Abstract
We examined age-related changes in the interactions among brain regions in children performing rhyming judgments on visually presented words. The difficulty of the task was manipulated by including a conflict between task-relevant (phonological) information and task-irrelevant (orthographic) information. The conflicting conditions included pairs of words that rhyme despite having different spelling patterns ( jazz–has), or words that do not rhyme despite having similar spelling patterns ( pint–mint). These were contrasted with nonconflicting pairs that have similar orthography and phonology (dime–lime) or different orthography and phonology ( press–list). Using fMRI, we examined effective connectivity among five left hemisphere regions of interest: fusiform gyrus (FG), inferior frontal gyrus (IFG), intraparietal sulcus (IPS), lateral temporal cortex (LTC), and medial frontal gyrus (MeFG). Age-related increases were observed in the influence of the IFG and FG on the LTC, but only in conflicting conditions. These results reflect a developmental increase in the convergence of bottom–up and top–down information on the LTC. In older children, top–down control process may selectively enhance the sensitivity of the LTC to bottom–up information from the FG. This may be evident especially in situations that require selective enhancement of task-relevant versus task-irrelevant information. Altogether these results provide a direct evidence for a developmental increase in top–down control processes in language processing. The developmental increase in bottom–up processing may be secondary to the enhancement of top–down processes.
INTRODUCTION
During development, improved cognitive abilities are associated with structural and functional changes in the brain (Casey, Tottenham, Liston, & Durston, 2005; Davidson, Thomas, & Casey, 2003; Johnson, 2001). In functional brain imaging studies, developmental changes are evident through increases and decreases in activation with age. However, accumulating evidence from functional and effective connectivity studies suggests that examination of the interaction among brain regions is crucial for understanding the neurocognitive changes that occur during development (Fair et al., 2007; Stephan, Fink, & Marshall, 2007; Berl, Vaidya, & Gaillard, 2006).
In a previous fMRI study that examined effective connectivity (the directional influence that one brain region exerts on another brain region) during language tasks, we found a weaker top–down effect of the left inferior frontal gyrus (IFG) on posterior regions in children compared to adults (Bitan et al., 2005, 2006). In these experiments, participants performed spelling and rhyming decisions on visually presented words. In both children and adults, task-specific regions (i.e., the left lateral temporal cortex [LTC] in the rhyming task and the left intraparietal sulcus [IPS] in the spelling task) served as convergence zones that integrated task-relevant information. The left IFG was the only region that was reciprocally connected with all other regions in the network, regardless of the task, suggesting it may have a role in exerting top–down control processes on posterior regions (Miller & Cohen, 2001). We hypothesized that the left IFG selectively enhanced the sensitivity of task-specific regions to the input they receive from the fusiform gyrus (FG) according to the requirements of the task. The effect of the left IFG in each task on the corresponding convergence zone was weaker in children compared to adults (Bitan et al., 2006). This developmental increase in the effect of frontal regions on posterior regions may reflect an increase in top–down control processes in adults compared to children. The goal of the current study is to examine whether the developmental increase in connectivity in linguistic processing reflects an increase in top–down control process, and to determine its relationship with bottom–up processes, in the previously depicted linguistic network. For this aim we manipulated the difficulty of the task and the demands on executive control processes, and examined developmental changes among children of different ages (9–15; rather than between children and adults).
Evidence from both structural and functional brain imaging studies are consistent with the notion of a developmental increase in top–down connectivity that continues into late childhood and adolescence. Anatomical studies show protracted development of regions throughout the prefrontal cortex and their connections with posterior regions (Zhang et al., 2007; Gogtay et al., 2004; Sowell, Delis, Stiles, & Jernigan, 2001; Klingberg, Vaidya, Gabrieli, Moseley, & Hedehus, 1999; Paus et al., 1999; Huttenlocher & Dabholkar, 1997; Stuss, 1992; Dobbing & Sands, 1973). Functional imaging studies of single word generation tasks show age-related increases in activation in the left IFG (Holland et al., 2001), specifically in BA 44 (Schapiro et al., 2004; Schlaggar et al., 2002), BA 44/45/47 (Gaillard et al., 2003), and BA 44/9 (Szaflarski et al., 2006). Increase in activation with age in the left IFG (BA 44/9) was also found in a rhyming judgment task of visually presented words (Bitan, Cheon, et al., 2007; Booth et al., 2004).
In addition to neuroimaging studies showing age-related increase in connectivity and functionality of prefrontal regions, cognitive and developmental psychology studies provide evidence for developmental improvement in top–down control processes. For example, distractibility from task-irrelevant information in the Wisconsin Card Sorting Test decreases by adulthood, and the ability to maintain a cognitive set reaches an adult level around the age of 13 to 15 years (Crone, Ridderinkhof, Worm, Somsen, & van der Molen, 2004). Similarly, impulsivity in a test of inhibitory control was less in adults than in 13-year-old children (Davidson, Amso, Anderson, & Diamond, 2006). A developmental increase in self-regulatory control was also found in a Stroop task, which is associated with increase in activation in right fronto-striatal circuits (BA 44/45) (Marsh et al., 2006). The medial frontal region is another area associated with cognitive control that shows age-related increases in activation. In a working memory task when distracting stimuli must be ignored, children were more distracted and showed less activation in this area than adults (Olesen, Macoveanu, Tegner, & Klingberg, 2007).
Although evidence suggests that lower-level regions mature earlier than higher-level association regions, and thus, show smaller developmental changes ( Johnson, 2001), lower-level perceptual processes may be influenced by the developmental increase in top–down control processes. Previous studies have shown modulation of lower-level regions, such as extrastriate visual areas, by top–down control processes such as attention ( Johnson, Mitchell, Raye, D’Esposito, & Johnson, 2007; Kotsoni, Csibra, Mareschal, & Johnson, 2007; Noesselt et al., 2002; Woldorff et al., 2002; Martinez et al., 1999). In the current study, we examine the hypothesis that the age-related increase in fronto-temporal effective connectivity found in linguistic processing (Bitan et al., 2006) reflects an increase in top–down control processes, and is more prominent in conditions with higher requirements of executive control. We further hypothesize that the increase in top–down modulation of task-relevant processing regions is accompanied by an increase in bottom–up modulation (i.e., the connectivity between the FG and the LTC). In the current study, the difficulty of the rhyming task was manipulated by including conditions with a conflict between orthographic and phonological information. The conflicting conditions require that participants respond to phonological information without distraction from task-irrelevant orthographic information. Previous studies have suggested that the left IFG is involved in biasing the processing in posterior regions to ensure that task-relevant information is selected (Milham, Banich, & Barad, 2003). We therefore predict that the conflicting conditions would show a larger developmental increase in both top–down connectivity (the inferior frontal cortex to the LTC) and bottom–up connectivity (the FG to the LTC) compared to conditions that do not entail a conflict.
METHODS
Subjects
Thirty-six healthy children (ages 9–15 years, mean = 11.7 years), including 22 female participants, participated in the study. Children were all right-handed (mean = 78, range 50–90) according to the 10-item Likert-scale questionnaire (−100 to 100, positive scores indicate right-hand dominance). All children were native English speakers with normal hearing and normal or corrected-to-normal vision. All children were free of neurological diseases or psychiatric disorders and were not taking medication affecting the central nervous system. Children were recruited from the Chicago metropolitan area. Parents of children were given an interview to ensure that they did not have a known deficit of intelligence, reading, attention, or oral-language skills. Children were given standardized intelligence tests (Wechsler Abbreviated Scale of Intelligence [WASI]; Wechsler, 1999) that showed a mean full-scale IQ = 113 (range = 85–130, SD = 15.3), verbal IQ = 114 (range = 79–142, SD = 14.8), and performance IQ = 108 (range = 78–140, SD = 14.7). The Institutional Review Board at Northwestern University and Evanston Northwestern Healthcare Research Institute approved the informed consent procedures.
Tasks
Word Judgment Tasks
Two words were presented visually in a sequential order and the participant had to determine whether the words rhymed. Each word was presented for 800 msec followed by a 200-msec blank interval. A red fixation cross appeared on the screen after the second word, indicating the need to make a response during the subsequent 2600-msec interval (Figure 1). Twenty-four word pairs were presented in each one of four lexical conditions that independently manipulated the orthographic and phonological similarity between words. In the two nonconflicting conditions, the two words were either similar in both orthography and phonology (O+P+, e.g., dime–lime), or different in both orthography and phonology (O−P−, e.g., press–list). In the two conflicting conditions, the two words had either similar orthography but different phonology (O+P−, e.g., pint–mint) or different orthography but similar phonology (O−P+, e.g., jazz–has). If there was a match, the participant pressed a button with the index finger; if there was no match, the participant pressed a different button with the middle finger.
Figure 1.
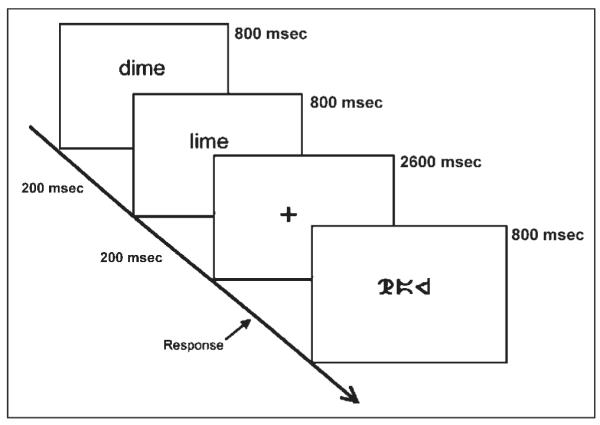
Time course of one word-judgment trial in the scanner.
Control Conditions
Two perceptual control conditions were used in which two symbol strings were presented visually in sequential order. In the “simple” condition, the symbol string consisted of a single symbol, whereas in the “complex” condition, the symbol string consisted of three different symbols; timing parameters were the same as for the lexical conditions. In both perceptual conditions, the participant had to determine whether the strings matched. Twenty-four items were presented in each perceptual condition, with half of them matching; in addition, 72 fixation trials were included as a baseline. In the fixation condition, a black fixation cross was presented for the same stimulus duration as in the lexical and perceptual conditions and a button was to be pressed when the black fixation cross turned red.
Stimulus Characteristics
All words were monosyllabic words and were matched across conditions for written word frequency in adults and children (Zeno, Ivens, Millard, & Duvvuri, 1996) and for written and spoken word frequency in adults (Baayen, Piepenbrock, & Gulikers, 1995). The symbols in the control conditions consisted of rearranged parts of lowercase courier letters. In the complex condition, a symbol did not repeat within any symbol string. Nonmatching pairs differed in one symbol, with the position of the nonmatching symbol equally distributed across the string. All words and symbols were presented in lowercase, at the center of the screen, with a 0.5 letter offset of position between the first and second stimulus.
Experimental Procedure
After informed consent was obtained and the standardized intelligence test was administered, participants were trained to minimize head movement using feedback from an infrared tracking device. In addition, they performed one run of the experimental task in a simulator scanner in order to make sure they understood the tasks and to acclimatize themselves to the scanner environment. Different stimuli were used in the practice and in the scanning sessions. Scanning took place within a week from the practice session. In the scanning session, two 8-min runs of 108 trials each were performed, in which lexical, perceptual, and fixation trials were intermixed and their pseudorandom order was optimized for event-related design (Burock, Buckner, Woldorff, Rosen, & Dale, 1998). The order of stimuli was fixed for all subjects.
MRI Data Acquisition
Images were acquired using a 1.5-Tesla GE scanner, using a standard head coil. Head movement was minimized using vacuum pillow (Bionix, Toledo, OH). The stimuli were projected onto a screen, and viewed through a mirror attached to the inside of the head coil. Participants’ responses were recorded using an optical response box (Current Designs, Philadelphia, PA). The BOLD functional images were acquired using the EPI method. The following parameters were used for scanning: TE = 35 msec, flip angle = 90°, matrix size = 64 × 64, field of view = 24 cm, slice thickness = 5 mm, number of slices = 24; TR = 2000 msec. Four runs, with 240 repetitions each, were administered for the functional images. In addition, structural T1-weighted 3-D image were acquired (SPGR, TR = 21 msec, TE = 8 msec, flip angle = 20°, matrix size = 256 × 256, field of view = 22 cm, slice thickness = 1 mm, number of slices = 124) using an identical orientation as the functional images.
Image Analysis
In the current study, we reanalyzed previously published data (Bitan, Burman, et al., 2007; Bitan, Cheon, et al., 2007). Data analysis was performed using SPM2 (Statistical Parametric Mapping) (www.fil.ion.ucl.ac.uk/spm). The images were spatially realigned to the first volume to correct for head movements. No individual runs had more than 4 mm maximum displacement, with an average of 1.2 mm per individual run. Sinc interpolation was used to minimize timing errors between slices (Henson, Buchel, Josephs, & Friston, 1999). The functional images were coregistered with the anatomical image, and normalized to the standard T1 template volume (MNI). The data were then smoothed with a 10-mm isotropic Gaussian kernel. Statistical analyses at the first level were calculated using an event-related design, with five conditions of interest: “conflicting,” “nonconflicting,” “perceptual,” “fixation,” and a “visual” condition that included “conflicting,” “nonconflicting,” and “perceptual” conditions. A high-pass filter with a cutoff period of 128 sec was applied. Word pairs were treated as individual events for analysis and modeled using a canonical HRF. Group results were obtained using random-effects analyses by combining subject-specific summary statistics across the group as implemented in SPM2 (Penny & Holmes, 2003). For descriptive purpose, all areas of activation are reported at the level of significance of uncorrected p < .001 at the voxel level and containing a cluster size greater than or equal to 10 voxels. The results of the lexical conditions versus fixation, which were used to identify VOIs for the effective connectivity analysis, are displayed with a more stringent threshold of uncorrected p < .0001 to enable the distinction among brain structures that otherwise comprise a single cluster. In all tables, clusters that are significant at p < .05 with an FWE correction are presented in bold.
Effective Connectivity Analysis
In order to avoid a bias toward conflicting or nonconflicting conditions, ROIs for the effective connectivity analysis were chosen based on activation in the contrast of all lexical conditions versus null. Four left hemisphere regions were chosen because they were identified in our previous effective connectivity studies as part of the language network involved in the phonological judgment of visually presented words (Bitan et al., 2005, 2006). These include the FG, previously associated with processing of visual word forms (Cohen et al., 2000); the IFG, associated with various linguistic processes including phonological and prearticulatory segmentation (Poldrack et al., 1999) as well as with executive control processes (Milham et al., 2003); the LTC, which was associated with phonological processing (Booth et al., 2004); and the IPS, which was associated with visual, spatial, and orthographic processing (Bitan, Burman, et al., 2007). The medial frontal gyrus (MeFG) was chosen as a fifth ROI because this region showed sensitivity to the conflict between orthography and phonology. The medial frontal and anterior cingulate gyri have been implicated in response monitoring and conflict resolution not specific for linguistic stimuli (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004; Milham et al., 2003).
Five ROIs were specified in the left hemisphere for each individual: FG, IFG, LTC, IPS, and MeFG. All ROIs were 6 mm radius spheres centered on the most significant voxel within 30 mm of the group maximum in the individual’s “lexicals versus null” activation map, restricted by an anatomical mask of the relevant region (i.e., IFG for IFG, superior and middle temporal gyri for LTC, and fusiform and inferior temporal gyri for FG, inferior and superior parietal lobules and precuneus for IPS, and medial frontal and anterior cingulate for MeFG). The relatively liberal criterion of 30 mm was chosen in order to account for intersubject variability in this potentially heterogeneous multiage sample. Because our main goal was to examine age correlations in connectivity measures, it was important to avoid suboptimal choice of regions for a particular age group. Although the use of an anatomical mask reduces the shortcomings of allowing such variability between individuals, there is still the risk that the same VOI will represent different functional regions in different individuals. A weaker voxel was chosen in individuals where the distance between the most significant voxels of the FG and the LTC were less than 26 mm apart. Three subjects were excluded because they had no significant clusters within 30 mm from the group reference voxel (two in the LTC and one in the IPS). Table 1 and Figure 2 show the results of the conventional analysis and the group reference for the ROIs.
Table 1.
Regions of Activation in All Lexical Conditions vs. Fixation
| Region | BA | H | Z Score | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|
| Fusiform/inferior occipital/middle occipital gyri | 37/18/19 | L | 7.62 | 641 | −45 | −69 | −12 |
| Inferior/middle frontal gyri | 47/45/9 | L | 7.32 | 1445 | −45 | 30 | 9 |
| Fusiform/inferior occipital/middle occipital gyri | 37/18/19 | R | 6.23 | 360 | 36 | −54 | −15 |
| Medial/superior frontal gyri | 8/6 | L | 5.74 | 235 | −6 | 15 | 48 |
| Superior temporal gyrus | 22 | L | 5.31 | 67 | −51 | −42 | 9 |
| Cuneus/calcarine sulci | 23/17 | L + R | 5.31 | 219 | −9 | −78 | 9 |
| Inferior frontal gyrus | 47 | R | 5.29 | 149 | 30 | 27 | 0 |
| Thalamus | – | L | 4.43 | 17 | −9 | −12 | 6 |
| Anterior cingulate gyrus | 24 | L | 4.31 | 26 | −3 | 3 | 27 |
| Superior parietal lobule/precuneus | 7 | L | 4.23 | 14 | −24 | −54 | 45 |
Clusters are presented with a threshold of uncorrected p < .0001, and extent of >10 voxels. Clusters significant at the threshold of corrected p < .05 are displayed in bold.
Figure 2.
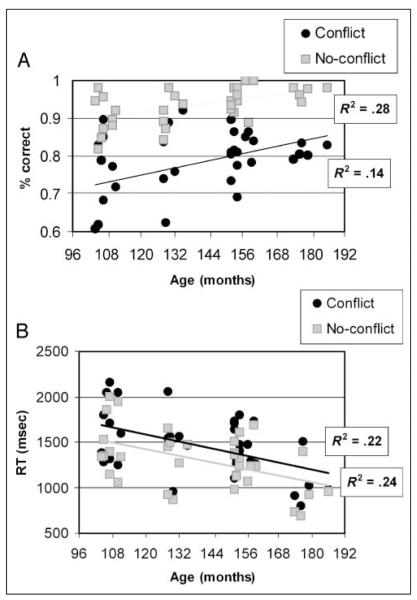
Correlation of age with performance in the scanner in terms of accuracy and reaction time.
Effective connectivity was examined using Dynamic Causal Modeling (DCM) in SPM2 (Penny, Stephan, Mechelli, & Friston, 2004; Friston, Harrison, & Penny, 2003), a system used for the estimation of directional effects among neural systems. In DCM, three sets of parameters are estimated: the direct influence of stimuli on regional activity, the intrinsic or latent connections between regions in the absence of modulating experimental effects, and the changes in the intrinsic connectivity between regions induced by the experimental design (modulatory effects) (Mechelli, Price, Noppeney, & Friston, 2003). Our analysis adopted a two-stage procedure that is formally identical to the summary statistic approach used in random effects analysis of neuroimaging data. The parameters from the subject-specific, first-level DCM models were taken to a second, between-subject level using the random effects approach (Bitan et al., 2005). Subject-specific DCMs were fully and reciprocally connected (resulting in 20 connections), with modulatory (bilinear) effects of the conflicting and the nonconflicting conditions specified on coupling among all regions. Direct input of the “visual” condition (which includes conflicting, nonconflicting and perceptual conditions) was specified on the FG. The validity of the effective connectivity analysis depends on the choice of regions accurately representing the nodes in the network involved in the task.
The second-level analysis was done on the bilinear effects of conflicting and nonconflicting conditions. Our main hypothesis was that top–down and bottom–up converging influences on the LTC increase with age, especially in conflicting conditions. To examine the effect of age on the converging connections from the IFG and the FG into the LTC, we performed a GLM analysis on the bilinear effects of conflicting and nonconflicting conditions on these connections. Within-subject variables for the model included 2 Conditions (conflict vs. no-conflict) × 2 Regions coupled to the LTC (IFG and FG) × 2 Directions (converging and diverging from LTC); three covariates were included as between-subject variables (age, accuracy in conflicting and accuracy in nonconflicting conditions). Accuracy was included in order to control for the level of performance while testing the effect of age. Because we found a significant four-way interaction of Age × Condition × Direction × Coupled region, we also conducted a separate GLM analysis within each coupled region: the IFG and FG ( p < .05 corrected for 2 comparisons), in order to ensure that the three-way interaction (Age × Condition × Direction) is significant in both top–down (IFG–LTC) and bottom–up (FG–LTC) connections. Within-subject variables for this model included 2 Conditions × 2 Directions, with age and accuracy as between-subject covariates.
Our second prediction was that we will replicate our previous findings showing greater bilinear effects of the rhyming task on converging influences on the LTC compared to converging influences on the IPS (Bitan et al., 2005, 2006). To examine this prediction, we performed a GLM analysis with three within-subject variables: 2 Conditions (conflict vs. no-conflict) × 2 Target regions (LTC vs. IPS) × 3 Source regions (FG, IFG, MeFG). The significant interactions were then followed up by separate analyses within each source region ( p < .05, corrected for 3 comparisons). Individual (intrinsic and bilinear) effects are reported with significance threshold of p < .05, corrected for 20 comparisons.
RESULTS
Behavioral
Figure 2 shows the correlation of performance accuracy and reaction time (RT) with age in conflicting and nonconflicting conditions. Accuracy increased with age, with a significant correlation, in both the conflicting (r = .38, p < .05) and nonconflicting (r = .52, p < .01) conditions. RT significantly decreased with age in both conflicting [r = (−.51), p < .01] and nonconflicting [r = (−.51), p < .01] conditions. GLM analyses on accuracy and RT were conducted, with two conditions (conflict vs. no-conflict) as within-subject variables and age as a between-subject covariate. These analyses revealed a significant effect of condition [F(1, 31) = 7.5; 4.44 for accuracy and RT respectively, p < .05] and a significant effect of age [F(1, 31) = 8.5; 11.27 for accuracy and RT, respectively, p < .001]. The interaction of age and condition was not significant for either accuracy or RT [F(1, 31) < 1]. [More details on the behavioral results are presented elsewhere (Bitan, Burman, et al., 2007; Bitan, Cheon, et al., 2007).]
fMRI Conventional Analysis
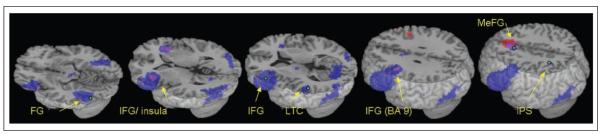
Table 1 and Figure 3 (blue) present regions that were active for all lexical conditions compared to fixation. These regions include left hemisphere clusters in the FG (including middle and inferior occipital gyri, BA 37, 18, 19); the IFG (including the middle frontal gyrus, BA 45, 47, 9); the LTC (including the superior temporal gyrus, BA 22); the IPS (including the superior parietal lobule and the precuneus, BA 7); and the MeFG (including the superior frontal gyrus, BA 8, 6). These clusters served as ROIs for the effective connectivity analysis. Table 2 and Figure 3 (red) present regions that were more active for conflicting compared to nonconflicting conditions. These are largely overlapping with regions that are active in all lexical conditions (purple) and consist of the MeFG and two bilateral clusters in the IFG (BA 47 and 9).
Figure 3.
Activation in all lexical conditions versus fixation (blue), p < .0001, overlaid on the differential activation for Conflicting vs. Nonconflicting conditions (red), p < .001 and their overlap (purple). Green dots indicate group reference for ROI specification. FG = fusiform gyrus; IFG = inferior frontal gyrus; IPS = intraparietal sulcus; LTC = lateral temporal cortex; and MeFG = medial frontal gyrus.
Table 2.
Regions of Greater Activation for Conflicting vs. Nonconflicting Conditions
| Region | BA | H | Z Score | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|
| Superior frontal/medial frontal/middle cingulate gyri | 6/8/31 | L | 4.75 | 301 | −3 | 21 | 48 |
| Inferior/middle frontal gyri | 9 | R | 4.04 | 17 | 42 | 9 | 30 |
| Insula/inferior frontal gyrus | 13/47 | L | 3.89 | 69 | −36 | 24 | 0 |
| Insula/inferior frontal gyrus | 13/47 | R | 3.79 | 46 | 36 | 27 | −3 |
| Inferior frontal gyrus | 9 | L | 3.69 | 32 | −39 | 6 | 27 |
| Middle frontal gyrus | 46 | R | 3.39 | 11 | 48 | 36 | 27 |
Clusters are presented with a threshold of uncorrected p < .001, and extent of >10 voxels. Clusters significant at the threshold of corrected p < .05 are displayed in bold.
fMRI Effective Connectivity
Table 3 shows that all intrinsic connections among regions were significant. Table 3 also shows the strength and significance of the bilinear effects of conflicting and nonconflicting conditions. Figure 4 shows the significant bilinear effects across ages. The nonconflicting conditions modulated all the effects converging into the IFG, and the effects of the FG and IFG on the LTC. The conflicting conditions modulated all converging effects on the IFG, the LTC, and the MeFG.
Table 3.
Strength of Intrinsic and Modulatory Effects in Dynamic Causal Modeling
| From: | FG | IFG | IPS | LTC | MeFG | |
|---|---|---|---|---|---|---|
| Intrinsic | ||||||
| To: | FG | 0.073 | 0.049 | 0.066 | 0.048 | |
| IFG | 0.247 | 0.121 | 0.174 | 0.144 | ||
| IPS | 0.171 | 0.118 | 0.093 | 0.097 | ||
| LTC | 0.149 | 0.158 | 0.092 | 0.097 | ||
| MeFG | 0.167 | 0.149 | 0.106 | 0.113 | ||
| Conflicting | ||||||
| To: | FG | 0.014 | 0.009 | 0.01 | 0.009 | |
| IFG | 0.163 | 0.014 | 0.018 | 0.015 | ||
| IPS | 0.063 | 0.009 | 0.007 | 0.009 | ||
| LTC | 0.147 | 0.02 | 0.012 | 0.013 | ||
| MeFG | 0.087 | 0.012 | 0.01 | 0.009 | ||
| Nonconflicting | ||||||
| To: | FG | 0.001 | 0.001 | 0.001 | 0 | |
| IFG | 0.118 | 0.014 | 0.016 | 0.015 | ||
| IPS | 0.025 | 0.005 | 0.003 | 0.004 | ||
| LTC | 0.09 | 0.016 | 0.011 | 0.011 | ||
| MeFG | 0.039 | 0.009 | 0.007 | 0.008 | ||
Significant effects (corrected p < .05) are presented in bold. Units are arbitrary.
Figure 4.
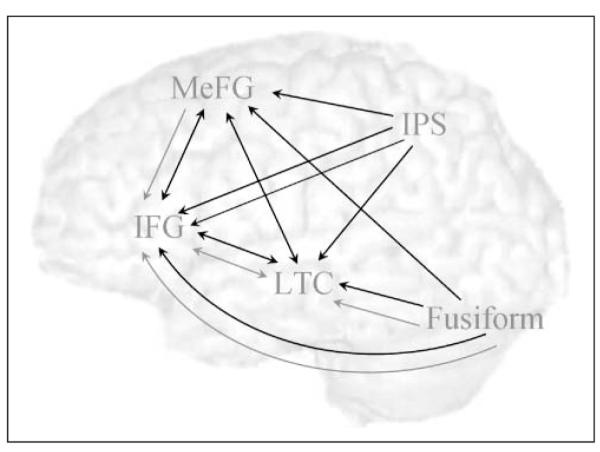
Significant bilinear conflicting (black) and nonconflicting (gray) effects.
The Effect of Age
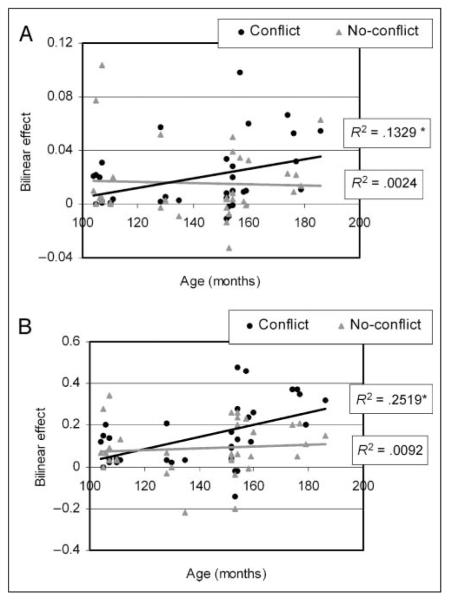
In order to examine our main hypothesis regarding the effect of age on the converging influences on the LTC from the IFG and the FG, we performed a GLM analysis with 2 Conditions (conflict vs. no-conflict) × 2 Coupled regions (IFG and FG) × 2 Directions (converging and diverging) as within-subject variables and three covariates (age, accuracy in conflicting and accuracy in nonconflicting) as between-subject variables. The results show that the interaction of age and condition was significant [F(1, 29) = 13.63, p < .01], showing an increase in strength of bilinear effect with age, but only in conflicting conditions with both coupled regions. The results also show a significant interaction of Condition × Age × Direction [F(1, 29) = 13.61, p < .01], indicating a stronger interaction of age and condition for converging connections on the LTC. Because the four-way interaction of Condition × Age × Direction × Coupled region was also significant [F(1, 29) = 11.47, p < .01], we performed a GLM analysis within each coupled region: the IFG and FG (2 conditions × 2 directions with age and accuracy as covariates), in order to ensure that the three-way interaction (Age × Condition × Direction) is significant in both top–down (IFG–LTC) and bottom–up (FG–LTC) connections. The interaction of Condition × Direction × Age was significant for the coupling of the LTC with the IFG [F(1, 29) = 7.9, p < .01] and for the coupling of the LTC with the FG [F(1, 29) = 12.81, p < .01]. Figure 5 shows that the effect of conflicting conditions on connections converging into the LTC increases with age (r = .47, p < .01 for FG–LTC; r = .37, p < .05 for IFG–LTC). The correlations of age with nonconflicting conditions were not significant [r = (−.12) for FG–LTC, r = (−.23) for IFG–LTC]. The effect of age on the diverging connections from the LTC was not significant for either conflicting or nonconflicting conditions. There was no significant main effect or interaction for accuracy.
Figure 5.
Correlation of age with the bilinear effects of conflicting and nonconflicting conditions on the converging connection from the IFG (A) and the FG (B) into the LTC. The proportion of variance in the bilinear effects explained by the correlation with age is presented (R2). * indicates a significant correlation.
Comparison of the LTC and the IPS
To test the replication of our previous results, we examined the hypothesis that converging influences on the LTC are stronger than converging influences on the IPS. To do that we performed a three-way analysis with 2 Conditions (conflict vs. no-conflict) × 2 Target regions (LTC vs. IPS) × 3 Source regions (FG, IFG, MeFG). Significant main effects of condition [F(1, 32) = 7.2], target region [F(1, 32) = 15.7], and source region [F(2, 64) = 22.2] were found, as well as significant two-way interaction effects of Condition by Source region [F(2, 64) = 9.3] and Target region by Source region [F(2, 64) = 13.4] ( p < .05). Separate analyses within each source region showed a significant effect of target region in all source regions [F(1, 32) = 14.5, 13.6 and 10.5 for the FG, IFG, and MeFG ,respectively; corrected p < .05]. Converging influences on the LTC were stronger compared to influences on the IPS.
DISCUSSION
In the current study, we examined the hypothesis that top–down control processes, involved in enhancing task-relevant information in a phonological judgment task, increases with age. We further hypothesized that this increase in top–down processes is associated with a similar increase in bottom–up influences from lower-level perceptual regions to task-relevant regions. Our results support these hypotheses, showing an age-related increase in the effect of the left IFG and the left FG on the left LTC, but only in conditions that entail a conflict between task-relevant and task-irrelevant information.
The results also replicate our previous findings (Bitan et al., 2005, 2006) showing convergence of information into the LTC in the rhyming task, and extend them to the age groups examined here. In the current study, the bilinear effects on the converging influences on the LTC were stronger than the influences on the IPS. These results are consistent with the involvement of the LTC in phonological processing (Booth et al., 2002; Xu et al., 2001; Kareken, Lowe, Chen, Lurito, & Mathews, 2000; Lurito, Kareken, Lowe, Chen, & Mathews, 2000; Crosson et al., 1999; Paulesu et al., 1996; Pugh et al., 1996), whereas the IPS was implicated in orthographic judgment in the spelling task (Bitan et al., 2005, 2006).
Developmental Increase in Top–down Control
The finding of age-related increase in the influence of the left IFG on the left LTC replicates our previous results found in the comparison of adults and children on the rhyming task. However, in the current study, our subjects were all children of different ages, and we manipulated the difficulty of the rhyming task by including conditions with a conflict between orthographic and phonological information. As expected, the developmental increase in effective connectivity was only evident for the conditions that entail a conflict between task-relevant (phonological) and task-irrelevant (orthographic) information. These results suggest that the effect of the IFG on the LTC in older children is selectively enhancing task-relevant information, and ignoring task-irrelevant information, thus contributing to the resolution of the conflict. These findings reflect a developmental increase in top–down control processes associated with the prefrontal cortex.
The role of different subregions in the prefrontal cortex in top–down control processes has been highlighted in neurophysiological, behavioral, and neuroimaging studies (Aron, Monsell, Sahakian, & Robbins, 2004; Miller, 2000; Duncan, Emslie, Williams, Johnson, & Freer, 1996; Watanabe, 1992; Fuster, 1989; Pandya & Barnes, 1987). It has been suggested that the prefrontal cortex provides biasing signals to other brain structures that guide the flow of activity in order to map between inputs and the appropriate outputs according to the requirements of a given task. This is especially important when multiple responses are possible, and when task-appropriate response must compete with stronger alternatives (Miller & Cohen, 2001). Furthermore, in an attempt to differentiate between specific executive control functions within the prefrontal cortex, Milham et al. (2003) have analyzed the involvement of specific regions in the Stroop task. The authors suggest that the dorsal left IFG is involved in top–down biasing of processing in posterior processing systems to ensure that task-relevant information is selected (Milham et al., 2003).
The developmental increase in top–down control processes is supported not only by behavioral studies showing an improvement in executive control functions with age (Davidson et al., 2006; Marsh et al., 2006; Crone et al., 2004) but also by functional neuroimaging studies showing increased activation with age in prefrontal regions during executive control tasks (Badre, Poldrack, Pare’-Blagoev, Insler, & Wagner, 2005; Konrad et al., 2005; Luna & Sweeney, 2004; Adleman et al., 2002; Tamm, Menon, & Reiss, 2002; Luna et al., 2001). Further support comes from anatomical studies showing protracted maturation of prefrontal association areas and their connectivity to posterior regions (Gogtay et al., 2004; Huttenlocher & Dabholkar, 1997). Recently, a structural connectivity study using DTI (Zhang et al., 2007) showed an age-related increase in the superior longitudinal fasciculus (SLF) known to be projecting to language areas such as the IFG, the superior temporal gyrus, and the supramarginal gyrus (Catani, Jones, & ffytche, 2005; Wakana, Jiang, Nagae-Poetscher, van Zijl, & Mori, 2004; Makris et al., 1997).
Effective and functional connectivity analyses enable a more direct measure of top–down influences. Few studies examined developmental changes in functional and effective connectivity. One MRI study that examined resting state functional connectivity of the control network showed a development increase in long-range connections including connections between temporal and prefrontal regions (Fair et al., 2007). In a narrative comprehension task, effective connectivity from the left IFG to the posterior bilateral superior temporal gyri increased with age in children 5 to 18 years (Schmithorst, Holland, & Plante, 2007). These studies are consistent with the results of the current study, which provides direct evidence for the developmental increase in top–down effective connectivity.
Developmental Increase in Bottom–up Processes in Conflict
Our results show a developmental increase in the bilinear effect of conflicting conditions not only on the connection from the IFG to the LTC but also on the bottom–up influence from the FG to the LTC. The FG is a lower-level region thought to be involved in orthographic processing (Starrfelt & Gerlach, 2007; Binder, Medler, Westbury, Liebenthal, & Buchanan, 2006; Booth et al., 2002; Cohen et al., 2000; Nobre, Allison, & McCarthy, 1994). Although lower-level regions appear to mature earlier than higher-level association areas ( Johnson, 2001), the developmental increase in bottom–up influences is consistent with the hypothesis that top–down modulation on the LTC affects its sensitivity to bottom–up information coming from the FG. The enhanced influence of the IFG on the LTC in older children in conflicting conditions may result in the enhancement of influence from the FG to the LTC in the same conditions. Previous studies have shown that activation in lower-level input regions was modulated by top–down control processes (Shulman et al., 1997). For example, studies that examined the effect of attention manipulation on visual detection tasks showed reactivation of input regions enhanced by attention ( Johnson et al., 2007; Kotsoni et al., 2007; Noesselt et al., 2002; Woldorff et al., 2002; Martinez et al., 1999). Similarly, attention modulated activation in the primary somatosensory cortex (Sterr, Shen, Zaman, Roberts, & Szameitat, 2007). Furthermore, functional connectivity analysis in an auditory and visual oddball task showed increased connectivity between the anterior cingulate associated with top–down attention control and primary regions in the respective modality (Crottaz-Herbette & Menon, 2006).
Conclusions
Our results show a developmental increase in the convergence of both top–down and bottom–up information into the LTC in the rhyming task. This increase is specific to conditions that entail a conflict between phonological and orthographic information. These results suggest that top–down control processes, involved in selective enhancement of task relevant information and exerted from the inferior frontal cortex on the LTC, increase with age. This modulation effect may enhance the sensitivity of the LTC to task-relevant information arriving from the FG, resulting in reactivation of orthographic representations in conflicting conditions. Our results also replicate our previous findings showing convergence of information into the LTC during a phonological judgment task, and show that this convergence of information increases with age.
REFERENCES
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, et al. A developmental fMRI study of the Stroop color–word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, Gulikers L. The CELEX Lexical Database (Version Release 2) [CD-ROM] Linguistic Data Consortium, University of Pennsylvania; Philadelphia, PA: 1995. [Google Scholar]
- Badre D, Poldrack RA, Pare’-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Berl MM, Vaidya CJ, Gaillard WD. Functional imaging of developmental and adaptive changes in neurocognition. Neuroimage. 2006;30:679–691. doi: 10.1016/j.neuroimage.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage. 2006;33:739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam M-M. Shifts of effective connectivity within a language network during rhyming and spelling. Journal of Neuroscience. 2005;25:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Chou T, Lu D, Cone NE, Cao F, et al. The interaction between orthographic and phonological information in children: An fMRI study. Human Brain Mapping. 2007;28:880–891. doi: 10.1002/hbm.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Lu D, Cone NE, Gitelman DR, Mesulam M-M, et al. Weaker top–down modulation from the left inferior frontal gyrus in children. Neuroimage. 2006;33:991–998. doi: 10.1016/j.neuroimage.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Gitelman DR, Mesulam M-M, et al. Developmental changes in activation and effective connectivity in phonological processing. Neuroimage. 2007;38:564–575. doi: 10.1016/j.neuroimage.2007.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. Neuroimage. 2002;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Development of brain mechanisms for processing orthographic and phonologic representations. Journal of Cognitive Neuroscience. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. NeuroReport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: What have we learned about cognitive development? Trends in Cognitive Sciences. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones KD, ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, et al. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Crone EA, Ridderinkhof KR, Worm M, Somsen RJ, van der Molen MW. Switching between spatial stimulus–response mappings: A developmental study of cognitive flexibility. Developmental Science. 2004;7:443–455. doi: 10.1111/j.1467-7687.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- Crosson B, Rao SM, Woodley SJ, Rosen AC, Bobholz JA, Mayer A, et al. Mapping of semantic, phonological, and orthographic verbal working memory in normal adults with functional magnetic resonance imaging. Neuropsychology. 1999;13:171–187. doi: 10.1037//0894-4105.13.2.171. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: Combined fMRI and ERP evidence. Journal of Cognitive Neuroscience. 2006;18:766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MC, Thomas KM, Casey BJ. Imaging the developing brain with fMRI. Mental Retardation and Developmental Disabilities Research Reviews. 2003;9:161–167. doi: 10.1002/mrdd.10076. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Archives of Disease in Childhood. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Emslie H, Williams P, Johnson R, Freer C. Intelligence and the frontal lobe: The organization of goal-directed behavior. Cognitive Psychology. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences, U.S.A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. Raven Press; New York: 1989. [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Human Brain Mapping. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, U.S.A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R, Buchel C, Josephs O, Friston K. The slice-timing problem in event-related fMRI. Neuroimage. 1999;9:S125. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars AW, Strawsburg RH, Schmithorst VJ, Ball WS. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nature Reviews Neuroscience. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Mitchell KJ, Raye CL, D’Esposito M, Johnson MK. A brief thought can modulate activity in extrastriate visual areas: Top–down effects of refreshing just-seen visual stimuli. Neuroimage. 2007;37:290–299. doi: 10.1016/j.neuroimage.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Lowe M, Chen SHA, Lurito J, Mathews V. Word rhyming as a probe of hemispheric language dominance with functional magnetic resonance imaging. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2000;13:264–270. [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. NeuroReport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, et al. Development of attentional networks: An fMRI study with children and adults. Neuroimage. 2005;28:429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Kotsoni E, Csibra G, Mareschal D, Johnson MH. Electrophysiological correlates of common-onset visual masking. Neuropsychologia. 2007;45:2285–2293. doi: 10.1016/j.neuropsychologia.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Lurito JT, Kareken DA, Lowe MJ, Chen SH, Mathews VP. Comparison of rhyming and word generation with fMRI. Human Brain Mapping. 2000;10:99–106. doi: 10.1002/1097-0193(200007)10:3<99::AID-HBM10>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Worth AJ, Papadimitriou GM, Stakes JW, Caviness VS, Kennedy DN, et al. Morphometry of in vivo human white matter association pathways with diffusion-weighted magnetic resonance imaging. Annals of Neurology. 1997;42:951–962. doi: 10.1002/ana.410420617. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, et al. A developmental fMRI study of self-regulatory control. Human Brain Mapping. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, et al. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nature Neuroscience. 1999;2:364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Noppeney U, Friston KJ. A dynamic causal modeling study on category effects: Bottom–up or top–down mediation? Journal of Cognitive Neuroscience. 2003;15:925–934. doi: 10.1162/089892903770007317. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V. Competition for priority in processing increases prefrontal cortex’s involvement in top–down control: An event-related fMRI study of the Stroop task. Brain Research, Cognitive Brain Research. 2003;17:212–222. doi: 10.1016/s0926-6410(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Hillyard SA, Woldorff MG, Schoenfeld A, Hagner T, Jancke L, et al. Delayed striate cortical activation during spatial attention. Neuron. 2002;35:575–587. doi: 10.1016/s0896-6273(02)00781-x. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Macoveanu J, Tegner J, Klingberg T. Brain activity related to working memory and distraction in children and adults. Cerebral Cortex. 2007;17:1047–1054. doi: 10.1093/cercor/bhl014. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Barnes CL. In: The frontal lobes revisited. Perecman E, editor. IRBN Press; New York: 1987. pp. 41–72. [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RSJ, et al. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, et al. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Penny WD, Holmes A. Random effects analysis. In: Frackowiak RSJ, Friston KJ, Frith CD, editors. Human brain function. 2nd ed Academic Press; San Diego: 2003. pp. 843–850. [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Modelling functional integration: A comparison of structural equation and dynamic causal models. Neuroimage. 2004;23(Suppl 1):S264–S274. doi: 10.1016/j.neuroimage.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, et al. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Schapiro MB, Schmithorst VJ, Wilke M, Byars AW, Strawsburg RH, Holland SK. BOLD fMRI signal increases with age in selected brain regions in children. NeuroReport. 2004;15:2575–2578. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E. Development of effective connectivity for narrative comprehension in children. NeuroReport. 2007;18:1411–1415. doi: 10.1097/WNR.0b013e3282e9a4ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM, et al. Top–down modulation of early sensory cortex. Cerebral Cortex. 1997;7:193–206. doi: 10.1093/cercor/7.3.193. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: A structural MRI study. Journal of the International Neuropsychological Society. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Starrfelt R, Gerlach C. The visual what for area: Words and pictures in the left fusiform gyrus. Neuroimage. 2007;35:334–342. doi: 10.1016/j.neuroimage.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Fink GR, Marshall JC. Mechanisms of hemispheric specialization: Insights from analyses of connectivity. Neuropsychologia. 2007;45:209–228. doi: 10.1016/j.neuropsychologia.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterr A, Shen S, Zaman A, Roberts N, Szameitat A. Activation of SI is modulated by attention: A random effects fMRI study using mechanical stimuli. NeuroReport. 2007;18:607–611. doi: 10.1097/WNR.0b013e3280b07c34. [DOI] [PubMed] [Google Scholar]
- Stuss DT. Biological and psychological development of executive functions. Brain and Cognition. 1992;20:8–23. doi: 10.1016/0278-2626(92)90059-u. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Annals of Neurology. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Frontal units of the monkey coding the associative significance of visual and auditory stimuli. Experimental Brain Research. 1992;89:233–247. doi: 10.1007/BF00228241. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Woldorff MG, Liotti M, Seabolt M, Busse L, Lancaster JL, Fox PT. The temporal dynamics of the effects in occipital cortex of visual–spatial selective attention. Brain Research, Cognitive Brain Research. 2002;15:1–15. doi: 10.1016/s0926-6410(02)00212-4. [DOI] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, et al. Conjoint and extended neural networks for the computation of speech codes: The neural basis of selective impairment in reading words and pseudowords. Cerebral Cortex. 2001;11:267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]
- Zeno SM, Ivens SH, Millard RT, Duvvuri R. The Educator’s Word Frequency Guide [CD-ROM, DOS version] Touchstone Applied Science Associates; Brewster, NY: 1996. [Google Scholar]
- Zhang J, Evans A, Hermoye L, Lee S-K, Wakana S, Zhang W, et al. Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. Neuroimage. 2007;38:239–247. doi: 10.1016/j.neuroimage.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]