Abstract
The midbrain periaqueductal gray (PAG) and its descending projections to the rostral ventromedial medulla (RVM) provides an essential neural circuit for the antinociceptive effects of opiates, and has been implicated in the development of tolerance to morphine. Systemic morphine activates a greater proportion of PAG–RVM neurons in male vs female rats, and induces tolerance to a greater degree in males. The present studies tested the hypothesis that if the PAG–RVM pathway is essential for the development of tolerance, then: (i) morphine activation of the PAG–RVM pathway should decline as tolerance develops; and (ii) sex differences in the development of tolerance to morphine should be reflected as a greater decline in the activation of this pathway in males. These hypotheses were tested in male and female rats using behavioral testing (hot-plate) and immunohistochemistry to map the activation of the PAG–RVM pathway following repeated morphine administration (5 mg/kg; s.c.). In males, morphine potency decreased from 3.0 to 6.3 mg/kg, indicating tolerance, and this was paralleled by a steady decline in the percentage of PAG–RVM output neurons activated by morphine. In contrast, in females the shift in morphine potency was significantly attenuated (D50 6–8.3 mg/kg), and no significant difference in the activity of PAG–RVM output neurons was noted. These results demonstrate that the greater development of tolerance to morphine administration in male rats corresponds with a significant reduction in the activation of the PAG–RVM circuit and suggest a central role for the PAG in the development of tolerance to morphine.
Keywords: analgesia, anatomy, descending modulatory pathway, pain
Introduction
It is estimated that as many as one in five adults currently experience chronic pain. Of that population, 41% are receiving treatment with opioid-based narcotics (Breivik et al., 2006). While morphine and other opioid-based narcotics are the most effective treatment for pain, opiates do not provide adequate pain relief for many people. The sex of the patient and the development of tolerance to morphine's analgesic effects are two particularly important factors that limit the effectiveness of opiates. Men tend to experience greater analgesia following opiate administration compared with women (Cepeda & Carr, 2003; Miller & Ernst, 2004), a difference that has been reported for a wide range of species using both somatic and visceral pain models (Craft, 2003; Ji et al., 2006; Loyd & Murphy, 2006; Wang et al., 2006).
Recent findings suggest that the midbrain periaqueductal gray (PAG) and its descending projections to the rostral ventromedial medulla (RVM) contribute to the sexually dimorphic actions of morphine. Microinjection of morphine directly into the PAG produces greater antinociception in male compared with female rats (Krzanowska & Bodnar, 1999; X. Wang and A.Z. Murphy, unpublished observations), and blocking PAG opioid receptors reduces morphine antinociception to a greater extent in female rats (Bernal et al., 2007). Furthermore, both the anatomy and physiology of the PAG–RVM pathway is sexually dimorphic, with males having a significantly greater percentage of PAG–RVM neurons activated by either inflammatory pain (Loyd & Murphy, 2006) or systemic morphine (Loyd et al., 2007).
Tolerance is known to occur with repeated or continuous administration of morphine into the ventrolateral PAG of male rats (Jacquet & Lajtha, 1976; Siuciak & Advokat, 1987; Tortorici et al., 1999; Tortorici & Morgan, 2002; Lane et al., 2005; Morgan et al., 2006a). In addition, blocking opioid binding in the ventrolateral PAG attenuates tolerance to systemically administered morphine (Lane et al., 2005). Tolerance appears to be mediated by a reduction in mu opioid receptor (MOR) signaling efficacy in PAG neurons (Bagley et al., 2005), an effect that is reversed when MOR coupling is enhanced via upregulated cyclase activity (Hack et al., 2003). These findings suggest that the PAG contributes to the decrease in antinociception that occurs with the development of tolerance.
Given that morphine activates a greater percentage of PAG–RVM output neurons in male compared with female rats (Loyd et al., 2007), our working hypothesis is that if the PAG–RVM pathway is essential for the development of tolerance to morphine, then: (i) activation of the PAG–RVM pathway should decline as tolerance to repeated injections of systemic morphine develops; and (ii) given that males show greater antinociception and greater tolerance (Craft et al., 1999; Barrett et al., 2001), these sex differences should be reflected as a greater decline in the activation of the PAG–RVM pathway in male compared with female rats. These hypotheses were tested using behavioral assessment of nociception in combination with immunohistochemistry to map the activation of the PAG–RVM pathway over the time course of the development of tolerance to repeated administration of morphine.
Materials and methods
Experimental subjects
Thirty-five adult male and 37 weight-matched (250–350 g) cycling female Sprague–Dawley rats were used in these experiments (behavior subjects from Harlan, Indianapolis, IN, USA and anatomy subjects from Zivic-Miller, Pittsburg, PA, USA). Rats were housed in same-sex pairs on a 12 : 12 h light : dark cycle. Access to food and water was ad libitum throughout the experiment, except during surgery and testing. These studies were performed in compliance with the Institutional Animal Care and Use Committees at Georgia State University and at Washington State University. All efforts were made to reduce the number of animals used.
Vaginal cytology
Vaginal lavages were performed daily to confirm that the female rats were cycling normally and to keep daily records on the stages of their cycle in respect to experimental testing. Stage of estrous was determined for 2 weeks prior to testing, and during the 3 days of morphine or saline injections. Proestrus was identified as a predominance of nucleated epithelial cells and estrus was identified as a predominance of cornified epithelial cells. Diestrus 1 was differentiated from Diestrus 2 by the presence of leucocytes. Rats that appeared between phases were noted as being in the more advanced stage.
Experiment 1: behavioral assessment of tolerance to morphine
Thirteen male and 14 female rats were handled daily for 5 days prior to initiation of drug administration. Morphine sulfate (5 mg/kg; s.c., provided by the National Institute on Drug Abuse, Bethesda, MD, USA) was prepared in a 0.9% saline vehicle and administered systemically twice a day for three consecutive days to male (n = 7) and female (n = 7) rats. The 5 mg/kg dose was chosen based on our previous studies demonstrating this to be the 50% effective dose (ED50) for systemic morphine in rats (Morgan et al., 2006b; Wang et al., 2006; Loyd et al., 2007). Control groups consisting of six male and seven female rats received daily injections of saline (1 mL/kg, s.c.). Tolerance was assessed on Day 4 by injecting cumulative doses of morphine every 20 min, resulting in quarter log doses of 1.8, 3.2, 5.6, 10.0 and 18.0 mg/kg. Nociception was assessed using the hot-plate test 15 min after each injection by an experimenter blind to the pretreatment condition. The hot-plate test measures the latency to lick the hindpaw when the rat is placed on a 52.5 °C plate. If the animal did not respond within 50 s, it was removed from the plate and given a score of 50 s. The mean latency at each dose was calculated for male and female rats pretreated with morphine or saline.
Behavioral data analysis and presentation
The half-maximal antinociceptive effect (D50; Tallarida, 2000) and 95% confidence intervals (CI) were calculated from dose–response curves generated using GraphPad software. The lower limit for calculating D50 values was the mean baseline score, and the upper limit was the mean hot-plate latency following administration of the highest morphine dose. Changes in D50 between groups were assessed using anova (GraphPad).
Experiment 2: anatomical assessment of tolerance to morphine
Retrograde tracer injections
Twenty-two male and 24 female rats were deeply anesthetized with ketamine/xylazine (50 mg/kg/10 mg/kg; s.c.). When a surgical plane of anesthesia was reached, the animal was placed in a stereotaxic frame with bregma and lambda at the same dorsal-ventral plane. A glass micropipette (10–20 μm) filled with the retrograde tracer Fluorogold (FG; 2% soln w/v in saline; Fluorochrome LLC, Denver, CO, USA) was lowered into the RVM using the following coordinates (in mm): AP: –2.0 Lambda; ML: 0.0; DV: –8.5). FG was iontophoresed (50/50 duty cycle, 7 s on/7 s off, 7.5 μA current) into the RVM for 25 min to facilitate neuronal uptake. The current was then turned off and the pipette remained in place for an additional 5 min prior to removal to minimize backflow of the tracer along the pipette track. Following the tracer injection, wounds were sutured closed, the antibiotic Neosporin was applied to the wound, and the animal was placed in a clean cage to recover under a heat lamp. Upon complete recovery from the anesthetic, the rat was returned to its original housing facility.
Morphine administration
Ten days following tracer injection, animals were administered morphine or saline for three consecutive days. Morphine sulfate (5 mg/kg, s.c.) was prepared fresh in a 0.9% saline vehicle within 1 h prior to administration. Animals were divided into two experimental groups: (i) eight male and eight female rats received one daily injection of morphine sulfate for three consecutive days; and (ii) five male and five female rats received two daily injections of morphine sulfate for three consecutive days. Injections were administered between the 10.00 h and 16.00 h, with multiple daily injections separated by 6 h. Two control groups were used: (iii) the morphine-naïve group consisted of five male and six female rats injected with saline twice daily for three consecutive days (no morphine injections); and (iv) the acute morphine group consisted of four male and five female rats injected with saline twice daily for 2.5 consecutive days followed by a final injection of morphine (Table 1).
Table 1.
Dosing schedule for experimental and control groups in Experiment 2
| Treatment group | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| Experimental | |||
| Morphine pretreatment | 1 Dose Morphine | 1 Dose Morphine | 1 Dose Morphine |
| Morphine pretreatment | 2 Doses Morphine | 2 Doses Morphine | 2 Doses Morphine |
| Control | |||
| Morphine naïve | 2 Doses Saline | 2 Doses Saline | 2 Doses Saline |
| Acute morphine | 2 Doses Saline | 2 Doses Saline | 1 Dose saline/1 Dose Morphine |
Perfusion fixation
All animals were given a lethal dose of Nembutal (160 mg/kg; i.p.) 1 h following the last injection of morphine or saline. This time point was chosen based on previous studies showing that expression of c-fos mRNA peaks within 30–60 min after increased neuronal activity, while the Fos protein is detectable after 60 min (Sharp et al., 1991; Murphy et al., 1995; Rizvi et al., 1996; Zimmer et al., 1997). Additionally, our previous studies show that Fos is expressed in a strong, consistent pattern in the PAG 1 h following systemic morphine administration (Loyd & Murphy, 2006; Loyd et al., 2007). The animals were transcardially perfused with 200–250 mL of 0.9% sodium chloride containing 2% sodium nitrite as a vasodilator to remove blood from the brain. Immediately following removal of blood, 300 mL of 4% paraformaldehyde in 0.1 m phosphate buffer containing 2.5% acrolein (Polyscience, Niles, IL, USA) was perfused through the brain as a fixative. A final rinse with 200–250 mL of the sodium chloride/sodium nitrate solution was perfused through the brain to remove any residual acrolein. Immediately following perfusion, the brains were carefully removed, placed in a 30% sucrose solution and stored at 4 °C for at least 1 week prior to sectioning. Sucrose solutions were changed daily to optimize saturation of sucrose into the tissue. To section the brain, the dura and pia mater were carefully removed, and the brains were cut into six series of 25-μm coronal sections with a Leica 2000R freezing microtome and stored free-floating in cryoprotectant-antifreeze solution (Watson et al., 1986) at –20 °C until immunocytochemical processing.
Immunocytochemistry
A 1 : 6 series through the rostrocaudal axis of each brain was processed for FG and Fos immunoreactivity as previously described (Murphy & Hoffman, 2001). Briefly, sections were rinsed extensively in potassium phosphate-buffered saline (KPBS) to remove cryoprotectant solution, immediately followed by a 20-min incubation in 1% sodium borohydride to remove excess aldehydes. The tissue was then incubated in primary antibody solution rabbit anti-Fos (Oncogene, Cambridge, MA, USA; lot no. 4194; 1 : 50 000) in KPBS containing 1.0% Triton X-100 for 1 h at room temperature followed by 48 h at 4 °C. After rinsing out the primary antibody with KPBS, the tissue was incubated for 1 h in biotinylated goat anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA, USA; 1 : 200), rinsed with KPBS, followed by a 1 h incubation in avidin-biotin peroxidase complex (1 : 10; ABC Elite Kit, Vector Laboratories). After rinsing in KPBS and sodium acetate (0.175 m; pH 6.5), Fos was visualized as a black reaction product using nickel sulfate-intensified 3,3′-diaminobenzidine solution containing 0.08% hydrogen peroxide in sodium acetate buffer. After rinsing, sections were placed in primary antibody solution rabbit anti-FG (Chemicon, Billerica, MA, USA; lot no. 25060005; 1 : 10 000) in KPBS containing 1.0% Triton X-100 for 1 h at room temperature followed by 48 h at 4 °C. FG was visualized as a brown reaction product using 3,3′-diaminobenzidine containing 0.08% hydrogen peroxide in Trizma buffer (pH 7.2). After 15–30 min, three rinses in sodium acetate buffer terminated the reaction. Sections were then mounted out of saline onto gelatin-subbed slides, air-dried overnight, dehydrated in a series of graded alcohols, cleared in xylene and coverslipped using Permount.
Anatomical data analysis and presentation
Data were analysed across six representative levels through the rostrocaudal axis of the PAG (Bregma –6.72, –7.04, –7.74, –8.00, –8.30, –8.80). As there was no significant effect of level in all analyses conducted, data are presented as rostral (Bregma –6.72, –7.04, –7.74) vs caudal (Bregma –8.00, –8.30, –8.80) PAG. The number of PAG–RVM output neurons (FG +), the total number of activated PAG neurons (Fos +) and the number of activated PAG–RVM output neurons (Fos + FG) were quantified by an experimenter blinded to the experimental condition. Cell counts were conducted unilaterally as there are no differences in the number of FG + cells for the left vs right side of PAG (Loyd & Murphy, 2006). The tissue was sectioned at 25 μm so that 125 μm separates each analysed level of the PAG, thus eliminating any possible bias of counting the same cell twice. Additionally, previous data have shown that there are no sex differences in total area (mm2) of the PAG between weight-matched male and female Sprague–Dawley rats (Loyd & Murphy, 2006).
Data are expressed as the mean ± standard error of the mean (SEM), from which percentages were calculated. As females have a greater number of PAG–RVM output neurons (Loyd & Murphy, 2006), data are presented as the percentage of Fos that was colocalized in PAG–RVM output neurons (%Fos in FG + cells). A three-way analysis of variance (anova) was used to test for significant main effects of sex (male, female), PAG level (Bregma –6.72 to –8.80) and treatment (two experimental groups of morphine administration and two control groups). A one-way anova was used for post hoc analysis to test for significant main effects of treatment, and the Fishers PLSD was used to determine significant interactions between treatment groups when there was a significant main effect independent of sex. P < 0.05 was considered significant for all analyses.
Photomicrographs were generated using a Synsys digital camera attached to a Nikon Eclipse E800 microscope. Images were captured with IP Spectrum software, adjusted to figure format by adjusting brightness and contrast levels using Adobe Photoshop 7.0.
Results
Experiment 1: behavioral assessment of tolerance to morphine
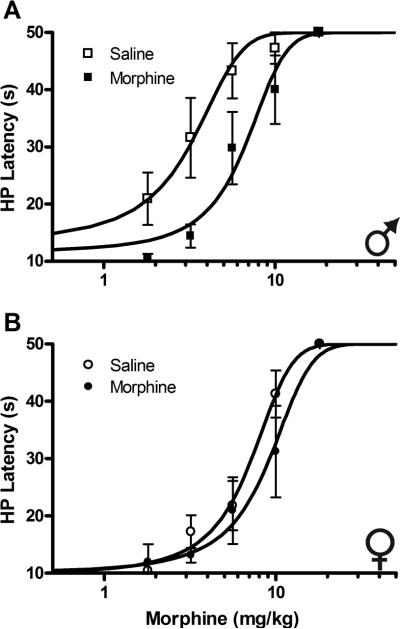
The objective of the present experiment was to compare the development of tolerance to morphine in male and female rats. Following 3 days of morphine or saline injections, administration of cumulative doses of morphine on Day 4 produced a dose-dependent increase in hot-plate latency in all groups tested (Fig. 1). The antinociceptive potency of morphine was greatest in male rats pretreated with saline on Days 1–3 (D50 = 3.0 mg/kg). In contrast, the D50 for female rats pretreated with saline was 6.1 mg/kg, a two-fold difference when compared with males. Consistent with the development of tolerance, male rats pretreated with morphine showed a significant rightward shift in the morphine dose–response curve compared with male rats pretreated with saline (F1,74 = 20.04, P < 0.001; Fig. 1A). Specifically, the D50 increased from 3.0 mg/kg (CI = 2.2–3.7) to 6.3 mg/kg (CI = 5.1–7.6) in rats pretreated with morphine, a 2.1-fold shift in the D50. Female rats showed significantly less tolerance than male rats. In females, pretreatment with morphine on Days 1–3 resulted in a small rightward shift (F1,115 = 5.63, P < 0.05) in the dose–response curve from 6.1 mg/kg (CI = 5.2–7.0) to 8.0 mg/kg (CI = 6.6–9.4; Fig. 1B), a 1.3-fold increase.
Fig. 1.
Comparison of tolerance to the antinociceptive effects of morphine in male (A) and female (B) rats. Rats were pretreated with morphine or saline twice a day for 3 days. Morphine dose–response curves for antinociception on the hot-plate (HP) test were generated on Day 4 using a cumulative dosing paradigm. Both antinociceptive potency and tolerance to morphine were greater in male compared with female rats.
Experiment 2a: anatomical assessment of tolerance to morphine in the PAG
The next series of experiments were conducted to determine if the activation of the PAG–RVM pathway decreases during the development of tolerance to repeated systemic morphine administration in male and female rats. Across all groups (Table 1), systemic morphine administration induced extensive Fos expression throughout the rostrocaudal axis of the PAG. Fos was present in both FG + and non-FG + PAG neurons. No significant main effect of sex (F1,208 = 0.764, n.s.) or PAG level (F5,208 = 0.0454, n.s.) was noted in the mean number of morphine-induced Fos-positive neurons in the PAG. However, there was a significant main effect of treatment (F3,208 = 8.733, P < 0.05) and a significant sex-by-treatment interaction (F3,208 = 10.617, P < 0.05). Male rats treated with saline had significantly less Fos expression compared with all other treatment groups in the caudal PAG (Fig. 2; P < 0.05). In females, administration of one dose of morphine resulted in significantly less Fos expression in comparison to the other treatment groups (Fig. 2; P < 0.05). Total Fos expression was comparable between the two repeated morphine administration groups and, in females, as compared with the morphine-naïve group.
Fig. 2.
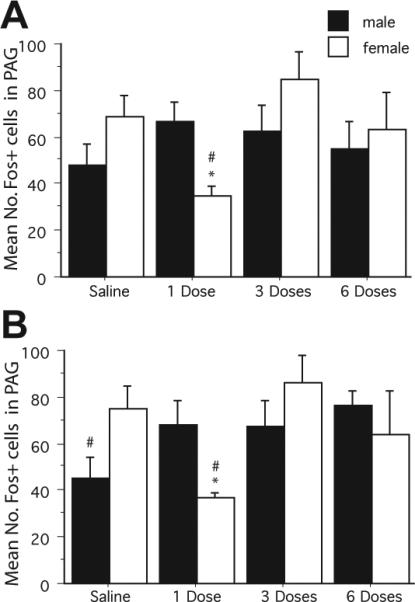
Mean number of Fos-positive cells in male and female rats injected with either morphine or saline once or twice daily for 3 days for the rostral (A; Bregma –6.72, –7.04, –7.74) and caudal (B; Bregma –8.00, –8.30, –8.80) PAG. The # indicates a significant main effect of treatment (morphine and saline), and the * indicates a significant main effect of sex. Saline: morphine naïve; 1 Dose: saline pretreatment followed by one dose of morphine; 3 Doses: one dose of morphine per day; 6 Doses: two doses of morphine per day.
Experiment 2b: anatomical assessment of tolerance to morphine in the PAG–RVM circuit
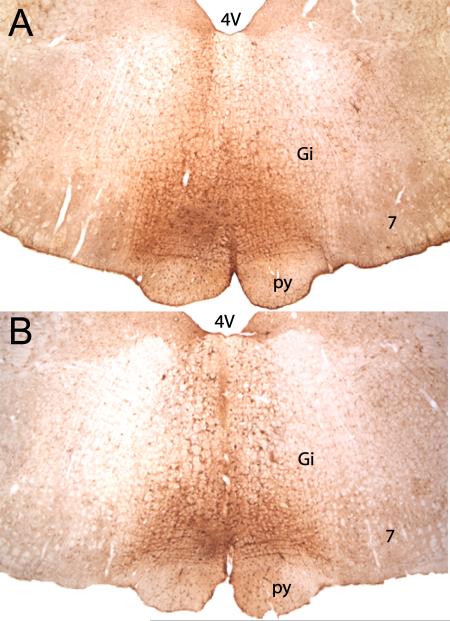
Figure 3 shows an example of a typical iontophoretic injection of retrograde tracer FG into the RVM of a male (top) and female (bottom) rat. All injections were located on the midline and dorsal to the pyramidal tract, at the level of the caudal pole of the facial nucleus (Lambda –2.0 mm). Injections outside of the RVM were not included for analysis. Injection of FG into the RVM produced dense retrograde labeling throughout the rostrocaudal axis of the PAG, with females having a significantly greater amount of labeling compared with males (F1,208 = 59.610, P < 0.05; data not shown; Loyd & Murphy, 2006; Loyd et al., 2007).
Fig. 3.
Color photomicrograph of representative examples of Fluorogold injection sites in the RVM of a male (A) and female (B) rat. Injection sites were limited to localization within the bottom third of the medulla along the midline between the facial nuclei and dorsal to the pyramidal tract. Gi, gigantocellularis; py, pyramidal tract; 4V, fourth ventricle; 7, facial nucleus.
Administration of one dose of morphine following 3 days of saline injections resulted in extensive Fos expression in PAG–RVM output neurons across all rostrocaudal levels of the PAG (Fig. 4). An analysis of the %Fos in FG + cells (PAG–RVM output neurons) revealed a significant main effect of sex (F1,208 = 41.194, P < 0.05), treatment (F3,208 = 43.607, P < 0.05) and a significant sex-by-treatment interaction (F3,208 = 19.440, P < 0.05). There was no significant main effect of PAG level (F5,208 = 0.1883, n.s.), indicating that these results were consistent across the rostrocaudal axis of the PAG. The %Fos in FG + neurons decreased as a function of morphine treatment in males only (F2,71 = 26.546, P < 0.05). Overall, the average %Fos in FG + neurons decreased from 52 ± 5% in males receiving one injection of morphine to 14 ± 6% in males following the sixth injection of morphine. In females, the %Fos in FG + cells was low across all rostrocaudal levels of the PAG, and no significant effect of treatment was observed (F2,83 = 1.919, n.s.). Together, these results parallel our behavioral data, indicating greater tolerance to morphine in male rats.
Fig. 4.
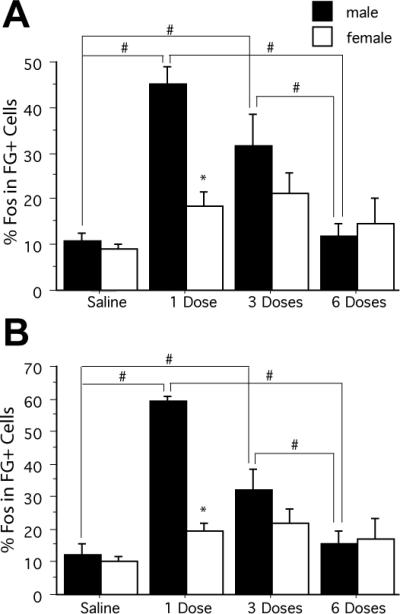
Percentage of Fos-positive neurons that were retrogradely labeled from the RVM (%Fos in FG + cells) in male (solid bars) and female (open bars) rats injected with either morphine or saline once or twice daily for 3 days for the rostral (A; Bregma –6.72, –7.04, –7.74) and caudal (B; Bregma –8.00, –8.30, –8.80) PAG. A decrease in labeling is evident with an increase in the number of morphine injections for male rats. The # indicates a significant effect of treatment and the * indicates a significant effect of sex. Saline: morphine naïve; 1 Dose: saline pretreatment followed by one dose of morphine; 3 Doses: one dose of morphine per day; 6 Doses: two doses of morphine per day.
Discussion
In the present study, behavioral and neuroanatomical techniques were used to examine the role of the PAG–RVM circuit in the development of tolerance to systemic morphine in male and female rats. The results demonstrate that repeated administration of systemic morphine induces tolerance in males to a much greater extent than in females. In parallel, repeated morphine administration significantly reduces the activation of PAG–RVM neurons by morphine in males but not females. The half-maximal antinociceptive effect of a single injection of morphine was twice as great for female compared with male rats, and is consistent with previous research (Cicero et al., 1997; Cook & Nickerson, 2005; Ji et al., 2006; Wang et al., 2006). This sex difference in morphine potency is reflected in the greater activation of PAG–RVM output neurons in males following morphine administration reported here and previously (Loyd et al., 2007). In addition, the greater tolerance to morphine in male compared with female rats also is consistent with previous research (Barrett et al., 2001; Bernal et al., 2007). Our data show that two injections of morphine per day for three consecutive days was sufficient to reduce morphine potency by 2.1 times in male rats. However, in females, the half-maximal effective dose of morphine only increased from 6.1 to 8.0 mg/kg. Thus, not only do female rats show less antinociception in response to morphine, but they also show much less tolerance to morphine.
Our data indicate that the PAG and its projections to the RVM likely contribute to the observed behavioral differences between male and female rats. Both the number of Fos + neurons in the PAG and the number of Fos + neurons in the PAG that were retrogradely labeled from the RVM following a single dose of morphine was greater in male compared with female rats. Although there was no significant main effect of sex in the number of Fos + neurons in the PAG observed following repeated morphine administration, there was a progressive decrease in the %Fos in FG + cells following three or six repeated injections of morphine in male rats. This decrease in activation of PAG–RVM output neurons in male rats led to a decrease in the %Fos in FG + cells so that male rats were comparable to female rats. Moreover, the slight tolerance observed in females is consistent with a lack of change in the %Fos in FG + cells following one, three or six repeated injections of morphine. These data correspond very closely with the behavioral data showing that morphine antinociception is greater in male compared with female rats, and tolerance causes a reduction in morphine potency so that tolerant male rats experience comparable analgesia to less tolerant female rats. These results demonstrate that the development of tolerance to morphine is associated with a reduction in the activation of the PAG–RVM circuit in male rats, and provide additional data demonstrating a central role for the PAG in tolerance to morphine.
It has been shown that tolerance develops with repeated microinjections of morphine directly into the ventrolateral PAG (Tortorici et al., 1999; Morgan et al., 2006a), and is associated with a reduction in mu opioid receptor (MOR) signaling in the PAG (Bagley et al., 2005). Importantly, blocking opioid binding in the PAG attenuates the development of tolerance to systemic morphine administration (Lane et al., 2005). While the PAG does not project directly to the spinal cord, it projects heavily to the RVM, which in turn projects to the spinal cord (Beitz, 1982; Beitz et al., 1983). RVM on-cells and off-cells that are normally inhibited and activated by morphine, respectively, show normal responses to nociceptive reflexes during the induction of tolerance following repeated intra-PAG administration of morphine (Lane et al., 2004). Given that tolerance develops to a greater degree when morphine is administered directly into the PAG compared with administration into the RVM (Morgan et al., 2005) and that there is a corresponding progressive decrease in the activation of PAG–RVM neurons, these data together indicate that a key mechanism for tolerance resides within the PAG.
Previous data have shown that one systemic injection of morphine induces extensive Fos expression in the PAG that is comparable in male and female rats (Loyd et al., 2007). Similarly, in the present study, total Fos expression in the PAG following morphine administration over 3 days was not sexually dimorphic. However, unlike our previous study, acute morphine administration following 3 days of saline injections in males did not induce significantly greater Fos labeling compared with saline-treated rats, and females rats showed a significant decline in Fos labeling. This difference in Fos induction by morphine in these two studies suggests that the multiple injections of saline over 3 days in the present study induce Fos expression that is independent of morphine. The significant decline in Fos labeling in the PAG following one dose of morphine in female rats indicates that there is a sexually dimorphic effect of multiple injections of saline on the type of stimuli inducing activation in the PAG; further studies are warranted to examine this result.
Given that Fos expression in the PAG can be induced by a wide range of stimuli including fear and anxiety (Kim et al., 1993), changes in respiration (Zhang et al., 1990; Harper et al., 1996), vocalization (Davis et al., 1993; Zhang et al., 1994), blood pressure (Carrive, 1991; Murphy et al., 1995), and in fight or flight responses (Bandler et al., 1985; Bandler & Carrive, 1988; Depaulis et al., 1992), it is not surprising that repeated daily injections of saline induced Fos in PAG neurons. In contrast, PAG neurons retrogradely labeled with FG from the RVM were more likely to express Fos following morphine administration than in saline-treated rats. Moreover, this increase in Fos expression was greater in male compared with female rats. The greater Fos expression in FG + cells correlates with the greater morphine antinociception produced in male compared with female rats. The limited Fos expression in the PAG–RVM output neurons of female rats is consistent with the low antinociceptive potency of morphine in these animals. Likewise, the lack of change in Fos expression with repeated morphine administration is consistent with the limited antinociceptive tolerance to repeated intra-PAG microinjections of morphine in female rats.
Sex differences in MOR distribution and function in the PAG (A.Z. Murphy, unpublished observations) may provide a mechanism for sex differences in the development of tolerance to morphine. Furthermore, cells that express both MOR and γ-aminobutyric acid (GABA) are common in the PAG (Williams & Beitz, 1990; Kalyuzhny & Wessendorf, 1998), suggesting that morphine directly modulates the GABAergic neurons that tonically inhibit PAG–RVM neurons (Behbehani & Fields, 1979; Chieng & Christie, 1994; Vaughan & Christie, 1997; Commons et al., 2000; Tortorici & Morgan, 2002). Administration of GABA agonists hyperpolarizes PAG–RVM neurons (Osborne et al., 1996), and microinjection of GABA antagonists into the PAG results in a net increase in membrane depolarization, firing frequency, frequency of excitatory postsynaptic potentials (Behbehani et al., 1990) and antinociception (Morgan et al., 2005). A potential mechanism of action is that morphine is hyperpolarizing GABA interneurons in the PAG. As tolerance develops, these GABAergic cells are no longer inhibited by morphine, resulting in a progressive increase in tonic inhibition on PAG–RVM neurons and thus a corresponding decrease in the antinociceptive effects of morphine. The decrease in Fos expression in PAG neurons projecting to the RVM is consistent with this model and indicates that tolerance is caused by a change in morphine-sensitive GABAergic neurons.
The PAG also contains a high density of estrogen receptors (ERα) (Murphy & Hoffman, 1999, 2001; Marson & Murphy, 2006), and approximately 25% of PAG–RVM neurons contain ERα (Loyd & Murphy, unpublished observations). Estradiol has been shown to uncouple MOR from G-protein-gated inwardly rectifying potassium channels, thus reducing morphine hyperpolarization of PAG–RVM neurons (Kelly et al., 2003). In the present study, circulating estrogens acting at ERα receptors may be uncoupling MOR in the PAG of female rats, resulting in an overall reduction in the activity of these cells and the analgesic effects of morphine. Interestingly, it has been shown that the excitability of neurons in the PAG is variable across the estrous cycle of the rat, such that during estrus and late diestrus, GABA antagonists greatly increase PAG neuronal firing rates, suggesting a decrease in GABAergic tone (Brack & Lovick, 2007).
Cycling females were used in these studies; all rats continued to cycle normally during the three consecutive days of morphine or saline administration. The activation of PAG neurons in females tended to be more variable compared with males, and stage of estrous may potentially affect morphine potency and tolerance. Unfortunately, in the present study we were unable to statistically analyse the effect of cycle on morphine potency due to limitations in the number of animals per stage of estrous. Upon looking for trends in the data, there appears to be an effect of cycle on the activation of the PAG following repeated administration of morphine. Female rats in diestrus 2 (n = 3) had on average twice as much Fos expression in the PAG following six repeated doses of morphine compared with rats in estrus (n = 2; data not shown). In addition, rats in diestrus 2 (n = 3) that received three repeated doses of morphine had on average twice the %Fos in FG + cells as compared with rats in estrus (n = 2) and in diestrus 1 (n = 3; data not shown). No other trends were observed; however, further studies are clearly warranted to examine the potential effect of estrous cycle on tolerance.
In summary, the results of the present study indicate that tolerance to repeated systemic injections of morphine occurs to a greater extent in male compared with female rats. As tolerance develops, there is a corresponding steady decline in the activation of the PAG–RVM pathway in male rats. Activation of this pathway by morphine in female rats is not significant, and it does not significantly decline following repeated administration of morphine. Together, these data provide additional evidence demonstrating a central role for the PAG in the development of tolerance to repeated administration of morphine.
Acknowledgements
The authors would like to acknowledge the technical assistance of Theresa Bau during preliminary data collection. This work was supported by NIH grants DA16272 and P50 AR49555 awarded to A.Z.M., and NIH grant DA015498 awarded to M.M.M.
Abbreviations
- CI
confidence interval
- ER
estrogen receptor
- FG
Fluorogold
- GABA
γ-aminobutyric acid
- KPBS
potassium phosphate-buffered saline
- MOR
mu opioid receptor
- PAG
periaqueductal gray
- RVM
rostral ventromedial medulla
References
- Bagley EE, Chieng BC, Christie MJ, Connor M. Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine. Br. J. Pharmacol. 2005;146:68–76. doi: 10.1038/sj.bjp.0706315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R, Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988;439:95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- Bandler R, Depaulis A, Vergnes M. Identification of midbrain neurones mediating defensive behaviour in the rat by microinjections of excitatory amino acids. Behav. Brain Res. 1985;15:107–119. doi: 10.1016/0166-4328(85)90058-0. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ. Importance of sex and relative efficacy at the mu opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology (Berl.) 2001;158:154–164. doi: 10.1007/s002130100821. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 1979;170:85–93. doi: 10.1016/0006-8993(79)90942-9. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Jiang MR, Chandler SD, Ennis M. The effect of GABA and its antagonists on midbrain periaqueductal gray neurons in the rat. Pain. 1990;40:195–204. doi: 10.1016/0304-3959(90)90070-T. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- Beitz AJ, Shepard RD, Wells WE. The periaqueductal gray-raphe magnus projection contains somatostatin, neurotensin and serotonin but not cholecystokinin. Brain Res. 1983;261:132–137. doi: 10.1016/0006-8993(83)91292-1. [DOI] [PubMed] [Google Scholar]
- Bernal SA, Morgan MM, Craft RM. PAG mu opioid receptor activation underlies sex differences in morphine antinociception. Behav. Brain Res. 2007;177:126–133. doi: 10.1016/j.bbr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack KE, Lovick TA. Neuronal excitability in the periaqueductal grey matter during the estrous cycle in female Wistar rats. Neuroscience. 2007;144:325–335. doi: 10.1016/j.neuroscience.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur. J. Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Carrive P. Functional organization of PAG neurons controlling regional vascular beds. In: Depaulis A, Bandler R, editors. The Midbrain Periaqueductal Gray Matter. Plenum Press; New York: 1991. pp. 67–100. [Google Scholar]
- Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth. Analg. 2003;97:1464–1468. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Inhibition by opioids acting on mu-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br. J. Pharmacol. 1994;113:303–309. doi: 10.1111/j.1476-5381.1994.tb16209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, Meyer ER. Sex-related differences in morphine's antinociceptive activity: relationship to serum and brain morphine concentrations. J. Pharmacol. Exp. Ther. 1997;282:939–944. [PubMed] [Google Scholar]
- Commons KG, Aicher SA, Kow LM, Pfaff DW. Presynaptic and postsynaptic relations of mu-opioid receptors to gamma-aminobutyric acid-immunoreactive and medullary-projecting periaqueductal gray neurons. J. Comp. Neurol. 2000;419:532–542. doi: 10.1002/(sici)1096-9861(20000417)419:4<532::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Cook CD, Nickerson MD. Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund's adjuvant-induced arthritic male and female rats. J. Pharmacol. Exp. Ther. 2005;313:449–459. doi: 10.1124/jpet.104.077792. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in drug- and non-drug-induced analgesia. Life Sci. 2003;72:2675–2688. doi: 10.1016/s0024-3205(03)00178-4. [DOI] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA, Bartok RE, Walpole TI, King SJ. Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacology (Berl.) 1999;143:1–7. doi: 10.1007/s002130050911. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Zhang SP, Bandler R. Pulmonary and upper airway afferent influences on the motor pattern of vocalization evoked by excitation of the midbrain periaqueductal gray of the cat. Brain Res. 1993;607:61–80. doi: 10.1016/0006-8993(93)91490-j. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Keay KA, Bandler R. Longitudinal neuronal organization of defensive reactions in the midbrain periaqueductal gray region of the rat. Exp. Brain Res. 1992;90:307–318. doi: 10.1007/BF00227243. [DOI] [PubMed] [Google Scholar]
- Hack SP, Vaughan CW, Christie MJ. Modulation of GABA release during morphine withdrawal in midbrain neurons in vitro. Neuropharmacology. 2003;45:575–584. doi: 10.1016/s0028-3908(03)00205-3. [DOI] [PubMed] [Google Scholar]
- Harper RM, Rector D, Poe G, Frysinger RC, Kristensen M, Gozal D. Rostral brain regions contributing to respiratory control. In: Holstege G, Bandler R, Saper CB, editors. The Emotional Motor System. Elsevier Science B.V.; 1996. pp. 145–158. [DOI] [PubMed] [Google Scholar]
- Jacquet YF, Lajtha A. The periaqueductal gray: site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Res. 1976;103:501–513. doi: 10.1016/0006-8993(76)90448-0. [DOI] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine-induced analgesia of visceral pain are supraspinally and peripherally mediated. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R307–R314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- Kalyuzhny AE, Wessendorf MW. Relationship of mu- and delta- opioid receptors to GABAergic neurons in the central nervous system, including antinociceptive brainstem circuits. J. Comp. Neurol. 1998;392:528–547. [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Ronnekleiv OK. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann. N Y Acad. Sci. 2003;1007:6–16. doi: 10.1196/annals.1286.001. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav. Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Bodnar RJ. Morphine antinociception elicited from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 1999;821:224–230. doi: 10.1016/s0006-8993(98)01364-x. [DOI] [PubMed] [Google Scholar]
- Lane DA, Patel PA, Morgan MM. Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience. 2005;135:227–234. doi: 10.1016/j.neuroscience.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Lane DA, Tortorici V, Morgan MM. Behavioral and electrophysiological evidence for tolerance to continuous morphine administration into the ventrolateral periaqueductal gray. Neuroscience. 2004;125:63–69. doi: 10.1016/j.neuroscience.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Morgan MM, Murphy AZ. Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience. 2007;147:456–468. doi: 10.1016/j.neuroscience.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J. Comp. Neurol. 2006;496:723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson L, Murphy AZ. Identification of neural circuits involved in female genital responses in the rat: a dual virus and anterograde tracing study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R419–R428. doi: 10.1152/ajpregu.00864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PL, Ernst AA. Sex differences in analgesia: a randomized trial of mu versus kappa opioid agonists. South Med. J. 2004;97:35–41. doi: 10.1097/01.smj.0000085743.68121.a9. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Clayton CC, Boyer-Quick JS. Differential susceptibility of the PAG and RVM to tolerance to the antinociceptive effect of morphine in the rat. Pain. 2005;113:91–98. doi: 10.1016/j.pain.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Fossum EN, Levine CS, Ingram SL. Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol. Biochem. Behav. 2006a;85:214–219. doi: 10.1016/j.pbb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Fossum EN, Stalding BM, King MM. Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J. Pain. 2006b;7:358–366. doi: 10.1016/j.jpain.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Ennis M, Rizvi TA, Behbehani MM, Shipley MT. Fos expression induced by changes in arterial pressure is localized in distinct, longitudinally organized columns of neurons in the rat midbrain periaqueductal gray. J. Comp. Neurol. 1995;360:286–300. doi: 10.1002/cne.903600207. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of androgen and estrogen receptor containing neurons in the male rat periaqueductal gray. Horm. Beh. 1999;36:98–108. doi: 10.1006/hbeh.1999.1528. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: a potential circuit for the initiation of male sexual behavior. J. Comp. Neurol. 2001;438:191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- Osborne PB, Vaughan CW, Wilson HI, Christie MJ. Opioid inhibition of rat periaqueductal grey neurones with identified projections to the rostral ventromedial medulla in vitro. J. Physiol. (Lond.) 1996;490:383–389. doi: 10.1113/jphysiol.1996.sp021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi TA, Murphy AZ, Ennis M, Behbehani MM, Shipley MT. Medial preoptic area afferents to periaqueductal gray medullo-output neurons: a combined Fos and tract tracing study. J. Neurosci. 1996;16:333–344. doi: 10.1523/JNEUROSCI.16-01-00333.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Sagar SM, Hicks K, Lowenstein D, Hisanaga K. c-fos mRNA, Fos, and Fos-related antigen induction by hypertonic saline and stress. J. Neurosci. 1991;11:2321–2331. doi: 10.1523/JNEUROSCI.11-08-02321.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Advokat C. Tolerance to morphine microinjections in the periaqueductal gray (PAG) induces tolerance to systemic, but not intrathecal morphine. Brain Res. 1987;424:311–319. doi: 10.1016/0006-8993(87)91476-4. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism and dose effect data analysis. Chapman & Hall/CRC; Boca Raton: 2000. [Google Scholar]
- Tortorici V, Morgan MM. Comparison of morphine and kainic acid microinjections into identical PAG sites on the activity of RVM neurons. J. Neurophysiol. 2002;88:1707–1715. doi: 10.1152/jn.2002.88.4.1707. [DOI] [PubMed] [Google Scholar]
- Tortorici V, Robbins CS, Morgan MM. Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not lateral-dorsal periaqueductal gray of the rat. Behav. Neurosci. 1999;113:833–839. doi: 10.1037//0735-7044.113.4.833. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J. Physiol. 1997;498:463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R300–R306. doi: 10.1152/ajpregu.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RE, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain longterm peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Williams FG, Beitz AJ. Ultrastructural morphometric analysis of GABA-immunoreactive terminals in the ventrocaudal periaqueductal grey: analysis of the relationship of GABA terminals and the GABAA receptor to periaqueductal grey-raphe magnus projection neurons. J. Neurocytol. 1990;19:686–696. doi: 10.1007/BF01188037. [DOI] [PubMed] [Google Scholar]
- Zhang SP, Bandler R, Carrive P. Flight and immobility evoked by excitatory amino acid microinjection within distinct parts of the subtentorial midbrain periaqueductal gray of the cat. Brain Res. 1990;520:73–82. doi: 10.1016/0006-8993(90)91692-a. [DOI] [PubMed] [Google Scholar]
- Zhang SP, Davis PJ, Bandler R, Carrive P. Brain stem integration of vocalization: role of the midbrain periaqueductal gray. J. Neurophysiol. 1994;72:1337–1356. doi: 10.1152/jn.1994.72.3.1337. [DOI] [PubMed] [Google Scholar]
- Zimmer LA, Ennis M, el-Etri M, Shipley MT. Anatomical localization and time course of Fos expression following soman-induced seizures. J. Comp. Neurol. 1997;278:468–481. [PubMed] [Google Scholar]