Abstract
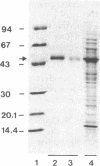
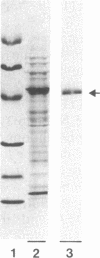
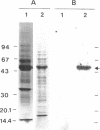
Phytoene is the first C40 intermediate in the biogenesis of carotenoids. It is formed by two enzyme activities, catalyzing (i) the coupling of two molecules of geranylgeranyl diphosphate to yield prephytoene diphosphate and (ii) the conversion of prephytoene diphosphate into phytoene. We show now, with Capsicum chromoplast stroma, that the overall activity resides in a single protein, which has been purified to homogeneity by affinity chromatography. The monomeric structure and the molecular size (Mr 47,500) were demonstrated by NaDodSO4/PAGE and glycerol gradient centrifugation. Further characterization was achieved by using specific antibodies which allowed immunofractionation and immunoprecipitation of the enzymatic activity from chromoplast stroma. The two reactions followed conventional Michaelis-Menten kinetics, with Km values of 0.30 μM and 0.27 μM, respectively, for geranylgeranyl diphosphate and prephytoene diphosphate. The activity of the enzyme depends strictly upon the presence of Mn2+. This selectivity may be one of the factors regulating the competition with potentially rival enzymes converting geranylgeranyl diphosphate into other plastid terpenoids. The two enzymatic reactions were inhibited by inorganic pyrophosphate and by the arginine-specific reagent hydroxyphenylglyoxal. In no instance were the two reactions kinetically uncoupled. These properties strongly suggest that the same enzyme catalyzes the two consecutive reactions, and we propose to name it phytoene synthase.
Keywords: Capsicum annuum, plastid terpenoids, geranylgeranyl-diphosphate geranylgeranyltransferase, phytoene synthase, enzyme regulation
Full text
PDF

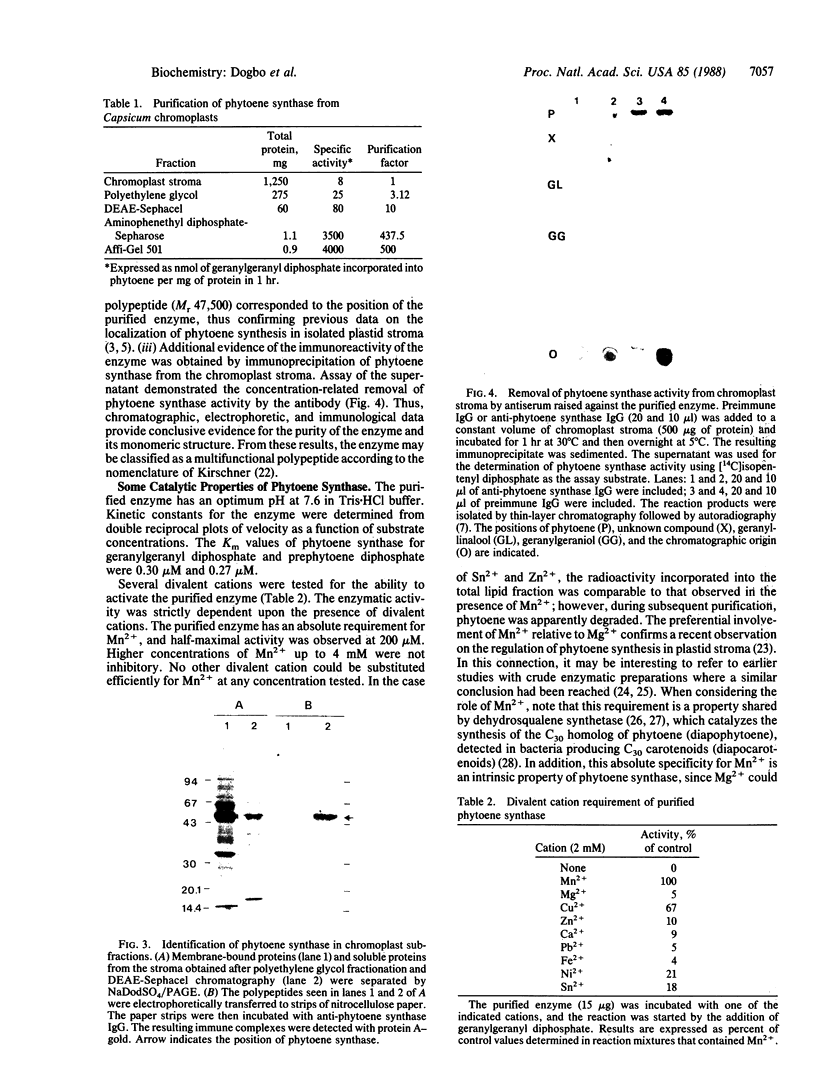

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S., Popják G. Squalene synthetase. Solubilization from yeast microsomes of a phospholipid-requiring enzyme. J Biol Chem. 1978 Jul 10;253(13):4574–4583. [PubMed] [Google Scholar]
- Altman L. J., Ash L., Kowerski R. C., Epstein W. W., Larsen B. R., Rilling H. C., Muscio F., Gregonis D. E. Prephytoene pyrophosphate. A new intermediate in the biosynthesis of carotenoids. J Am Chem Soc. 1972 May 3;94(9):3257–3259. doi: 10.1021/ja00764a073. [DOI] [PubMed] [Google Scholar]
- Brada D., Roth J. "Golden blot"--detection of polyclonal and monoclonal antibodies bound to antigens on nitrocellulose by protein A-gold complexes. Anal Biochem. 1984 Oct;142(1):79–83. doi: 10.1016/0003-2697(84)90518-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Camara B., Bardat F., Monéger R. Sites of biosynthesis of carotenoids in Capsicum chromoplasts. Eur J Biochem. 1982 Oct;127(2):255–258. doi: 10.1111/j.1432-1033.1982.tb06863.x. [DOI] [PubMed] [Google Scholar]
- Camara B. Terpenoid metabolism in plastids : sites of phytoene synthetase activity and synthesis in plant cells. Plant Physiol. 1984 Jan;74(1):112–116. doi: 10.1104/pp.74.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswik-Rabiega G., Rilling H. C. Squalene synthetase. Solubilization and partial purification of squalene synthetase, copurification of presqualene pyrophosphate and squalene synthetase activities. J Biol Chem. 1987 Feb 5;262(4):1505–1509. [PubMed] [Google Scholar]
- Maudinas B., Bucholtz M. L., Papastephanou C., Katiyar S. S., Briedis A. V., Porter J. W. The partial purification and properties of a phytoene synthesizing enzyme system. Arch Biochem Biophys. 1977 Apr 30;180(2):354–362. doi: 10.1016/0003-9861(77)90049-2. [DOI] [PubMed] [Google Scholar]
- Moeremans M., Daneels G., Van Dijck A., Langanger G., De Mey J. Sensitive visualization of antigen-antibody reactions in dot and blot immune overlay assays with immunogold and immunogold/silver staining. J Immunol Methods. 1984 Nov 30;74(2):353–360. doi: 10.1016/0022-1759(84)90303-x. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Ogura K., Koyama T., Shibuya T., Nishino T., Seto S. Inhibitory effect of substrate analogs on isopentenyl pyrophosphate isomerase and prenyltransferase. J Biochem. 1969 Jul;66(1):117–118. doi: 10.1093/oxfordjournals.jbchem.a129113. [DOI] [PubMed] [Google Scholar]
- Riordan J. F., McElvany K. D., Borders C. L., Jr Arginyl residues: anion recognition sites in enzymes. Science. 1977 Mar 4;195(4281):884–886. doi: 10.1126/science.190679. [DOI] [PubMed] [Google Scholar]
- Sasiak K., Rilling H. C. Purification to homogeneity and some properties of squalene synthetase. Arch Biochem Biophys. 1988 Feb 1;260(2):622–627. doi: 10.1016/0003-9861(88)90490-0. [DOI] [PubMed] [Google Scholar]
- Suzue G., Tsukada K., Tanaka S. Occurrence of dehydrosqualene (C30 phytoene) in Staphylococcus aureus. Biochim Biophys Acta. 1968 Sep 2;164(1):88–93. doi: 10.1016/0005-2760(68)90074-x. [DOI] [PubMed] [Google Scholar]
- Takatsuji H., Nishino T., Izui K., Katsuki H. Formation of dehydrosqualene catalyzed by squalene synthetase in Saccharomyces cerevisiae. J Biochem. 1982 Mar;91(3):911–921. doi: 10.1093/oxfordjournals.jbchem.a133780. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartak H. G., Rele M. V., Rao M., Deshpande V. V. A method for concentrating dilute solutions of macromolecules. Anal Biochem. 1983 Aug;133(1):260–263. doi: 10.1016/0003-2697(83)90252-x. [DOI] [PubMed] [Google Scholar]
- Welch G. R. On the free energy "cost of transition" in intermediary metabolic processes and the evolution of cellular infrastructure. J Theor Biol. 1977 Sep 21;68(2):267–291. doi: 10.1016/0022-5193(77)90165-5. [DOI] [PubMed] [Google Scholar]