Abstract
The greatest challenge in cancer treatment is to achieve the highest levels of specificity and efficacy. Cancer gene therapy could be designed specifically to express therapeutic genes to induce cancer cell destruction. Cancer-specific promoters are useful tools to accomplish targeted expression; however, high levels of gene expression are needed to achieve therapeutic efficacy. Incorporating an imaging reporter gene in tandem with the therapeutic gene will allow tangible proof of principle that gene expression occurs at the correct location and at a sufficient level. Gene-based imaging can advance cancer detection and diagnosis. By combining the cancer-targeted imaging and therapeutic strategies, the exciting prospect of a ‘one-two punch’ to find hidden, disseminated cancer cells and destroy them simultaneously can potentially be realized.
Multiple genetic alterations that confer growth advantages to tumor cells are accumulated during the transformation from normal to neoplastic growth [1]. The loss of growth suppressive genes or gain of oncogenes constitutes a common mechanism of oncogenesis. Based on this information, a rational therapeutic approach is to re-introduce the defective growth control genes into tumor cells. In addition, approaches of inducing apoptotic responses and enhancing anti-tumor immune responses have also been employed [2]. Due to the availability of multiple flexible therapeutic strategies, gene therapy is being actively investigated in clinical settings.
Among the ~ 40 ongoing gene therapy clinical trials for cancer (searched via www.clinicaltrials.gov/), 18 of the trial protocols involve an immune activating scheme, ten studies employ a genetic correction strategy and five use a cytotoxic gene. With regard to genetic correction strategies, p53 is a major target. The recombinant adenovirus is the dominant viral gene delivery vector, and it is employed in 18 protocols. However, none of 40 protocols use a cancer-specific gene expression strategy. An oncolytic adenovirus CN706 containing prostate-specific antigen (PSA) promoter driven viral replication [3] is being evaluated in phase II clinical trial for prostate cancer [4].
Because the goal of cancer gene therapy is to eradicate cancer cells, many therapeutic genes could be detrimental if unintentionally expressed in normal cells. Selectively targeting the cancer cells is useful to achieve safety and efficacy, especially when the gene therapy vector is directly delivered into patients. Based on features that distinguish cancerous from normal cells, three targeting strategies could be employed. Transcriptional targeting takes advantage of the fact that some cancer cells express a subset of exclusive genes, and uses these cancer-specific promoters to express the desired transgenes [5]. Transductional targeting refers to surface modification on the gene delivery vehicle to enhance interactions with the cancer cell membrane antigen, thereby improving gene transfer into the cancerous cell. A third promising approach is to exploit cancer-associated cellular pathways to activate therapy. For example, the attenuated adenovirus dl1520 (ONXY-015), lacking viral E1B 55k protein, was reported to selectively replicate and kill p53 deficient tumor cells and not normal cells ([2] and references within). In another example, the cytotoxic activity of a fusogenic glycoprotein (GALV) was engineered to be activated by matrix metalloproteinase (MMP) cleavage of a blocking domain [6]. This modulated GALV exhibited selective cytotoxicity to MMP-expressing glioma cells, while sparing normal human astrocytes.
In this review, we will focus on transcriptional targeting for cancer, and discuss strategies to amplify the magnitude of specific expression and the use of imaging modalities to monitor transgene expression in living animals.
Transcriptional targeting
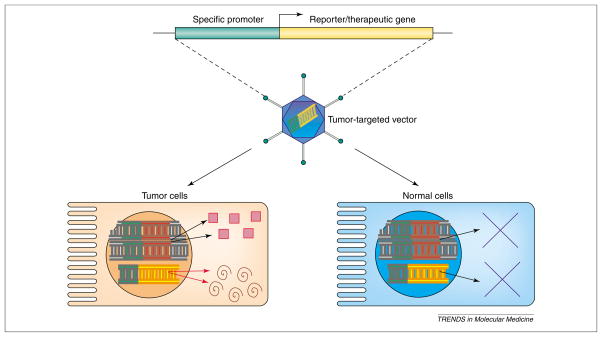
The transcriptional regulatory regions of a gene control the kinetics and levels of mRNA production. Typically, the gene regulatory regions can be subdivided into proximal promoter and distal enhancer elements, gauged by the distance from the start site of transcription [7]. A complex array of transcription factors bind to these regulatory regions. Complex coordinated actions of the activators recruit the RNA polymerase II general machinery to the promoter and initiate transcription of the gene. Some activators are ubiquitously expressed, whereas others are restricted to certain cell types [7]. Cell-specific expression can be thought of as being mediated by a unique subset of ubiquitous and specific activators present in the cell. Transcriptional targeting is feasible because the tissue- or cancer-specific promoter can be activated in the targeted cancer cell in the presence of the proper subset of activators but would remain silent in the non-targeted cell (Figure 1).
Figure 1.
Schematic representation of transcriptionally targeted gene expression. Tissue- or cancer-specific promoter-driven reporter or therapeutic gene is incorporated into a gene delivery vector (depicted as recombinant adenovirus here). Gene transfer can occur in both targeted cancer cells and non-targeted normal cells. However, trans-gene expression can only occur in cancer cells due to the presence of transcription factors competent to mediate expression of the specific promoter. Red squares denote the endogenous gene product expressed from the specific promoter. The swirls denote the reporter/therapeutic gene product.
Many tissue-specific promoters have been applied to targeted gene therapy (Table 1). Testing in animal models showed that specific promoters exhibit a clear advantage of reduced cytotoxicity, compared with a strong constitutive promoter such as the human cytomegalovirus (CMV) promoter currently used in clinical trials. For example, when Fas ligand expression was driven by neuronal tissue-specific promoters such as glial fibrillary acidic protein (GFAP) or neuronal-specific enolase (NSE), no hepatocyte apoptosis manifested as acute liver hemorrhage was observed [8]. Despite the tumor-directed injection of the CMV-driven interleukin (IL)-12 adenoviral vector, serious side effects resulted from systemic immune activation [9]. The use of an inducible promoter based on the heat shock proteins (hsp70B) greatly limited IL-12 transgene-mediated toxicity [9].
Table 1.
Tissue- and cancer-specific promoters used in targeted gene therapya
| Promoter | Target tumor type | Summary | Refs. |
|---|---|---|---|
| GFAP | Glioma | Ad with GFAP or NSE promoter driving FasL exhibited diminished liver toxicity | [7] |
| Tyrosinase | Melanoma | Tissue-specific expression combined with E1A mutation exhibited highly selective oncolysis of melanoma | [9] |
| PSA | Prostate cancer | Ad with E1A driven by PSA promoter destroyed LNCaP tumors | [16] |
| Ad with chimeric PSA promoter driving luciferase-visualized metastasized prostate cancer lesions | [18] | ||
| ALA | Breast cancer | Ad with ALA or β-lactoglobulin promoter driving HSV-TK exhibited regression of breast cancer in animal model | [12] |
| CEA | Digestive tract cancer | Cytotoxic gene therapy approach with viral vector using CEA promoter | [23,24] |
| AFP | Hepatocellular carcinoma | Cytotoxic or immunotherapy for hepatocellular carcinoma with viral vector regulated by AFP promoter | [20,21] |
| HIF-1α | Tumor (fibrosarcoma) | Specific expression with hypoxia-responsive element in HT1080 transfectant in vitro and in vivo | [30] |
| HTERT | Tumor (glioma) | Constitutive active caspase-6 driven by hTERT promoter suppressed glioma in nude mice | [35] |
| E2F | Tumor | Tumor-selective oncolytic adenovirus | [38] |
| Oncolytic effect of conditional-replicating Ad in Rb pathway-defective cells | [39] | ||
| Osteocalcin | Prostate cancer | Conditional-replicating Ad co-targeting tumor and associated osteoblasts kills cancer cells in vitro and skeletal metastasis in animal model | [40] |
| Muc-1 | Breast cancer | Ad with E1A driven by DF3/MUC1 promoter and CMV promoter driven TNF induced tumor regression | [41] |
| (DF3) | Ovarian cancer | Ad with the BAX driven by DF3 promoter showed cytotoxicity in vitro and in vivo | [70] |
| L-plastin | Breast and ovarian cancer | Conditional-replicating Ad with E1A driven by truncated L-plastin promoter showed cytotoxic effect in animal model | [10] |
Abbreviations: ALA, α-lactalbumin; BAX, pro-apoptotic member of the Bcl-2 family; DF3/Muc-1, mucin core protein-1; E2F, E2A-binding factor; GFAP, glial fibrillary acidic protein; NSE, neuronal-specific enolase; PSA, prostate-specific antigen; CEA, carcinoembryonic antigen; AFP, α-fetoprotein; HIF-1α, α-subunit of hypoxia-inducible transcription factor-1; hTERT, human telomerase reverse transcriptase; CMV, cytomegalovirus; TNF, tumor necrosis factor
Tissue-targeted gene therapy has been investigated for melanoma [10,11], prostate cancer [12] and breast cancer [13]. Tyrosinase, a key enzyme in melanin synthesis, is highly expressed in melanoma cells; therefore, its promoter has been used in melanoma targeted gene therapy [14]. The fidelity of the tyrosinase promoter was demonstrated in a melanoma-targeted oncolytic scheme, in which restricted adenoviral E1A gene expression led to greater than 200-fold selective viral replication and cytolysis [10].
The PSA gene encodes a serine protease that is expressed in normal and cancerous prostatic epithelial cells, and is an important serum marker for prostate cancer [12]. Due to its highly specific nature, the PSA promoter has been frequently used in prostate cancer-targeted gene therapy approaches [3,15,16]. A modified PSA promoter-driven luciferase reporter adenoviral vector construct showed preferential expression in prostate gland and tumors, by three orders of magnitude, compared with liver tissue [17,18]. Human α-lactalbumin (hALA) is an enzyme involved in lactose production, and it is expressed in the lactating mammary gland and in a high proportion of breast cancer cases [19]. Selected expression in breast cancer cells was demonstrated with an hALA promoter-driven reporter and therapeutic gene [13]. Tissue-specific promoter-based cytotoxic gene therapy can damage normal tissue at the site where the promoter is active. This therapeutic approach is feasible in situations in which the normal targeted tissue is dispensable.
The carcinoembryonic antigen (CEA) and α-fetoprotein (AFP) are embryonic proteins that become reactivated in carcinomas. Because these two genes are dormant in normal adult tissues, their promoters are highly tumor selective. The AFP promoter has been employed to target hepatic cancers in many therapeutic strategies, including expression of cytosine deaminase [20], the immunostimulatory IL-2 gene [21] and in oncolytic adenovirus [22]. The CEA promoter can be applied to diverse cancers in which this gene is overexpressed, such as gastric carcinoma [23] and colorectal cancer [24]. Adenoviral vectors carrying CEA promoter-driven therapeutic genes were able to mediate targeted expression, tumor regression and prolonged survival in CEA + tumor-bearing mice [25], with minimal liver toxicity [26]. Because AFP and CEA are used as serum diagnostic markers in several carcinomas [27,28], employing these promoters as targets would be supported in expression-positive carcinomas.
Promoters that are upregulated in cancer-specific conditions can be exploited for targeting. The abnormal tumor microenvironment can induce altered gene expression; for example, inadequate vascular supply relative to the rapid growth of cancer cells leads to hypoxia [29], which initiates a cascade of gene expression mediated by the hypoxia-inducible transcription factor (HIF). The α-subunit of HIF-1 is the inducible component of the heterodimeric HIF, which binds to the hypoxia response element (HRE) and activates target gene expression in response to hypoxia [30,31]. Incorporation of HRE into the adenovirus E1A gene regulatory region resulted in a virus that replicates and lyses tumor cells in a hypoxia-dependent manner [32].
Telomerase plays an important role in cell immortalization and tumorigenesis, and its activity is highly dependent on the catalytic subunit, human telomerase reverse transcriptase (hTERT) [33]. High levels of hTERT expression regulated at the transcription step are observed in malignant tumors but not in normal cells [34]. Therefore, use of the hTERT promoter has achieved targeted therapeutic results in experimental bladder cancer and glioma models [35].
Dysregulated cell cycle control and unrestricted growth of cancer is frequently caused by disruption of the retinoblastoma (Rb)/E2F/p16 pathway [36], which in turn activates E2F. E2F is a transcription factor that activates its own promoter and other genes involved in cell-cycle transition [37]. This elevated E2F-1 activity in cancer cells was exploited to control adenoviral E1A gene expression and viral replication. E2F appears to be a feasible target to mediate tumor lysis of multiple Rb-defective cancers [38,39]. A similar cancer-targeted oncolytic virus has been developed with the osteocalcin promoter for prostate cancer [40], the Muc-1 promoter for breast cancer [41] and the L-plastin promoter for breast and ovarian cancer [11]. It is desirable to use a specific promoter to direct viral lytic replication because an intrinsic amplification of therapeutic response is incorporated into this approach. Upon viral replication, a burst of progeny viruses can infect additional tumor cells.
Augmenting cancer-specific expression
Although the use of specific promoters will be likely to improve the safety of gene-based therapy, the activities of specific promoters are weaker than the current benchmark CMV promoter. Given that in vivo gene delivery to the tumor cells might be limited, a concern for employing weak specific promoters is that therapeutic efficacy might decline. However, any attempt to enhance the potency of promoters will need to retain the specificity of the promoter in order to maintain potential therapeutic benefits.
Simple manipulations of known regulatory elements, such as removal of a negative and inert regulatory sequence or multimerization of positive elements, can promote synergistic and cooperative interactions of activators to enhance transcription. For example, the activity of native PSA promoter and enhancer (PSE) can be augmented by modifying the androgen receptor (AR) elements, which serve key activating functions for the PSA gene [15,16]. By insertion of four tandem copies of the synthetic androgen-responsive element, or by duplication of a 400-base pair enhancer core element, a nearly 20-fold enhancement of activity over the parental PSE was achieved [17]. Similar approaches have been successful in improving the activity of the tyrosinase promoter [14] and the CEA promoter [42]. An interesting and more extreme approach would be to generate a complete synthetic promoter by multimerization and shuffling of known regulatory elements, then selecting the most active and properly regulated construct. This approach has been applied to the chicken skeletal α-actin promoter to achieve muscle-specific expression that exceeds the level of the CMV promoter [43].
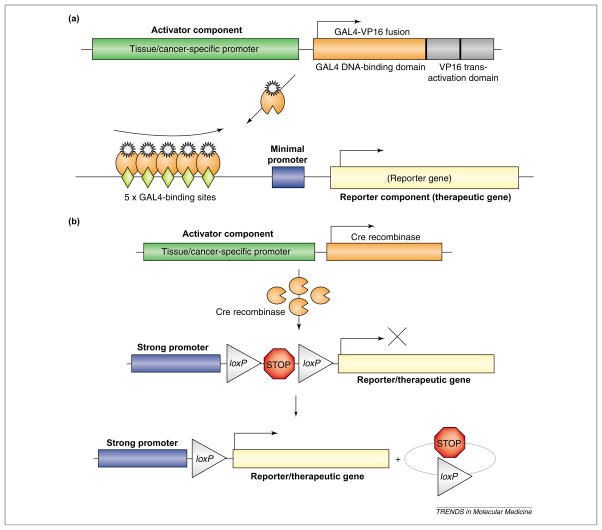
Two strategies have frequently been employed to amplify weak promoter activity in a two-tiered manner (Figure 2). In an approach known as ‘two-step transcriptional activation’ (TSTA, Figure 2a), the specific promoter directed the potent transcription activator, GAL4-VP16, which in turn acted upon a second GAL-4-responsive reporter or therapeutic gene. This TSTA approach, based on the original ‘enhancer trap’ methodology to study gene expression in Drosophila melanogaster development, can boost the activity of the PSA promoter over a range of up to 1000-fold [44,45]. Optimal TSTA constructs displayed activity levels significantly higher than those of the CMV promoter, while maintaining prostate cell specificity and androgen responsiveness [44,45]. The fidelity of this prostate-targeted TSTA-firefly luciferase (FL) expression cassette was maintained when inserted into an adenoviral vector, AdTSTA-FL [46]. Many applications of this two-tiered amplification strategy have been documented, including amplification of PSA promoter-mediated polyglutamine expression to treat prostate cancer [47], enhancement of the CEA promoter [48] and Muc-1-mediated expression for colon cancer [49].
Figure 2.
Binary approaches to amplify activity of weak promoter. (a) Two-step transcriptional amplification. The specific promoter drives expression of a synthetic transcriptional activator, GAL4-VP16, which in turn binds to and activates the GAL4 responsive promoter of the reporter or therapeutic gene. Multiple GAL4-VP16 activators work in concert to synergistically activate transcription of the downstream gene (denoted by upward sweeping arrow). (b) Cre-mediated activation of gene expression. Cre recombinase expression is regulated by a tissue- or cancer-specific promoter. Activation of transgene expression is induced by removal of the translational inhibition sequence via a Cre-specific recombination between the two loxP sites.
In another approach, a cancer-specific promoter controls the expression of Cre site-specific recombinase [50], which activates the reporter or therapeutic gene expression in a second step (Figure 2b). The desired transgene is linked to a strong constitutive promoter that is interrupted by expression termination sequences flanked by two loxP sites, the cognate site of Cre. Commonly, the two components of this scheme are incorporated into two separate adenoviral vectors [51–54]. By co-infection into target cells, the cell-specific, Cre-dependent activation of trans-gene expression was demonstrated in CEA-targeted systems [51], in thyroid carcinoma-targeted therapy [52], in a growth hormone promoter-mediated strategy targeting pituitary tumor [53] and in AFP promoter-based therapy for liver tumor [54]. The specificity of this system is maintained when the Cre and the loxP component are inserted in two separate vectors. However, the activation of this system requires co-delivery of two vectors into the same cell, which could be inefficient in vivo [54].
Imaging-specific expression in animals
Non-invasive gene-based imaging is a powerful tool to assess the performance of targeted gene therapy in vivo. Due to the multitude of therapeutic genes used, direct imaging of each therapeutic gene is not feasible. Thus, an imaging reporter gene delivered and expressed in conjunction with the therapeutic gene becomes a generalizable approach to monitor expression in vivo. Rapid advances in imaging technologies have accomplished repetitive monitoring of detailed location, magnitude and kinetics of reporter gene expression in living animals [55]. Two such modalities that will be discussed, luciferase-based bioluminescence imaging (BLI) and positron emission tomography (PET) have frequently been applied in pre-clinical small animal models.
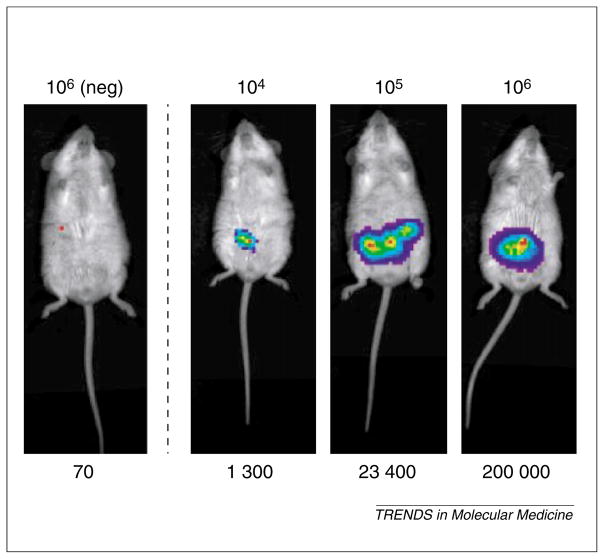
BLI [56] has the distinct advantage of low background signal, ease of use, and low cost, in comparison with radionuclide imaging; however, it is limited by light scatter and absorption, presenting difficulties in detecting and localizing signals in deep tissues. Luciferase is a generic term for a family of photo-proteins that can be isolated from insects, marine organisms and prokaryotes [57]. Biochemically, all luciferases are oxygenases that use molecular oxygen to oxidize a substrate, with the formation of product in an electronically excited state. The bioluminescent systems are not evolutionarily conserved; thus, each luciferase isolated from a particular organism catalyzes a unique substrate, with emission spectra ranging between 400 and 620 nm [57]. Imaging of FL expression in living mice has been accomplished using a highly sensitive charged coupled device (CCD) camera [56,58]. Light is produced through the interaction of FL with its substrate, D-luciferin, injected peritoneally in the presence of magnesium and ATP [56]. An estimate of the sensitivity of this FL-based CCD imaging is illustrated in Figure 3. Optical signals from 104 or more cells expressing FL driven by the CMV promoter can be easily detected.
Figure 3.
Estimation of the sensitivity of optical imaging. Prostate cancer cells, LNCaP, were infected by recombinant adenovirus expressing FL driven by CMV promoter (AdCMV-FL) at MOI 10. Two days after infection, cells were harvested and resuspended in 50 μl sterile phosphate-buffered saline + 50 μl Matrigel (BD Biosciences), and injected intra-peritoneally. On the same day of cell implantation, mice were imaged in Xenogen IVIS charged coupled device camera after administration of 200 μl D-luciferin substrate (15 mg/ml). Optical signal intensity (listed below images in relative luminescent units per min of acquisition, RLU/min) is roughly proportional to the number of AdCMV-FL-infected cells (listed above the images). The negative control cells were infected with AdCMV-TK.
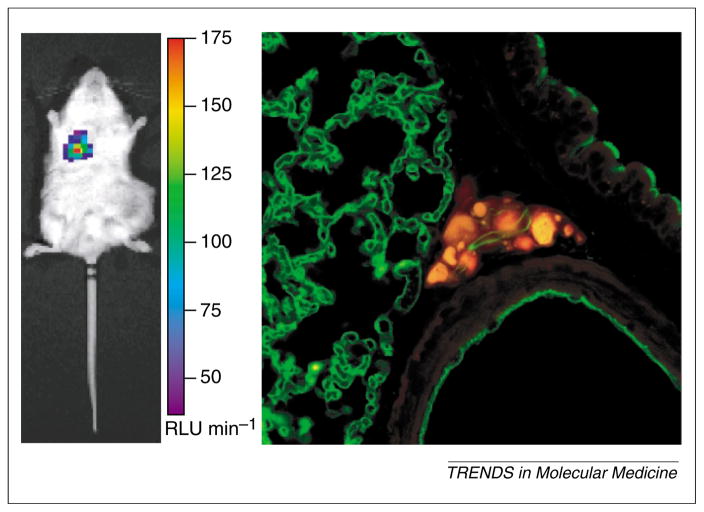
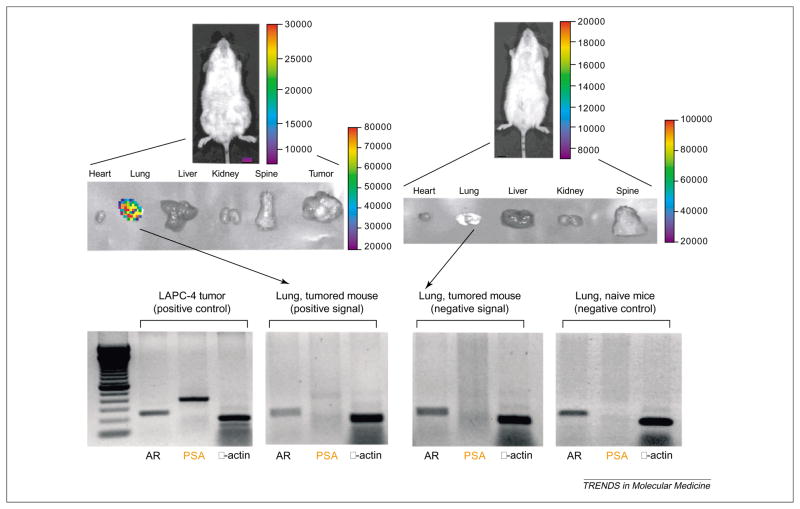
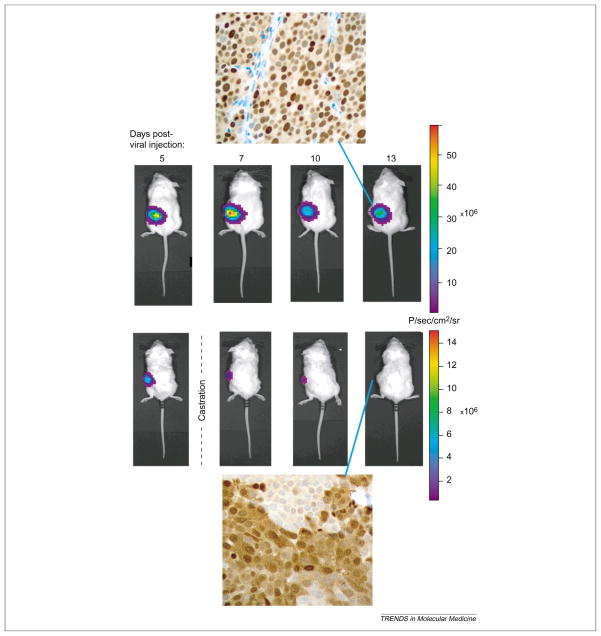
The specificity of transcriptionally targeted gene delivery vectors can be investigated by BLI. One advantage of non-invasive imaging is that sequential detection of signals in the same animal in a time-dependent manner often alleviates some of the uncertainty due to inter-animal technical variations. To overcome the limitation of precise three-dimensional signal localization in the animal, postmortem imaging of isolated organs was applied [18]. The specificity of a prostate-targeted adenoviral vector (AdPSE-BC-FL) has permitted detection of metastatic lesions in living mice [18]. Because these vector-based gene imaging approaches are relatively new, extra caution is needed to assure the reliability of the optical signals. As shown in Figures 4 and 5, the positive imaging signals were confirmed with detailed histological and pathological analyses of the tissues [18] (Figure 4) and with the sensitive polymerase chain reaction technology (Figure 5). Moreover, the highly amplified prostate-specific AdTSTA-FL can be applied to interrogate AR function during prostate cancer progression [46]. The real-time optical signals mediated by AdTSTA-FL correlated with cellular transcription complex formation by chromatin immunoprecipitation and AR cellular localization [46] (Figure 6). Depletion of testicular androgen in castrated animals resulted in rapid decay of the AdTSTA-FL mediated signal, which correlated with diffusion of AR into the cytoplasm from the nucleus (Figure 6).
Figure 4.
Verification of positive optical signals. Animals bearing prostate tumor received 3.6 or 7.2 × 107 infectious units of prostate-targeted adenovirus, AdPSE-BC-FL, administered via the tail vein. (a) Histological analysis to assess positive signal. Twelve days after administration, an optical signal was observed in the lung (left panel) of an animal that received 3.6 × 107 infectious units of virus. The positive optical signal in this animal was correlated with the presence of metastatic human cancer cells in the lung (right panel). Cancer cells in the lung sections were visualized by confocal microscopy using CY-3 conjugated (red) human-specific pan-cytokeratin antibody (BioGenex Laboratories). Lung blood vessels were visualized by FITC-lectin (green).
Figure 5.
Reverse transcriptase polymerase chain reaction (RT-PCR) to detect the presence of tumor cells in the lung. Semiquantitative RT–PCR was used to detect the presence of prostate-specific antigen (PSA)-expressing human cancer cells. The animals in the left and right panel received 3.6 × 107 and 7.2 × 107 infectious units of AdPSE-BC-FL, respectively. At 21 days after viral injection, the animal in the left and right panel displayed positive and negative lung signals, respectively, after organ isolation. Thirty-five cycles of PCR amplifications performed on the lung extract revealed a low-intensity positive band detected by PSA-specific primers (the animal in the left panel). No PSA transcripts were detected in the lung of the optically silent animal (right panel). Androgen receptor RNA was detected in the lung of non-tumor bearing (naïve) animals (negative control). Tumor tissue extract served as positive control.
Figure 6.
Dynamic imaging of androgen receptor (AR) function in tumors. LAPC-9 tumors grafted in SCID mice were injected with 107 infectious units of AdTSTA-FL. Optical signals were monitored by charged coupled device imaging repetitively at the specified days following injection. Animals castrated at 5 days following viral administration displayed a more rapid decay of intratumoral signal, compared with the non-castration animal. AR immunohistochemical staining of the respective tumors showed that depletion of testicular androgen correlated with the diffusion of AR into cytoplasm, compared with a predominant nuclear localization in the intact animals. Heterogeneity in AR staining was observed in tumors, both in intact or castrated animals.
Multiple distinct luciferases could potentially be developed for BLI. This exciting development means that it will be possible simultaneously to monitor multiple pathways or cell populations in the animal. In fact, Gambhir’s group has demonstrated the feasibility of this principle by simultaneously monitoring Renilla luciferase (RL) and FL [59]. RL is purified from sea pansy, a bioluminescent soft coral. This enzyme has an origin, enzyme structure and substrate requirements distinct from FL, and it catalyzes coelenterazine oxidation. Thus, by injecting coelenterazine or D-luciferin, respective levels of RL and FL expression can be imaged simultaneously in the same mouse, to track two different cell populations or gene therapy vectors [59]. Although BLI is widely applicable to investigating many biological processes in small animals [46], it cannot be applied to human studies because of the loss of signal penetration with increased tissue depth. To translate optical imaging results in animals to clinical settings, a high-energy imaging modality will be needed.
PET is a radionuclide imaging modality widely used in clinical settings. Our institution has acquired substantial experience in adapting this modality to gene-based imaging in small animals, using the herpes simplex virus thymidine kinase (HSV-TK) or the dopamine type 2 receptor gene [60]. Compared with optical imaging, PET has the distinct advantage of providing tomographic, quantitative image signals and adaptability for human imaging [60]. However, optical imaging is several orders of magnitude higher in sensitivity than PET [61]. Thus, gene expression amplification such as TSTA might be required to successfully implement PET imaging to monitor vector-mediated transgene expression in vivo.
One widely used PET reporter gene system is based on the HSV-TK gene. In contrast to human thymidine kinase, which phosphorylates thymidine selectively, HSV-TK has a relaxed substrate specificity for other nucleoside analogs, and can phosphorylate a variety of acycloguanosine and uracil derivatives. Radionuclide reporter probes derived from uracil [2′-fluoro-2′-deoxy-1-β-D-arabinofuranosyl-5-iodouracil (FIAU) labeled with radioactive iodine (131I)] and from guanosine (18F-labeled penciclovir, PCV) have been applied to single-photon emission computed tomography (SPECT) and PET. The success of these imaging approaches in many mouse models is based on the ability of HSV-TK to selectively phosphorylate and sequester the probes in cells expressing this gene [60]. Active site HSV-TK variants have been generated by random mutagenesis of the binding site amino acids, and selected for increased affinity for the acycloguanosine analogs, compared with thymidine [62]. One HSV-TK variant, sr39tk, displayed enhanced 18F-labeled PCV substrate uptake and improved sensitivity of PET imaging compared with wild-type HSV-TK [63].
The HSV-TK gene has been used in suicide cancer therapy for more than ten years, with ongoing clinical trials [64]. Therefore, the HSV-TK gene has the unique property of being able to function both as an imaging reporter gene and as a cytotoxic therapeutic gene. In practice, different dosages of HSV-TK substrate are used to achieve the two modes of action. The 18F-labeled PCV level administered for PET imaging is three to four orders of magnitude lower than the toxic pharmacological dose of ganciclovir (GCV). The toxicity of GCV is a result of HSV-TK-mediated conversion to GCV monophosphate, which undergoes further phosphorylation to the triphosphate form; this in turn disrupts DNA synthesis and induces apoptosis [65,66]. Among a panel of adenoviral vectors expressing either the wild-type HSV-TK or active site variants, sr39tk showed improved therapeutic efficacy in response to GCV in prostate cancer cell lines and tumors [67].
Molecular imaging should play an important role in gene-based therapeutic and diagnostic studies. Imaging the expression levels of therapeutic genes by indirect methods [reporter genes] or direct methods (HSV-TK with PET) can assess the performance and verify the specificity of the cancer-targeted vector in vivo. For example, the magnitude of HSV-TK-mediated PET imaging signals correlate directly with the gene expression level [60]. Thus, PET imaging before GCV instillation should permit localization and assessment of transduction efficiency of HSV-TK gene therapy in vivo. Based on this information, the magnitude of transgene expression can be modulated to achieve optimal expression in the tumor target, to enhance therapeutic efficacy. Moreover, imaging approaches could be developed to assess treatment response in real time. In the case of suicide gene therapy, the HSV-TK-transduced tumor cells should be eradicated after GCV administration; consequently, PET signals should drop precipitously. The kinetics of PET signal diminution can be monitored in real time. In addition, if the cancer-targeted gene expression vector is truly specific in vivo, imaging of the reporter gene can be adapted to detect disseminated cancer cells [18]. By combining targeted gene-based imaging and therapeutic approaches, the potential to detect and treat metastatic cancer could be developed in the future.
Concluding remarks
To achieve highly precise cell-specific targeting remains a great challenge in the future of cancer therapy. Transcriptional targeting is a feasible means of improving the specificity and efficacy of gene therapy. However, significant obstacles remain in seeking out and destroying the hidden metastatic cancer cells in the whole organism. The well documented abnormal tumor angiogenesis [68] might prevent sufficient drug and gene therapy vector delivery to the tumor [69]. To further enhance tumor selectivity, approaches which target cell surface or vascular antigens [70] or biochemical pathways unique to tumors can be incorporated. We foresee that combining different targeting strategies into the cancer-specific vector could achieve synergistic selectivity. Non-invasive imaging will be a useful tool to assess the performance of the targeted vector in vivo. Meticulous design and stringent testing of cancer-targeted gene therapy in preclinical settings should facilitate a clear path for future applications in clinics.
Acknowledgments
We appreciate the assistance of Wendy Aft on article preparation. Our active collaboration and interactions with the laboratories of Sanjiv Gambhir and Michael Carey have led to the generation of the amplified systems of prostate-specific gene expression and imaging. This work is supported by RO1 CA101904 (to L.W.), Jonsson Comprehensive Cancer Center and Department of Defense Prostate Cancer Research Program DAMD17-03-1-0095 postdoctoral fellowship (to M.S.).
References
- 1.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–1603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 2.McCormick F. Cancer gene therapy: fringe or cutting edge. Nat Rev Cancer. 2001;1:130–141. doi: 10.1038/35101008. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez R, et al. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559–2563. [PubMed] [Google Scholar]
- 4.Mabjeesh NJ, et al. Gene therapy of prostate cancer: current and future directions. Endocr Relat Cancer. 2002;9:115–139. doi: 10.1677/erc.0.0090115. [DOI] [PubMed] [Google Scholar]
- 5.Nettelbeck DM, et al. Gene therapy designer promoters for tumour targeting. Trends Genet. 2000;16:174–181. doi: 10.1016/s0168-9525(99)01950-2. [DOI] [PubMed] [Google Scholar]
- 6.Johnson KJ, et al. Targeting the cytotoxicity of fusogenic membrane glycoproteins in gliomas through protease-substrate interaction. Gene Ther. 2003;10:725–732. doi: 10.1038/sj.gt.3301951. [DOI] [PubMed] [Google Scholar]
- 7.Smale ST, Carey M. Transcriptional Regulation in Eukaryotes. Laboratory Press; Cold Spring Harbor: 2000. [Google Scholar]
- 8.Morelli AE, et al. Neuronal and glial cell type-specific promoters within adenovirus recombinants restrict the expression of the apoptosis-inducing molecule Fas ligand to predetermined brain cell types, and abolish peripheral liver toxicity. J Gen Virol. 1999;80:571–583. doi: 10.1099/0022-1317-80-3-571. [DOI] [PubMed] [Google Scholar]
- 9.Lohr F, et al. Systemic vector leakage and transgene expression by intratumorally injected recombinant adenovirus vectors. Clin Cancer Res. 2001;7:3625–3628. [PubMed] [Google Scholar]
- 10.Nettelbeck DM, et al. Novel oncolytic adenoviruses targeted to melanoma: specific viral replication and cytolysis by expression of E1A mutants from the tyrosinase enhancer/promoter. Cancer Res. 2002;62:4663–4670. [PubMed] [Google Scholar]
- 11.Zhang L, et al. Adenoviral vectors with E1A regulated by tumor-specific promoters are selectively cytolytic for breast cancer and melanoma. Mol Ther. 2002;6:386–393. doi: 10.1006/mthe.2002.0680. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Sato M. Integrated, molecular engineering approaches to develop prostate cancer gene therapy. Curr Gene Ther. doi: 10.2174/1566523034578230. (in press) [DOI] [PubMed] [Google Scholar]
- 13.Anderson LM, et al. Adenovirus-mediated tissue-targeted expression of the HSVtk gene for the treatment of breast cancer. Gene Ther. 1999;6:854–864. doi: 10.1038/sj.gt.3300909. [DOI] [PubMed] [Google Scholar]
- 14.Siders WM, et al. Transcriptional targeting of recombinant adenoviruses to human and murine melanoma cells. Cancer Res. 1996;56:5638–5646. [PubMed] [Google Scholar]
- 15.Schuur ER, et al. Prostate-specific antigen expression is regulated by an upstream enhancer. J Biol Chem. 1996;271:7043–7051. doi: 10.1074/jbc.271.12.7043. [DOI] [PubMed] [Google Scholar]
- 16.Pang S, et al. Identification of a positive regulatory element responsible for tissue-specific expression of prostate-specific antigen. Cancer Res. 1997;57:495–499. [PubMed] [Google Scholar]
- 17.Wu L, et al. Chimeric PSA enhancers exhibit augmented activity in prostate cancer gene therapy vectors. Gene Ther. 2001;8:1416–1426. doi: 10.1038/sj.gt.3301549. [DOI] [PubMed] [Google Scholar]
- 18.Adams JY, et al. Visualization of advanced human prostate cancer lesions in living mice by a targeted gene transfer vector and optical imaging. Nat Med. 2002;8:891–897. doi: 10.1038/nm743. [DOI] [PubMed] [Google Scholar]
- 19.Walker RA. The demonstration of alpha-lactalbumin in human breast carcinomas. J Pathol. 1979;129:37–42. doi: 10.1002/path.1711290107. [DOI] [PubMed] [Google Scholar]
- 20.Kanai F, et al. In vivo gene therapy for alpha-fetoprotein-producing hepatocellular carcinoma by adenovirus-mediated transfer of cytosine deaminase gene. Cancer Res. 1997;57:461–465. [PubMed] [Google Scholar]
- 21.Bui LA, et al. In vivo therapy of hepatocellular carcinoma with a tumor-specific adenoviral vector expressing interleukin-2. Hum Gene Ther. 1997;8:2173–2182. doi: 10.1089/hum.1997.8.18-2173. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M, et al. E1B-55K-deleted adenovirus expressing E1A-13S by AFP-enhancer/promoter is capable of highly specific replication in AFP-producing hepatocellular carcinoma and eradication of established tumor. Mol Ther. 2002;5:627–634. doi: 10.1006/mthe.2002.0589. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka T, et al. Adenovirus-mediated prodrug gene therapy for carcinoembryonic antigen-producing human gastric carcinoma cells in vitro. Cancer Res. 1996;56:1341–1345. [PubMed] [Google Scholar]
- 24.Cao G, et al. Effective and safe gene therapy for colorectal carcinoma using the cytosine deaminase gene directed by the carcino-embryonic antigen promoter. Gene Ther. 1999;6:83–90. doi: 10.1038/sj.gt.3300823. [DOI] [PubMed] [Google Scholar]
- 25.Lan KH, et al. In vivo selective gene expression and therapy mediated by adenoviral vectors for human carcinoembryonic antigen-producing gastric carcinoma. Cancer Res. 1997;57:4279–4284. [PubMed] [Google Scholar]
- 26.Brand K, et al. Tumor cell-specific transgene expression prevents liver toxicity of the adeno-HSVtk/GCV approach. Gene Ther. 1998;5:1363–1371. doi: 10.1038/sj.gt.3300728. [DOI] [PubMed] [Google Scholar]
- 27.Fujiyama S, et al. Tumor markers in early diagnosis, follow-up and management of patients with hepatocellular carcinoma. Oncology. 2002;62 (Suppl 1):57–63. doi: 10.1159/000048277. [DOI] [PubMed] [Google Scholar]
- 28.Hammarstrom S. The carcinoembryonic antigen [CEA] family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 29.Brown JM. Exploiting the hypoxic cancer cell: mechanisms and therapeutic strategies. Mol Med Today. 2000;6:157–162. doi: 10.1016/s1357-4310(00)01677-4. [DOI] [PubMed] [Google Scholar]
- 30.Dachs GU, et al. Targeting gene expression to hypoxic tumor cells. Nat Med. 1997;3:515–520. doi: 10.1038/nm0597-515. [DOI] [PubMed] [Google Scholar]
- 31.Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- 32.Post DE, Van Meir EG. A novel hypoxia-inducible factor [HIF] activated oncolytic adenovirus for cancer therapy. Oncogene. 2003;22:2065–2072. doi: 10.1038/sj.onc.1206464. [DOI] [PubMed] [Google Scholar]
- 33.Meyerson M, et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 34.Takakura M, et al. Cloning of human telomerase catalytic subunit [hTERT] gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–557. [PubMed] [Google Scholar]
- 35.Komata T, et al. Treatment of malignant glioma cells with the transfer of constitutively active caspase-6 using the human telomerase catalytic subunit [human telomerase reverse transcriptase] gene promoter. Cancer Res. 2001;61:5796–5802. [PubMed] [Google Scholar]
- 36.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 37.Johnson DG, et al. Autoregulatory control of E2F1 expression in response to positive and negative regulators of cell cycle progression. Genes Dev. 1994;8:1514–1525. doi: 10.1101/gad.8.13.1514. [DOI] [PubMed] [Google Scholar]
- 38.Johnson L, et al. Selectively replicating adenoviruses targeting deregulated E2F activity are potent, systemic antitumor agents. Cancer Cell. 2002;1:325–337. doi: 10.1016/s1535-6108(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 39.Jakubczak JL, et al. An oncolytic adenovirus selective for retinoblastoma tumor suppressor protein pathway-defective tumors: dependence on E1A, the E2F-1 promoter, and viral replication for selectivity and efficacy. Cancer Res. 2003;63:1490–1499. [PubMed] [Google Scholar]
- 40.Matsubara S, et al. A conditional replication-competent adenoviral vector, Ad-OC-E1a, to cotarget prostate cancer and bone stroma in an experimental model of androgen-independent prostate cancer bone metastasis. Cancer Res. 2001;61:6012–6019. [PubMed] [Google Scholar]
- 41.Kurihara T, et al. Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen. J Clin Invest. 2000;106:763–771. doi: 10.1172/JCI9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards CA, et al. Transcriptional regulatory sequences of carcinoembryonic antigen: identification and use with cytosine deaminase for tumor-specific gene therapy. Hum Gene Ther. 1995;6:881–893. doi: 10.1089/hum.1995.6.7-881. [DOI] [PubMed] [Google Scholar]
- 43.Li X, et al. Synthetic muscle promoters: activities exceeding naturally occurring regulatory sequences. Nat Biotechnol. 1999;17:241–245. doi: 10.1038/6981. [DOI] [PubMed] [Google Scholar]
- 44.Ng L, et al. Molecular engineering of a two-step transcription amplification (TSTA) system for transgene delivery in prostate cancer. Mol Ther. 2002;5:223–232. doi: 10.1006/mthe.2002.0551. [DOI] [PubMed] [Google Scholar]
- 45.Lyer M, et al. Two-step transcriptional amplification as a method for imaging reporter gene expression utilizing weak promoters. Proc Natl Acad Sci U S A. 2001;98:14595–14600. doi: 10.1073/pnas.251551098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L, et al. Interrogating androgen receptor function in recurrent prostate cancer. Cancer Res. (in press) [PubMed] [Google Scholar]
- 47.Segawa T, et al. Prostate-specific amplification of expanded polyglutamine expression: a novel approach for cancer gene therapy. Cancer Res. 1998;58:2282–2287. [PubMed] [Google Scholar]
- 48.Qiao J, et al. Tumor-specific transcriptional targeting of suicide gene therapy. Gene Ther. 2002;9:168–175. doi: 10.1038/sj.gt.3301618. [DOI] [PubMed] [Google Scholar]
- 49.Block A, et al. Amplified Muc1-specific gene expression in colon cancer cells utilizing a binary system in adenoviral vectors. Anticancer Res. 2002;22:3285–3292. [PubMed] [Google Scholar]
- 50.Austin SM, et al. A novel role for site-specific recombination in maintenance of bacterial replicons. Cell. 1981;25:729–736. doi: 10.1016/0092-8674(81)90180-x. [DOI] [PubMed] [Google Scholar]
- 51.Ueda K, et al. Carcinoembryonic antigen-specific suicide gene therapy of cytosine deaminase/5-fluorocytosine enhanced by the cre/loxP system in the orthotopic gastric carcinoma model. Cancer Res. 2001;61:6158–6162. [PubMed] [Google Scholar]
- 52.Nagayama Y, et al. Enhanced efficacy of transcriptionally targeted suicide gene/prodrug therapy for thyroid carcinoma with the Cre-loxP system. Cancer Res. 1999;59:3049–3052. [PubMed] [Google Scholar]
- 53.Lee EJ, Jameson JL. Cell-specific Cre-mediated activation of the diphtheria toxin gene in pituitary tumor cells: potential for cytotoxic gene therapy. Hum Gene Ther. 2002;13:533–542. doi: 10.1089/10430340252809829. [DOI] [PubMed] [Google Scholar]
- 54.Sakai Y, et al. Gene therapy for hepatocellular carcinoma using two recombinant adenovirus vectors with alpha-fetoprotein promoter and Cre/lox P system. J Virol Methods. 2001;92:5–17. doi: 10.1016/s0166-0934(00)00240-8. [DOI] [PubMed] [Google Scholar]
- 55.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 56.Contag PR, et al. Bioluminescent indicators in living mammals. Nat Med. 1998;4:245–247. doi: 10.1038/nm0298-245. [DOI] [PubMed] [Google Scholar]
- 57.Hastings JW. Chemistries and colors of bioluminescent reactions: a review. Gene. 1996;173:5–11. doi: 10.1016/0378-1119(95)00676-1. [DOI] [PubMed] [Google Scholar]
- 58.Wu JC, et al. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol Ther. 2001;4:297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]
- 59.Bhaumik S, Gambhir SS. Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci U S A. 2002;99:377–382. doi: 10.1073/pnas.012611099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 61.Ray P, et al. Optical bioluminescence and positron emission tomography imaging of a novel fusion reporter gene in tumor xenografts of living mice. Cancer Res. 2003;63:1160–1165. [PubMed] [Google Scholar]
- 62.Black ME, et al. Creation of drug-specific herpes simplex virus type 1 thymidine kinase mutants for gene therapy. Proc Natl Acad Sci U S A. 1996;93:3525–3529. doi: 10.1073/pnas.93.8.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gambhir SS, et al. A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. Proc Natl Acad Sci U S A. 2000;97:2785–2790. doi: 10.1073/pnas.97.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herman JR, et al. In situ gene therapy for adenocarcinoma of the prostate: a phase I clinical trial. Hum Gene Ther. 1999;10:1239–1249. doi: 10.1089/10430349950018229. [DOI] [PubMed] [Google Scholar]
- 65.Mesnil M, Yamasaki H. Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: role of gap-junctional intercellular communication. Cancer Res. 2000;60:3989–3999. [PubMed] [Google Scholar]
- 66.Rubsam LZ, et al. Cytotoxicity and accumulation of ganciclovir triphosphate in bystander cells cocultured with herpes simplex virus type 1 thymidine kinase-expressing human glioblastoma cells. Cancer Res. 1999;59:669–675. [PubMed] [Google Scholar]
- 67.Pantuck A, et al. Optimizing prostate cancer suicide gene therapy using herpes simplex virus thymidine kinase active site variants. Hum Gene Ther. 2002;13:777–789. doi: 10.1089/10430340252898966. [DOI] [PubMed] [Google Scholar]
- 68.McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 69.Jain RK. Delivery of molecular medicine to solid tumors: lessons from in vivo imaging of gene expression and function. J Control Release. 2001;74:7–25. doi: 10.1016/s0168-3659(01)00306-6. [DOI] [PubMed] [Google Scholar]
- 70.Ruoslahti E. Drug targeting to specific vascular sites. Drug Discov Today. 2002;7:1138–1143. doi: 10.1016/s1359-6446(02)02501-1. [DOI] [PubMed] [Google Scholar]