Abstract
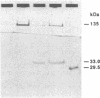
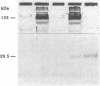
Uroporphyrinogen III synthase [URO-synthase; hydroxymethylbilane hydro-lyase (cyclizing), EC 4.2.1.75], the fourth enzyme in the heme biosynthetic pathway, is responsible for conversion of the linear tetrapyrrole, hydroxymethylbilane, to the cyclic tetrapyrrole, uroporphyrinogen III. The deficient activity of URO-synthase is the enzymatic defect in the autosomal recessive disorder congenital erythropoietic porphyria. To facilitate the isolation of a full-length cDNA for human URO-synthase, the human erythrocyte enzyme was purified to homogeneity and 81 nonoverlapping amino acids were determined by microsequencing the N terminus and four tryptic peptides. Two synthetic oligonucleotide mixtures were used to screen 1.2 x 10(6) recombinants from a human adult liver cDNA library. Eight clones were positive with both oligonucleotide mixtures. Of these, dideoxy sequencing of the 1.3 kilobase insert from clone pUROS-2 revealed 5' and 3' untranslated sequences of 196 and 284 base pairs, respectively, and an open reading frame of 798 base pairs encoding a protein of 265 amino acids with a predicted molecular mass of 28,607 Da. The authenticity of this clone was established by colinearity of the predicted amino acid sequence with 81 microsequenced residues from the purified enzyme. In addition, high levels of enzymatic activity and immunoreactive protein were expressed when a blunt-ended 971-base-pair Ava II cDNA fragment containing the entire coding region was inserted into vectors for expression in Escherichia coli. The isolation and expression of this full-length cDNA for human URO-synthase should facilitate studies of the structure, organization, and chromosomal localization of this heme biosynthetic gene as well as the characterization of the molecular lesions causing congenital erythropoietic porphyria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. II. Uroporphyrin III. J Biol Chem. 1958 Aug;233(2):510–515. [PubMed] [Google Scholar]
- Battersby A. R., Fookes C. J., Matcham G. W., McDonald E. Biosynthesis of the pigments of life: formation of the macrocycle. Nature. 1980 May 1;285(5759):17–21. doi: 10.1038/285017a0. [DOI] [PubMed] [Google Scholar]
- Berget S. M. Are U4 small nuclear ribonucleoproteins involved in polyadenylation? Nature. 1984 May 10;309(5964):179–182. doi: 10.1038/309179a0. [DOI] [PubMed] [Google Scholar]
- Bilofsky H. S., Burks C., Fickett J. W., Goad W. B., Lewitter F. I., Rindone W. P., Swindell C. D., Tung C. S. The GenBank genetic sequence databank. Nucleic Acids Res. 1986 Jan 10;14(1):1–4. doi: 10.1093/nar/14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogorad L., Granick S. The Enzymatic Synthesis of Porphyrins from Porphobilinogen. Proc Natl Acad Sci U S A. 1953 Dec;39(12):1176–1188. doi: 10.1073/pnas.39.12.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buikema W. J., Szeto W. W., Lemley P. V., Orme-Johnson W. H., Ausubel F. M. Nitrogen fixation specific regulatory genes of Klebsiella pneumoniae and Rhizobium meliloti share homology with the general nitrogen regulatory gene ntrC of K. pneumoniae. Nucleic Acids Res. 1985 Jun 25;13(12):4539–4555. doi: 10.1093/nar/13.12.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien S., Dubart A., Beaupain D., Raich N., Grandchamp B., Rosa J., Goossens M., Romeo P. H. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci U S A. 1988 Jan;85(1):6–10. doi: 10.1073/pnas.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp B., De Verneuil H., Beaumont C., Chretien S., Walter O., Nordmann Y. Tissue-specific expression of porphobilinogen deaminase. Two isoenzymes from a single gene. Eur J Biochem. 1987 Jan 2;162(1):105–110. doi: 10.1111/j.1432-1033.1987.tb10548.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hart G. J., Battersby A. R. Purification and properties of uroporphyrinogen III synthase (co-synthetase) from Euglena gracilis. Biochem J. 1985 Nov 15;232(1):151–160. doi: 10.1042/bj2320151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidekamp F., Dirkse W. G., Hille J., van Ormondt H. Nucleotide sequence of the Agrobacterium tumefaciens octopine Ti plasmid-encoded tmr gene. Nucleic Acids Res. 1983 Sep 24;11(18):6211–6223. doi: 10.1093/nar/11.18.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Wieczorek L., Altendorf K., Reicin A. S., Dorus E., Epstein W. Sequence homology between two membrane transport ATPases, the Kdp-ATPase of Escherichia coli and the Ca2+-ATPase of sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4746–4750. doi: 10.1073/pnas.81.15.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Protein sequence analysis: automated microsequencing. Science. 1983 Feb 11;219(4585):650–659. doi: 10.1126/science.6687410. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Mgbeje B. I., Thomas S. D., Alwan A. F. Nucleotide sequence for the hemD gene of Escherichia coli encoding uroporphyrinogen III synthase and initial evidence for a hem operon. Biochem J. 1988 Jan 15;249(2):613–616. doi: 10.1042/bj2490613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Klein P., Greif P., DeLisi C. Computer analysis and structure prediction of nucleic acids and proteins. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):417–428. doi: 10.1093/nar/12.1part1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Orkin S. H. The 3' untranslated regions of the duplicated human alpha-globin genes are unexpectedly divergent. Cell. 1980 Nov;22(2 Pt 2):371–377. doi: 10.1016/0092-8674(80)90347-5. [DOI] [PubMed] [Google Scholar]
- Priestle J. P., Grütter M. G., White J. L., Vincent M. G., Kania M., Wilson E., Jardetzky T. S., Kirschner K., Jansonius J. N. Three-dimensional structure of the bifunctional enzyme N-(5'-phosphoribosyl)anthranilate isomerase-indole-3-glycerol-phosphate synthase from Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5690–5694. doi: 10.1073/pnas.84.16.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich N., Romeo P. H., Dubart A., Beaupain D., Cohen-Solal M., Goossens M. Molecular cloning and complete primary sequence of human erythrocyte porphobilinogen deaminase. Nucleic Acids Res. 1986 Aug 11;14(15):5955–5968. doi: 10.1093/nar/14.15.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roméo P. H., Raich N., Dubart A., Beaupain D., Pryor M., Kushner J., Cohen-Solal M., Goossens M. Molecular cloning and nucleotide sequence of a complete human uroporphyrinogen decarboxylase cDNA. J Biol Chem. 1986 Jul 25;261(21):9825–9831. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasarman A., Nepveu A., Echelard Y., Dymetryszyn J., Drolet M., Goyer C. Molecular cloning and sequencing of the hemD gene of Escherichia coli K-12 and preliminary data on the Uro operon. J Bacteriol. 1987 Sep;169(9):4257–4262. doi: 10.1128/jb.169.9.4257-4262.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanley K. K. Solubilization and immune-detection of beta-galactosidase hybrid proteins carrying foreign antigenic determinants. Nucleic Acids Res. 1983 Jun 25;11(12):4077–4092. doi: 10.1093/nar/11.12.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Bishop D. F., Desnick R. J. Coupled-enzyme and direct assays for uroporphyrinogen III synthase activity in human erythrocytes and cultured lymphoblasts. Enzymatic diagnosis of heterozygotes and homozygotes with congenital erythropoietic porphyria. Anal Biochem. 1987 Oct;166(1):120–133. doi: 10.1016/0003-2697(87)90554-9. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Bishop D. F., Desnick R. J. Purification and properties of uroporphyrinogen III synthase from human erythrocytes. J Biol Chem. 1987 Jan 25;262(3):1268–1273. [PubMed] [Google Scholar]
- Wetmur J. G., Bishop D. F., Cantelmo C., Desnick R. J. Human delta-aminolevulinate dehydratase: nucleotide sequence of a full-length cDNA clone. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7703–7707. doi: 10.1073/pnas.83.20.7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Markham A. F., Ricker A. T., Goldberger G., Colten H. R. Isolation of cDNA clones for the human complement protein factor B, a class III major histocompatibility complex gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5661–5665. doi: 10.1073/pnas.79.18.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Yew N. S., Federspiel M., Dodgson J. B., Hayashi N., Engel J. D. Isolation of recombinant cDNAs encoding chicken erythroid delta-aminolevulinate synthase. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3702–3706. doi: 10.1073/pnas.82.11.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]