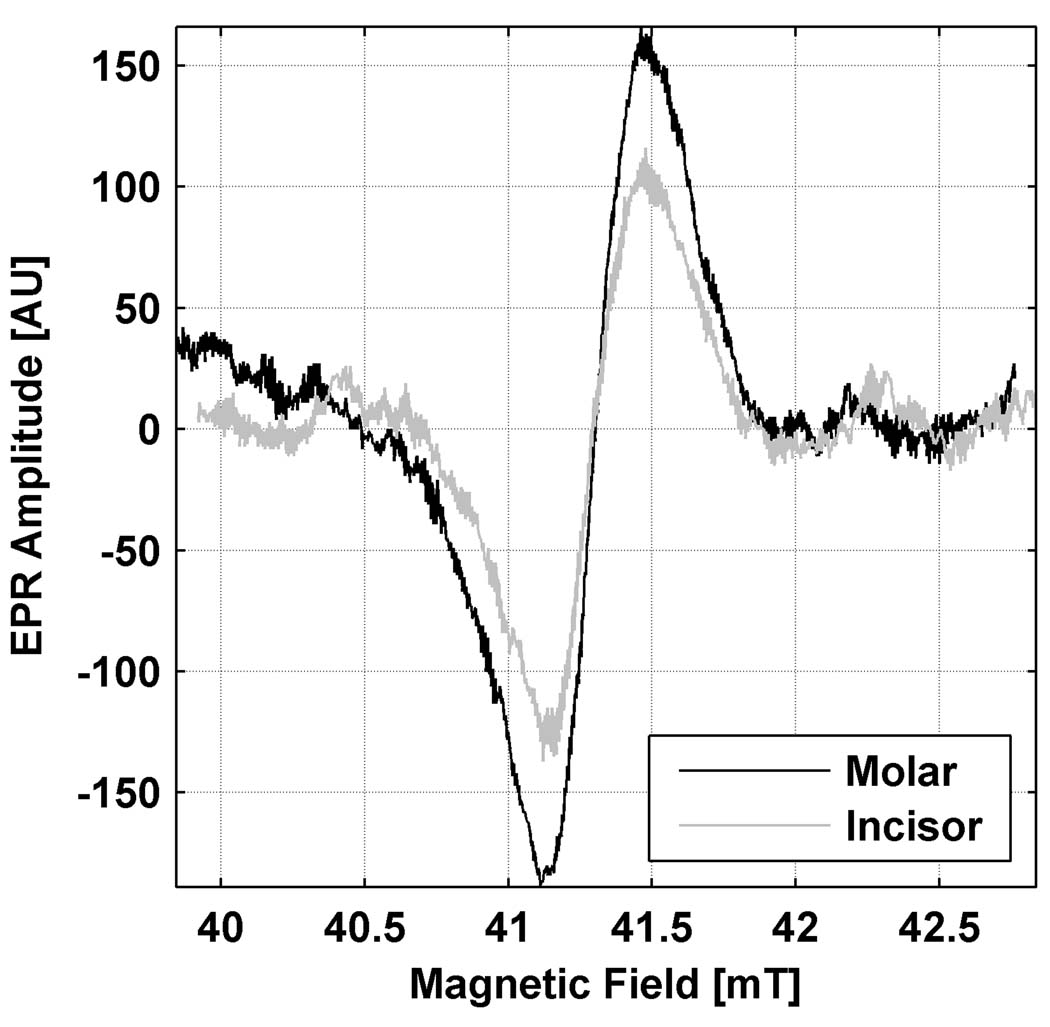
Fig. 5 illustrates a representative result where a 30 Gy incisor was measured using a 10 mm loop resonator and compared to the measurement of a similarly irradiated molar using the standard 12 mm loop resonator. Resonator dimensions were chosen to match the sizes of the respective teeth and optimize sensitivity. The resonators were placed over the biting surface of the molar tooth and on the lingual surface of the incisor. While the amplitude measured here from the incisor is approximately 35% lower than that of the molar, this result is a key indicator that it may be feasible to make the desired in vivo measurements in teeth other than molars, including incisors.
Fig. 5.
Spectra recorded for molar and incisor teeth that had been irradiated to equal doses of 30 Gy, each with an appropriate size resonator, have comparable EPR signal amplitudes. The incisor amplitude is 70% of the amplitude for the molar. No size correction has been applied. This indicates that measurements can be made with incisors with sensitivity approaching that achieved with molars.
Measurements in Teeth Irradiated In situ
In addition to the in vivo dose response studies with volunteer subjects and inserted teeth described above, measurements have been performed using natural teeth in the mouths of volunteer patients who have received radiation therapy. Some patients have volunteered for repeated measurements, as illustrated in the three examples below. The measurements in these patients presented a special challenge because none had molar teeth suitable for measurements and therefore we had to adapt the method to measure in their canine or premolar teeth. While this problem is less likely in a normal population, there clearly is a need to be able to adapt the technique for the types of teeth that are present and satisfactory for EPR dosimetry (i.e. intact, with no or minor amounts of restorations).
In one series of measurements a resonator with a large detection loop, designed for molar teeth, was placed over neighboring premolar and canine teeth in a single patient on seven different days and the dose was calculated using the calibration curve derived for the same resonator from measurements in the normal volunteers who had irradiated single molar teeth placed in their mouths. As usual, a correction was applied to account for the size of the teeth being measured. Based on this independent calibration, the dose to the patient’s teeth was estimated to be 25±5 Gy (n=7). The dose delivered to the teeth was estimated from the radiation treatment plan to be 25.5±0.8 Gy, so even under these less than ideal developmental conditions, the absolute dose estimate provided by in vivo EPR was in agreement with the delivered dose.
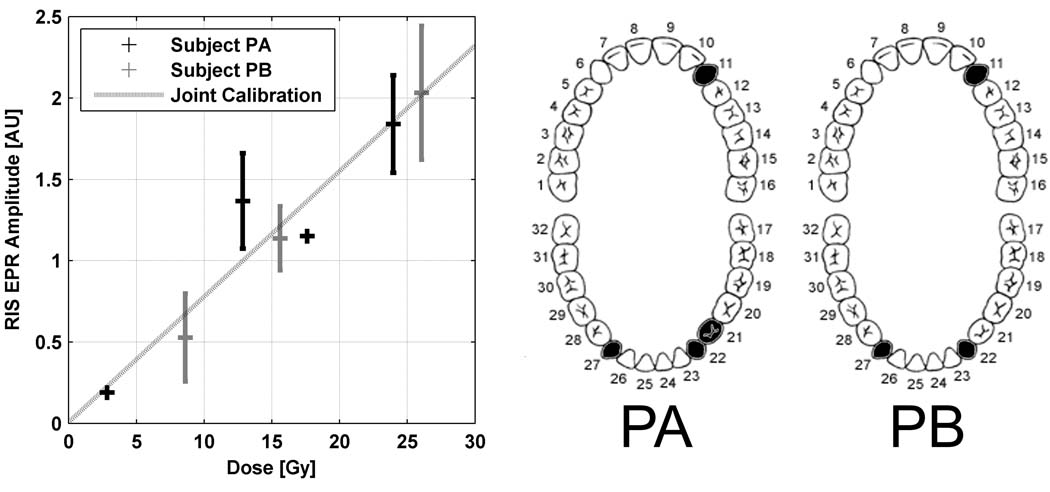
Measurements were made on teeth in two patients, referred to as Patient A and Patient B, whose treatment plan and remaining dentition provided the potential for measuring different doses in the mouths of the individual subjects. These data summarize multiple measurements in different canine teeth in two patients. These result shown in Table 2 and Fig. indicate that the doses as measured were consistent with the doses that were calculated by the medical physicists and that the relationship between dose and EPR signal is linear, as expected. The quantitative results for the several teeth and in the two different patients suggest that a universal calibration may exist. Based on the joint calibration, the standard error of prediction is 2.4 Gy, which may be higher than is required for the goals of screening and triage, but this is not surprising in view of the rather irregular size and shape of whole canine teeth and state of development of the measurement technique at the time.
Table 2.
Canine tooth doses for patients A and B based on treatment planning and EPR dosimetry. Doses are given in Gy and EPR estimates are given as the mean with SE.
| Prescribed Dose A |
Measured Dose A |
N | Prescribed Dose B |
Measured Dose B |
N | |
|---|---|---|---|---|---|---|
| UR Canine (#11) | 2.8 | 2 | 1 | 8.6 | 7±3 | 4 |
| LR Bicuspid (#21) | 24.0 | 24±4 | 2 | N/A | N/A | N/A |
| LR Canine (#22) | 17.6 | 15 | 1 | 15.5 | 15±3 | 7 |
| LL Canine (#27) | 12.8 | 18±4 | 2 | 26.0 | 26±5 | 5 |
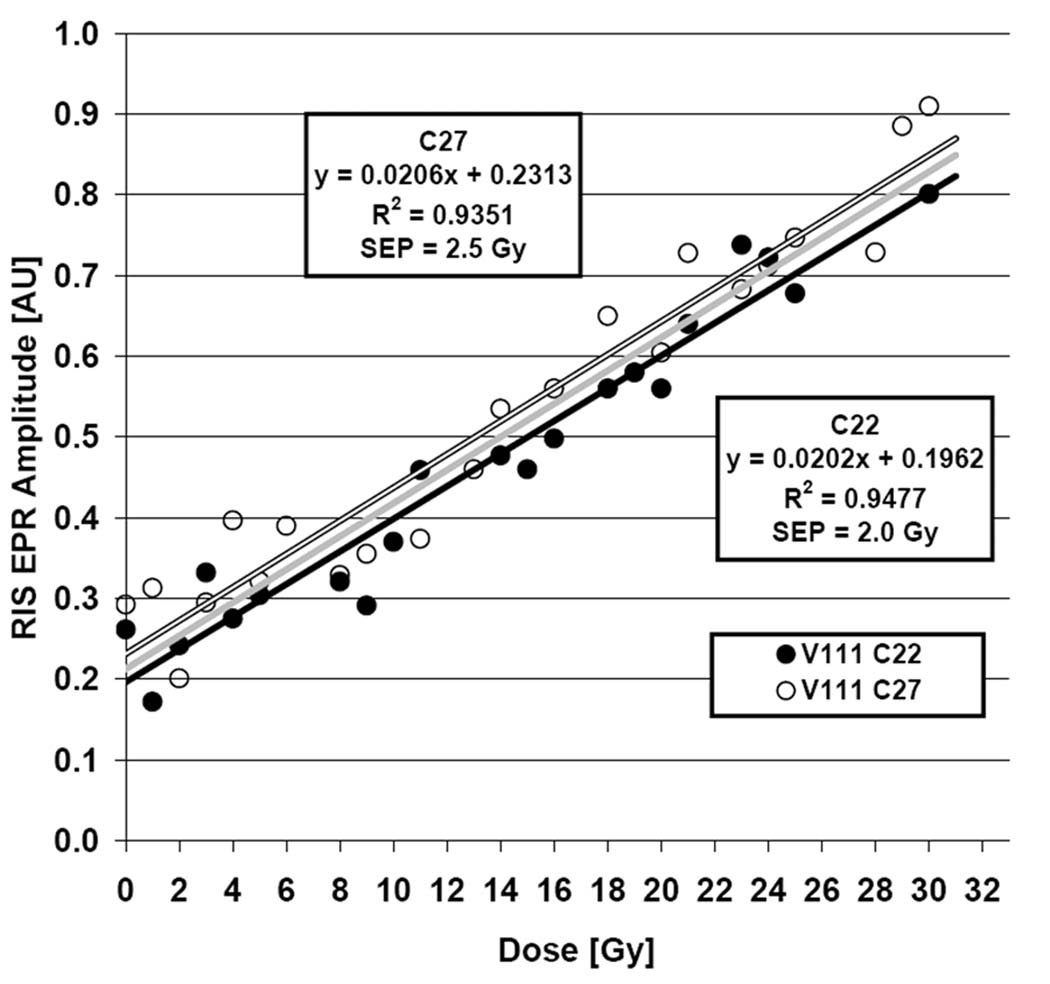
An extensive set of measurements was recently performed on 2 lower canine teeth, #22 and #27, of a patient volunteer receiving radiation treatment involving fractionated irradiation of the oral cavity. The treatment was delivered in 31 fractions over a period of 43 days, with the lower canines each receiving volumetric mean doses of 1.0 Gy (SD ±0.1 Gy) per fraction. Based on modeling within the treatment planning system, the gradient in dose spanned ±12% for #22 and ±30% for #27, with the maximum doses at the bases of the teeth and the minima at the tips. This highlights the inhomogeneity of the dose within single teeth, a situation that is not expected to occur in the mass exposure scenario. The standard EPR measurement procedure was used throughout the course of radiation treatment and measurements were tolerated with no complications. EPR dosimetry measurements were performed for each tooth four times each week and analyzed to estimate the RIS and dose response. Given the large degree of heterogeneity in dose across the teeth, the dose response analysis was performed Responses for both teeth are shown in Fig‥ Based on individual analyses of each tooth, the SEP values were 2.1 Gy and 2.5 Gy.
DISCUSSION
These results demonstrate that EPR dosimetry can be applied in vivo, using a variety of tooth types, and linear dose responses are observed. Common dose responses were observed for measurements made under consistent conditions, specifically including the tooth type and resonator in use. The existence of a commonly applicable dose calibration is crucial for application in the general population where dose-added methods to derive individual calibrations would not be practical. Common dose calibrations were observed separately for both the high and low dose response measurements using inserted molar teeth and indicated by the canine tooth measurements made in subjects who had completed courses of radiation therapy. For the single subject measured during the course of radiation therapy, we note that consistent dose responses were observed for the two canine teeth measured in this subject, but not equal to those measured in prior subjects due to instrumental changes. The consistency of dose responses is also observed among the in vitro measurements.
With the current instrumentation, measurements in molar teeth are the most reliable, with the lowest estimates for the SEP. This observation is related to the more advanced state of development of the resonators used for molar teeth and the availability of the large extended biting surface of these teeth over which the detector loop can be precisely positioned. While resonators with smaller loops have been designed for measurements of smaller teeth, such as canines, the loops are larger than the teeth that they partially encompass, which leads to an increased amount of position-related variability in the dose estimates. This variability may appear as either an increase or decrease relative to the expected dose as the proximity of the detection loop, or portion of the loop, varies with respect to the enamel surfaces (Williams et al. 2007) (Pollock et al. 2009 (In Press)). While the molds are useful in achieving accurate and reproducible positioning, the detection loops are increasingly sensitive as the distance between the sample and the loop is decreased, and perturbations in positioning can give rise to significant changes in the EPR amplitude for these smaller teeth (Williams et al. 2007) (Pollock et al. 2009 (In Press)). This problem could be overcome through the use of even smaller loops, which would rest directly on the teeth, and active development is underway to produce such resonators. Additional optimization of resonator positioning is being explored through finite element modeling of the electronic properties of resonators and their sensitivity to neighboring enamel and lossy tissue (Pollock et al. 2009 (In Press)). This includes the development of resonators and procedures to allow multiple teeth, either neighboring or opposing teeth of the upper and lower jaws, to be measured simultaneously. Preliminary considerations and measurements indicate that the simultaneous measurement of upper and lower teeth may increase the sensitivity by as much as a factor of 2, with an additional decrease in the sensitivity to resonator position.
Consistent with prior reports, the precision of dose estimates made in vivo were not observed to be significantly degraded when compared to analogous measurements in isolated teeth. While the EPR signal amplitudes are reduced due to the presence of increased amounts of lossy tissue and a decrease in the quality (Q) of the resonator, the baseline noise is also reduced resulting in similar SNR values and precision of dose estimation.
Throughout the development process, we have maintained focus on the eventual field deployment of the technology, the need for high throughput, and use by minimally trained operators (Flood et al. 2007, Gougelet et al. 2009 (In Press), Nicolalde et al. 2009 (In Press)). To this end, field deployable systems, based on smaller permanent magnet designs, are being developed and prototypes are currently being tested. These magnets range from an <1 kg intraoral magnet to miniaturized dipole systems, similar to ½ and ⅓ scale versions of the existing clinical magnet. Because they are based on permanent magnets, there are no special power or cooling requirements needed for their operation. The electronics for EPR detection and sweeping of the magnetic field can be powered using standard 120 V AC supplied through the grid or from a generator or through the use of batteries. The measurement procedure has been designed to allow parallel performance of measurement procedures, and it is expected that several spectrometers would be in operation following a mass-exposure incident. Finally, future versions of the instrument, procedures, and software are being designed to for robust operation by operators who may have only been given cursory instructions to operate the machine and who have no technical background or EPR experience. Examples of such design elements include automated tuning and matching of the detection circuitry, automated online data analysis, and self-positioning resonator loop configurations.
CONCLUSIONS
The development of in vivo EPR has made it feasible to make the measurements in teeth in situ, greatly expanding the potential for using this approach for immediate screening after radiation exposures. In vivo measurements can be made in molar, premolar and canine teeth, and no impediments for the lingual surfaces of incisors are expected. Tooth type specific, but general, dose calibrations appear to exist. Dose response curves have been generated for volunteers with inserted molar teeth and for patients using intact canine teeth. Using the current methodology and instrument, and based on single in vivo 4.5 min measurements, the standard error of prediction achieved with inserted molar teeth is approximately 1.5 Gy and between 2.0 and 2.5 Gy for intact canine teeth. Developments to reduce these errors are underway, focusing on geometric optimization of the resonators, detector positioning techniques, and optimal data averaging approaches. Developments are also underway toward miniaturization of the instrumentation for field use. It seems likely that in vivo EPR dosimetry techniques will play an important role in retrospective dosimetry and screening following exposures involving large numbers of individuals.
Fig. 6.
Dose-response relationship for two patient volunteers with the magnitude of the RIS EPR signal adjusted for the tooth size and PDT-derived modulation amplitude, averaged across canine tooth measurements. Error bars, where given, denote the SEM and the number of measurements per tooth ranged from 1 to 7. The dose responses from the individual volunteers was not found to be statistically different (p=0.66) and the joint dose response was estimated to be y=0.077x+0.008. The locations of the teeth for each volunteer are noted in the diagrams to the right.
Fig. 7.
In vivo EPR dose response for lower canine teeth (C22 and C27) measured during the course of radiation therapy where each tooth received approximately 1 Gy/fraction. Individual dose responses are shown and described for each tooth, along with SEP estimates. The joint dose response is shown in gray.
Acknowledgements
This research was supported by NIH (U19AI067733) and DARPA (HR0011-08-C-0023). We would also like to sincerely thank each of the volunteer subjects for their participation in this study.
Footnotes
Personal communication from L. Jackson Brown, D.D.S., M.Phil., Ph.D. Currently Editor of Journal of Dental Education, Dr. Brown was Associate Executive Director, Health Policy Resources Center, at the American Dental Association (ADA) and Chief of Staff of the ADA's 2001 Future of Dentistry Project when he provided us with the data on the age, gender, tooth breakdown of caries and fillings. These data were from the NHANES data for the year 2003–2004, and were provided in the ADA's role as principal site for these data.
Contributor Information
Benjamin B. Williams, Dartmouth Medical School, Radiology, 704 Vail, HB 7785, Hanover, NH 03755, UNITED STATES, 603-650-1806, Admin Assistant: 603-650-1784, FAX: 603-650-1717, benjamin.williams@dartmouth.edu.
Ruhong Dong, Dartmouth Medical School
Maciej Kmiec, Dartmouth Medical School.
Greg Burke, Dartmouth Medical School.
Eugene Demidenko, Dartmouth Medical School
David Gladstone, Dartmouth Medical School
Roberto J Nicolalde, Dartmouth Medical School.
Piotr Lesniewski, Dartmouth Medical School.
Harold M Swartz, Dartmouth Medical School.
REFERENCES
- Brady JM, Aarestad NO, Swartz HM. In vivo dosimetry by electron spin resonance spectroscopy. Health Physics. 1968;15:43–47. doi: 10.1097/00004032-196807000-00007. [DOI] [PubMed] [Google Scholar]
- Chumak V, Likhtarev I, Shalom S, Meckbach R, Krjuchkov V. Chernobyl Experience in Field of Retrospective Dosimetry: Reconstruction of Doses to the Population and Liquidators Involved in the Accident. Radiat Prot Dosimetry. 1998;77:91–95. [Google Scholar]
- Demidenko E, Williams BB, Sucheta A, Dong R, Swartz HM. Radiation dose reconstruction from L-band in vivo EPR spectroscopy of intact teeth: Comparison of methods. Radiation Measurements. 2007;42:1089–1093. doi: 10.1016/j.radmeas.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers M. In vivo assessment of radiation exposure. Health Physics. 1991;61:859–861. doi: 10.1097/00004032-199112000-00018. [DOI] [PubMed] [Google Scholar]
- Desrosiers M, Schauer DA. Electron paramagnetic resonance (EPR) biodosimetry. Nuclear Inst. and Methods in Physics Research, B. 2001;184:219–228. [Google Scholar]
- Flood AB, Bhattacharyya S, Nicolalde RJ, Swartz HM. Implementing EPR Dosimetry for Life-Threatening Incidents: Factors Beyond Technical Performance. Radiat Meas. 2007;42:1099–1109. doi: 10.1016/j.radmeas.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordy W, Ard WB, Shields H. Microwave Spectroscopy of Biological Substances. I. Paramagnetic Resonance in X-Irradiated Amino Acids and Proteins. Proc Natl Acad Sci U S A. 1955;41:983–996. doi: 10.1073/pnas.41.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougelet RM, Rea ME, Nicolalde RJ, Swartz HM. The View from the Trenches: Emergency Medical Response Plans. Health Physics. 2009 doi: 10.1097/HP.0b013e3181a6de7d. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güttler A, Wieser A. EPR-dosimetry with tooth enamel for low doses. Radiation Measurements. 2008;43:819–822. [Google Scholar]
- Ikeya M, Miki T, Kai A, Hoshi M. ESR Dosimetry of A-Bomb Radiation Using Tooth Enamel and Granite Rocks. Radiation Protection Dosimetry. 1986;17:181–184. [Google Scholar]
- Ikeya M, Miyajima J, Okajima S. ESR dosimetry for atomic bomb survivors using shell buttons and tooth enamel. Jpn. J. Appl. Phys. 1984;23:697–699. [Google Scholar]
- Ivannikov AA, Skvortzov VG, Stepanenko VF, Tikunov DD, Fedosov IM, Romanyukha AA, Wieser A. Wide Scale EPR Retrospective Dosimetry: Results and Problems. Radiation Protection Dosimetry. 1997;71:175–180. [Google Scholar]
- Iwasaki A, Grinberg O, Walczak T, Swartz HM. In vivo measurements of EPR signals in whole human teeth. Appl Radiat Isot. 2005;62:187–190. doi: 10.1016/j.apradiso.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Walczak T, Grinberg O, Swartz HM. Differentiation of the observed low frequency (1200MHz) EPR signals in whole human teeth. Appl Radiat Isot. 2005;62:133–139. doi: 10.1016/j.apradiso.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Miyake M, Liu KJ, Walczak TM, Swartz HM. In vivo EPR dosimetry of accidental exposures to radiation: experimental results indicating the feasibility of practical use in human subjects. Appl Radiat Isot. 2000;52:1031–1038. doi: 10.1016/s0969-8043(00)00053-1. [DOI] [PubMed] [Google Scholar]
- Nicolalde RJ, Gougelet RM, Rea M, Williams BB, Dong R, Kmiec MM, Lesniewski PN, Swartz HM. EPR Dosimetry as a Screening Tool for the Triage of Mass Casualties after a Nuclear Catastrophic Event: Technical and System Considerations. Health Physics. 2009 (In Press) [Google Scholar]
- Onori S, Aragno D, Fattibene P, Petetti E, Pressello MC. ISS protocol for EPR tooth dosimetry. Radiation Measurements. 2000;32:787–792. [Google Scholar]
- Pollock JD, Williams BB, Sidabras J, Grinberg O, Salikhov I, Lesniewski P, Kmiec M, Swartz HM. Surface Loop Resonator Design for In Vivo EPR Tooth Dosimetry using Finite Element Analysis. Health Physics. 2009 doi: 10.1097/HP.0b013e3181a6dd08. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BH, Mailer C, Reese AW. Linewidth analysis of spin labels in liquids. I. Theory and data analysis. Journal of Magnetic Resonance. 1999;138:199–209. doi: 10.1006/jmre.1999.1737. [DOI] [PubMed] [Google Scholar]
- Romanyukha A, Schauer DA, Malikov YK. Analysis of Current Assessments and Perspectives of ESR Tooth Dosimetry for Radiation Dose Reconstruction of the Population Residing Near the Semipalatinsk Nuclear Test Site. Journal of Radiation Research. 2006;47:55–60. doi: 10.1269/jrr.47.a55. [DOI] [PubMed] [Google Scholar]
- Salikhov I, Hirata H, Walczak T, Swartz HM. An improved external loop resonator for in vivo L-band EPR spectroscopy. J Magn Reson. 2003;164:54–59. doi: 10.1016/s1090-7807(03)00175-7. [DOI] [PubMed] [Google Scholar]
- Salikhov I, Walczak T, Lesniewski P, Khan N, Iwasaki A, Comi R, Buckey J, Swartz HM. EPR spectrometer for clinical applications. Magn Reson Med. 2005;54:1317–1320. doi: 10.1002/mrm.20689. [DOI] [PubMed] [Google Scholar]
- Steinberg F, Rohrborn HJ, Otto T, Scheufler KM, Streffer C. NIR reflection measurements of hemoglobin and cytochrome aa3 in healthy tissue and tumors. Correlations to oxygen consumption: preclinical and clinical data. Advances in Experimental Medicine and Biology. 1997;428:69–77. doi: 10.1007/978-1-4615-5399-1_11. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Burke G, Coey M, Demidenko E, Dong R, Grinberg O, Hilton J, Iwasaki A, Lesniewski P, Kmiec M, Lo K-M, Javier Nicolalde R, Ruuge A, Sakata Y, Sucheta A, Walczak T, Williams BB, Mitchell CA, Romanyukha A, Schauer DA. In vivo EPR for dosimetry. Radiation Measurements. 2007;42:1075–1084. doi: 10.1016/j.radmeas.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HM, Iwasaki A, Walczak T, Demidenko E, Salikhov I, Khan N, Lesniewski P, Thomas J, Romanyukha A, Schauer D, Starewicz P. In vivo EPR dosimetry to quantify exposures to clinically significant doses of ionising radiation. Radiat Prot Dosimetry. 2006;120:163–170. doi: 10.1093/rpd/nci554. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Iwasaki A, Walczak T, Demidenko E, Salikov I, Lesniewski P, Starewicz P, Schauer D, Romanyukha A. Measurements of clinically significant doses of ionizing radiation using non-invasive in vivo EPR spectroscopy of teeth in situ. Appl Radiat Isot. 2005;62:293–299. doi: 10.1016/j.apradiso.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Tolstykh EI, Degteva MO, Kozheurov VP, Shishkina EA, Romanyukha AA, Wieser A, Jacob P. Strontium metabolism in teeth and enamel dose assessment: analysis of the Techa river data. Radiation and Environmental Biophysics. 2000;39:161–171. doi: 10.1007/s004110000059. [DOI] [PubMed] [Google Scholar]
- Vanhaelewyn G, Amira S, Debuyst R, Callens F, Glorieux T, Leloup G, Thierens H. A critical discussion of the 2nd intercomparison on electron paramagnetic resonance dosimetry with tooth enamel. Radiation Measurements. 2001;33:417–426. [Google Scholar]
- Vanhaelewyn GC, Sadlo J, Matthys PF, Callens FJ. Comparative X- and Q-band EPR study of radiation-induced radicals in tooth enamel. Radiat Res. 2002;158:615–625. doi: 10.1667/0033-7587(2002)158[0615:cxaqbe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wieser A, Debuyst R, Fattibene P, Meghzifene A, Onori S, Bayankin SN, Blackwell B, Brik A, Bugay A, Chumak V. The 3rd international intercomparison on EPR tooth dosimetry: Part 1, general analysis. Applied Radiation and Isotopes. 2005;62:163–171. doi: 10.1016/j.apradiso.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Williams BB, Sucheta A, Dong R, Sakata Y, Iwasaki A, Burke G, Grinberg O, Lesniewski P, Kmiec M, Swartz HM. Experimental procedures for sensitive and reproducible in situ EPR tooth dosimetry. Radiation Measurements. 2007;42:1094–1098. doi: 10.1016/j.radmeas.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdravkova M, Crokart N, Trompier F, Asselineau B, Gallez B, Gaillard-Lecanu E, Debuyst R. Retrospective dosimetry after criticality accidents using low-frequency EPR: a study of whole human teeth irradiated in a mixed neutron and gamma-radiation field. Radiat Res. 2003;160:168–173. doi: 10.1667/rr3026. [DOI] [PubMed] [Google Scholar]
- Zdravkova M, Denis JM, Gallez B, Debuyst R. Sensitivity of whole human teeth to fast neutrons and gamma-rays estimated by L-band EPR spectroscopy. Radiation Measurements. 2002;35:603–608. doi: 10.1016/s1350-4487(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Zdravkova M, Wieser A, El-Faramawy N, Gallez B, Debuyst R. An in vitro L-band electron paramagnetic resonance study of highly irradiated whole teeth. Radiat Prot Dosimetry. 2002;101:497–502. doi: 10.1093/oxfordjournals.rpd.a006036. [DOI] [PubMed] [Google Scholar]
- Zdravkova M, Wieser A, El-Faramawy N, Ivanov D, Gallez B, Debuyst R. An in vitro L-band EPR study with whole human teeth in a surface coil resonator. Radiation Measurements. 2003;37:347–353. [Google Scholar]