Abstract
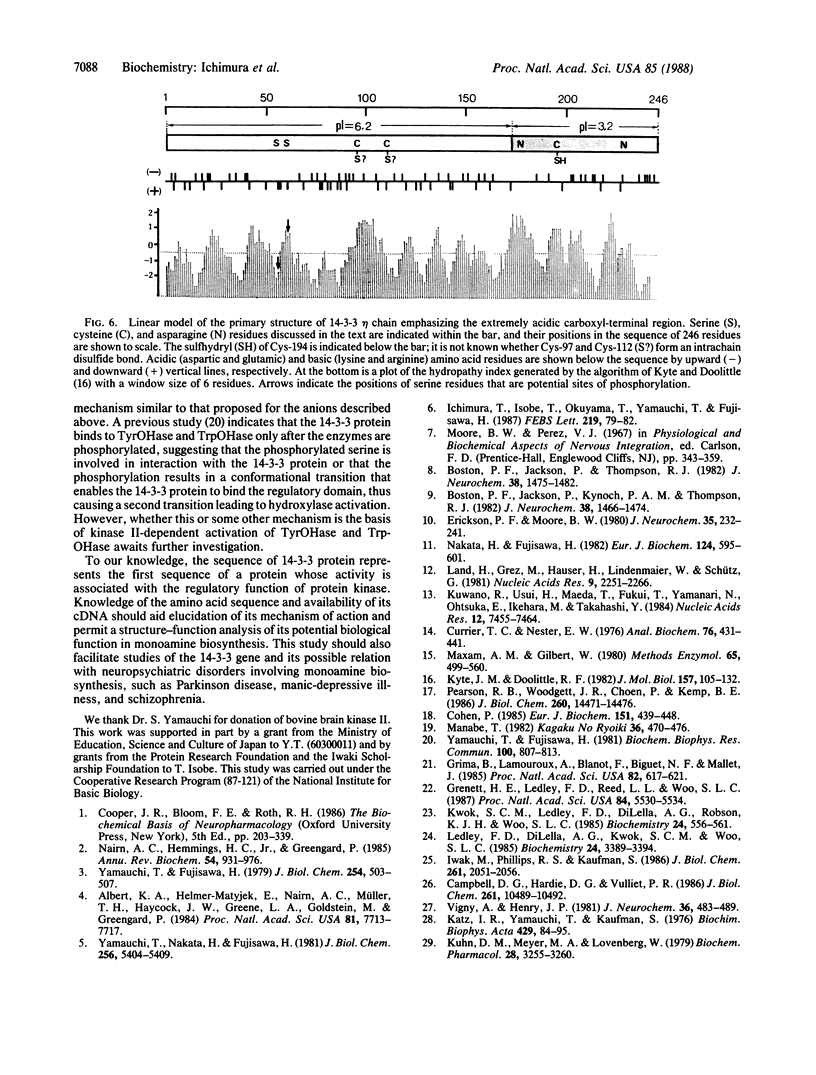
The 14-3-3 protein is a family of acidic proteins present exclusively in the brain and is believed to have a function in monoamine biosynthesis because of its ability to activate tyrosine hydroxylase and tryptophan hydroxylase in the presence of Ca2+/calmodulin-dependent protein kinase type II. In this study, we resolved bovine brain 14-3-3 protein into seven polypeptide components by means of reversed-phase chromatography and determined the amino acid sequence of one of these components (eta chain) by cloning its cDNA from a bovine cerebellum cDNA library. The eta-chain mRNA is 1.8 kilobases long and encodes a polypeptide of 246 amino acids and Mr 28,221. Computer-assisted analysis of the sequence indicates that the eta chain exhibits no internal sequence repeats, nor does it have significant sequence similarity to other proteins with known amino acid sequence. However, the eta chain appears to consist of two structural regions that are distinguishable in their clearly different charge characteristics: the almost neutral amino-terminal region and the strongly acidic carboxyl-terminal region. The structural features of the eta chain and the domain organization of tyrosine and tryptophan hydroxylases suggest that the 14-3-3 protein binds to the regulatory domain of the phosphorylated hydroxylases through its acidic carboxyl-terminal region and activates the hydroxylases by inducing an active conformation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert K. A., Helmer-Matyjek E., Nairn A. C., Müller T. H., Haycock J. W., Greene L. A., Goldstein M., Greengard P. Calcium/phospholipid-dependent protein kinase (protein kinase C) phosphorylates and activates tyrosine hydroxylase. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7713–7717. doi: 10.1073/pnas.81.24.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston P. F., Jackson P., Kynoch P. A., Thompson R. J. Purification, properties, and immunohistochemical localisation of human brain 14-3-3 protein. J Neurochem. 1982 May;38(5):1466–1474. doi: 10.1111/j.1471-4159.1982.tb07927.x. [DOI] [PubMed] [Google Scholar]
- Boston P. F., Jackson P., Thompson R. J. Human 14-3-3 protein: radioimmunoassay, tissue distribution, and cerebrospinal fluid levels in patients with neurological disorders. J Neurochem. 1982 May;38(5):1475–1482. doi: 10.1111/j.1471-4159.1982.tb07928.x. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Hardie D. G., Vulliet P. R. Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J Biol Chem. 1986 Aug 15;261(23):10489–10492. [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in the hormonal control of enzyme activity. Eur J Biochem. 1985 Sep 16;151(3):439–448. doi: 10.1111/j.1432-1033.1985.tb09121.x. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Erickson P. F., Moore B. W. Investigation of the axonal transport of three acidic, soluble proteins (14-3-2, 14-3-3, and S-100) in the rabbit visual system. J Neurochem. 1980 Jul;35(1):232–241. doi: 10.1111/j.1471-4159.1980.tb12509.x. [DOI] [PubMed] [Google Scholar]
- Grenett H. E., Ledley F. D., Reed L. L., Woo S. L. Full-length cDNA for rabbit tryptophan hydroxylase: functional domains and evolution of aromatic amino acid hydroxylases. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5530–5534. doi: 10.1073/pnas.84.16.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
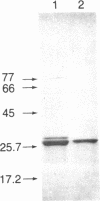
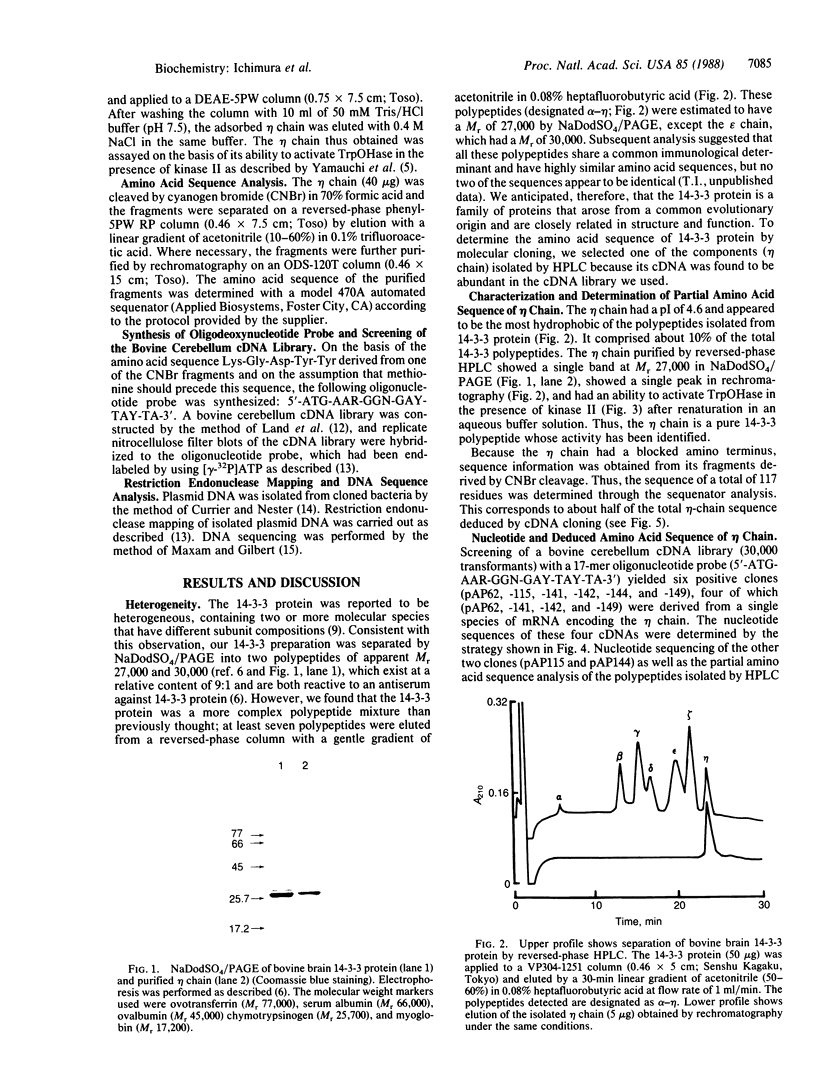
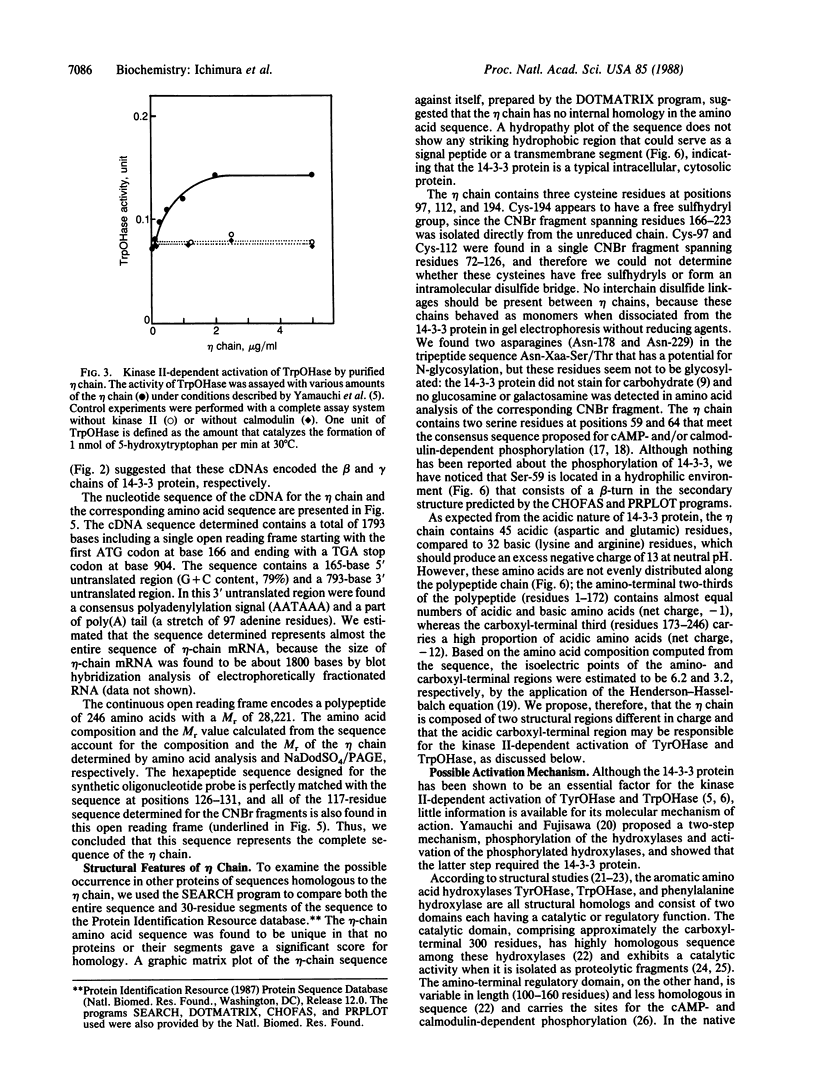
- Ichimura T., Isobe T., Okuyama T., Yamauchi T., Fujisawa H. Brain 14-3-3 protein is an activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+,calmodulin-dependent protein kinase II. FEBS Lett. 1987 Jul 13;219(1):79–82. doi: 10.1016/0014-5793(87)81194-8. [DOI] [PubMed] [Google Scholar]
- Iwaki M., Phillips R. S., Kaufman S. Proteolytic modification of the amino-terminal and carboxyl-terminal regions of rat hepatic phenylalanine hydroxylase. J Biol Chem. 1986 Feb 15;261(5):2051–2056. [PubMed] [Google Scholar]
- Katz I. R., Yamauchi T., Kaufman S. Activation of tyrosine hydroxylase by polyanions and salts. An electrostatic effect. Biochim Biophys Acta. 1976 Mar 11;429(1):84–95. doi: 10.1016/0005-2744(76)90032-2. [DOI] [PubMed] [Google Scholar]
- Kuhn D. M., Meyer M. A., Lovenberg W. Activation of rat brain tryptophan hydroxylase by polyelectrolytes. Biochem Pharmacol. 1979 Nov 15;28(22):3255–3260. doi: 10.1016/0006-2952(79)90118-7. [DOI] [PubMed] [Google Scholar]
- Kuwano R., Usui H., Maeda T., Fukui T., Yamanari N., Ohtsuka E., Ikehara M., Takahashi Y. Molecular cloning and the complete nucleotide sequence of cDNA to mRNA for S-100 protein of rat brain. Nucleic Acids Res. 1984 Oct 11;12(19):7455–7465. doi: 10.1093/nar/12.19.7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S. C., Ledley F. D., DiLella A. G., Robson K. J., Woo S. L. Nucleotide sequence of a full-length complementary DNA clone and amino acid sequence of human phenylalanine hydroxylase. Biochemistry. 1985 Jan 29;24(3):556–561. doi: 10.1021/bi00324a002. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D., DiLella A. G., Kwok S. C., Woo S. L. Homology between phenylalanine and tyrosine hydroxylases reveals common structural and functional domains. Biochemistry. 1985 Jul 2;24(14):3389–3394. doi: 10.1021/bi00335a001. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Greengard P. Protein kinases in the brain. Annu Rev Biochem. 1985;54:931–976. doi: 10.1146/annurev.bi.54.070185.004435. [DOI] [PubMed] [Google Scholar]
- Nakata H., Fujisawa H. Tryptophan 5-monooxygenase from mouse mastocytoma P815. A simple purification and general properties. Eur J Biochem. 1982 Jun;124(3):595–601. doi: 10.1111/j.1432-1033.1982.tb06636.x. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Woodgett J. R., Cohen P., Kemp B. E. Substrate specificity of a multifunctional calmodulin-dependent protein kinase. J Biol Chem. 1985 Nov 25;260(27):14471–14476. [PubMed] [Google Scholar]
- Vigny A., Henry J. P. Bovine adrenal tyrosine hydroxylase: comparative study of native and proteolyzed enzyme, and their interaction with anions. J Neurochem. 1981 Feb;36(2):483–489. doi: 10.1111/j.1471-4159.1981.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. In vitro phosphorylation of bovine adrenal tyrosine hydroxylase by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1979 Jan 25;254(2):503–507. [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Tyrosine 3-monoxygenase is phosphorylated by Ca2+-, calmodulin-dependent protein kinase, followed by activation by activator protein. Biochem Biophys Res Commun. 1981 May 29;100(2):807–813. doi: 10.1016/s0006-291x(81)80246-x. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Nakata H., Fujisawa H. A new activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+-, calmodulin-dependent protein kinase. Purification and characterization. J Biol Chem. 1981 Jun 10;256(11):5404–5409. [PubMed] [Google Scholar]