Abstract
Background:
C-reactive protein (CRP) is an important risk factor for cardiovascular disease. Furthermore, it has been reported that levels of CRP are increased in patients with obstructive sleep apnea (OSA). The aim of this study was to examine the effects of long-term therapy with nasal continuous positive airway pressure (nCPAP) on CRP levels and to investigate whether compliance with nCPAP therapy more effectively attenuated markers of systemic inflammation in patients with OSA.
Methods and results:
Fifty-five patients (mean [± SEM] age, 55 ± 2 years; 44 male patients, 11 female patients) with newly diagnosed moderate-to-severe OSA (apnea-hypopnea index > 20 events/h) were studied before and after 6 months of nCPAP treatment. There was a significant reduction in CRP levels after nCPAP therapy (before nCPAP therapy, 0.23 ± 0.03 mg/dL; after nCPAP therapy, 0.17 ± 0.02 mg/dL; p < 0.01). Additionally, we divided these patients into two groups based on adherence to nCPAP therapy. A group of patients using nCPAP > 4 h/d and > 5 d/wk were designated as the good compliance group. The decrease in CRP concentration was significant (before nCPAP therapy, 0.23 ± 0.04 mg/dL; after nCPAP therapy, 0.16 ± 0.03 mg/dL; p < 0.05) in the good compliance group but not in the poor compliance group (before nCPAP therapy, 0.24 ± 0.05 mg/dL; after nCPAP therapy, 0.20 ± 0.05 mg/dL; p = 0.21). Furthermore, we divided those patients into a high CRP group (≥ 0.2 mg/dL) and a normal CRP group (< 0.2 mg/dL) before nCPAP therapy. The significant decrease in CRP levels in the good compliance group was evident only in those patients with an initially elevated CRP level (before nCPAP therapy, 0.48 ± 0.08 mg/dL; after nCPAP therapy, 0.29 ± 0.06 mg/dL; p < 0.05).
Conclusion:
Appropriate use of nCPAP in patients with OSA may be required to decrease elevated CRP levels, with possible implications for cardiovascular morbidity and mortality.
Obstructive sleep apnea (OSA) has been increasingly linked to cardiovascular disease.1,2 C-reactive protein (CRP), an important serum marker of systemic inflammation, has been considered to be a predictor of cardiovascular events.3,4 CRP levels have been reported to be increased in adults5 and in children6 with OSA. Punjabi and Beamer7 reported that a strong association was found between the degree of sleep-disordered breathing and serum levels of CRP, with or without adjustment for age and several measures of adiposity. Nasal continuous positive airway pressure (nCPAP) therapy improves cardiac function in chronic heart failure patients with OSA,8 lowers BP,9 and may reduce their mortality.10 In a previous study,11 nCPAP also decreased levels of CRP and interleukin (IL)-6 in patients with OSA. The improvement of apnea-related hypoxemia by nCPAP may decrease neurohumoral activation and cytokines and systemic inflammation, leading to decreased cardiovascular morbidity and mortality. Although nCPAP is effective in the management of OSA, it is also intrusive and poorly accepted by some patients. There are few data regarding responses of systemic inflammation according to compliance with nCPAP, and the available data are controversial.10,12 Variations in nCPAP therapy compliance may contribute to the inconsistency found in prior studies. We tested the hypotheses that nCPAP therapy would lower CRP in patients with moderate-to-severe OSA and that better compliance with nCPAP therapy would more effectively lower CRP.
Materials and Methods
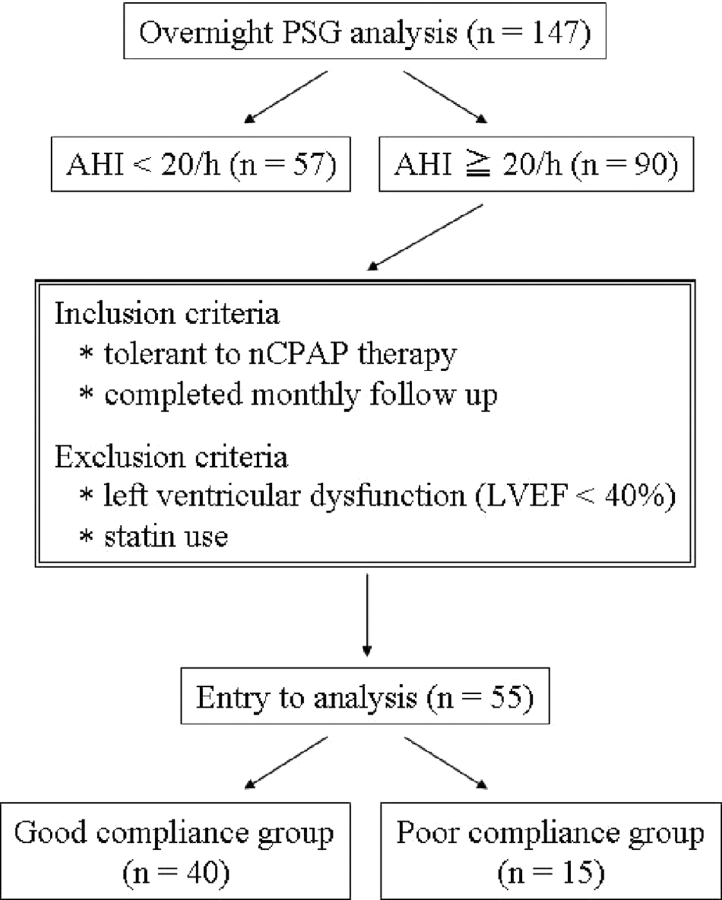
One hundred forty-seven patients (mean [± SEM] age, 57 ± 1 years; 109 male patients, 38 female patients) with suspected OSA underwent overnight polysomnography. Ninety of 147 patients were newly diagnosed with moderate-to-severe OSA (apnea hypopnea index, > 20 events per hour). Fifty-five patients with OSA (mean age, 55 ± 2 years; 44 male patients, 11 female patients) who tolerated nCPAP therapy and completed monthly follow-ups were recruited to participate in our present study. BP and heart rate were measured in the sitting position after at least a 15-min rest in the early morning. Baseline blood samples were obtained while the patient was fasting, in the early morning. The hours per day and percentage of days that nCPAP was used were monitored (IC card; Respironics, Inc; Murrysville, PA) with monthly clinical assessment. Additionally, we divided these patients into two groups based on usage of nCPAP. The group of patients using nCPAP > 4 h/d and > 5 d/wk was designated as the good compliance group. After nCPAP treatment for a mean duration of 6.1 ± 0.5 months, blood samples were drawn again. Serum levels of CRP were measured with latex agglutination immunoassay (Mitsubishi Kagaku Yatoron; Tokyo, Japan) [detection range, 0.01 to 40 mg/dL; normal range, < 0.2 mg/dL]. None of the patients was receiving statin therapy. The study was approved by the Institutional Review Board for Human Investigation, and written informed consent was obtained from all subjects before the study.
Statistical Analysis
The difference between the good compliance and poor compliance groups was tested by an unpaired t test. Categorical variables were compared using a χ2 test. The change in CRP concentration before and after nCPAP therapy was tested by a paired t test. A value of p < 0.05 was considered significant.
Results
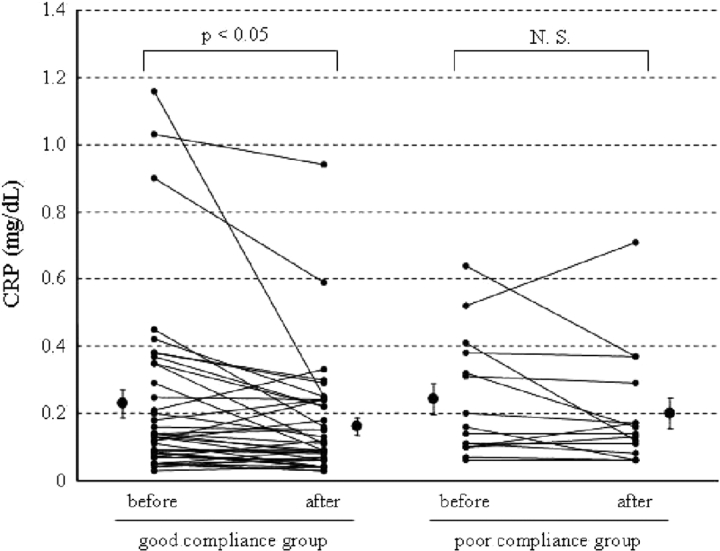
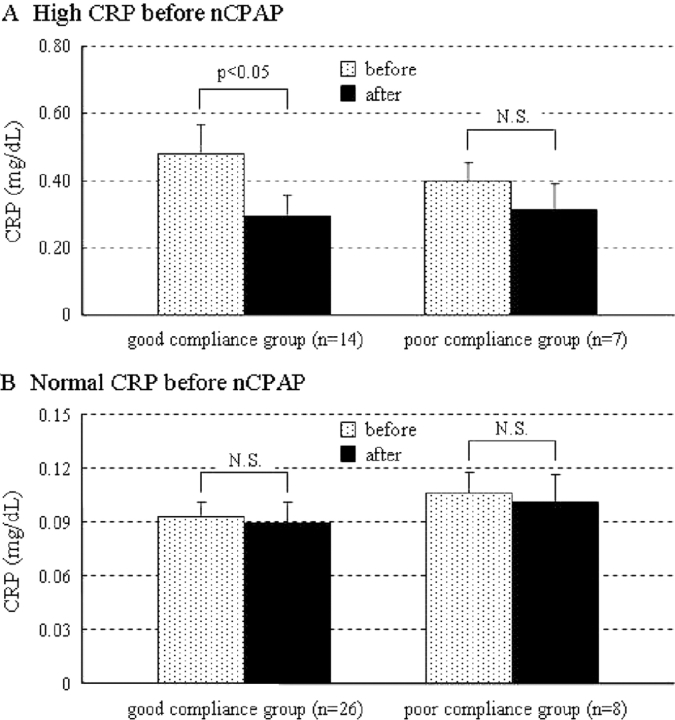
Fifty-five patients with OSA were treated with nCPAP (duration, 6.1 ± 0.5 months). Overall, there was a significant reduction in CRP levels after nCPAP therapy (before nCPAP therapy, 0.23 ± 0.03 mg/dL; after nCPAP therapy, 0.17 ± 0.02 mg/dL; p < 0.01) [Fig 1]. Additionally, we divided these patients into two groups by usage of nCPAP. There was no significant difference between the good compliance group and poor compliance group in the demographic data (Table 1), with the exception of triglyceride levels. The decrease in CRP was significant (before nCPAP therapy, 0.23 ± 0.04 mg/dL; after nCPAP therapy, 0.16 ± 0.03 mg/dL; p < 0.05) in the good compliance group (n = 40) but not in the poor compliance group (n = 15) [before nCPAP therapy, 0.24 ± 0.05 mg/dL; after nCPAP therapy, 0.20 ± 0.05 mg/dL; p = 0.21] (Fig 2). However, nCPAP therapy did not decrease CRP levels in patients with OSA whose CRP concentration was already within normal limits (< 0.2 mg/dL). A reduction of CRP levels was seen only in patients with OSA whose CRP levels were high (≥0.2 mg/dL), and only in the good compliance group (Fig 3). Body mass index (BMI) did not change significantly in either the good compliance group or the poor compliance group. Systolic BP decreased significantly after nCPAP therapy only in the good compliance group. Lipid profiles decreased significantly in both groups (Table 2).
Figure 1.
Flowchart of patient recruitment.
Table 1.
Demographic Data in Patients With Moderate-to-Severe OSA
| Data | Good Compliance (n = 40) | Poor Compliance (n = 15) | p Value |
|---|---|---|---|
| Age, yr | 57 ± 2 | 50 ± 4 | 0.068 |
| Gender | 0.999 | ||
| Male | 32 | 12 | |
| Female | 8 | 3 | |
| AHI, events/h | 47.8 ± 4.3 | 46.3 ± 6.4 | 0.853 |
| Lowest Spo2, % | 77 ± 2 | 78 ± 3 | 0.665 |
| Arousal index, arousals/h | 37.0 ± 3.5 | 31.9 ± 6.2 | 0.459 |
| BMI, kg/m2 | 29.3 ± 1.1 | 31.5 ± 1.3 | 0.273 |
| Systolic BP, mm Hg | 141 ± 3 | 141 ± 5 | 0.952 |
| Diastolic BP, mm Hg | 84 ± 2 | 86 ± 4 | 0.606 |
| Heart rate, beats/min | 72 ± 2 | 77 ± 5 | 0.242 |
| Total cholesterol, mg/dL | 211 ± 6 | 211 ± 8 | 0.985 |
| TG, mg/dL | 138 ± 14 | 208 ± 25 | 0.019 |
| HDL cholesterol, mg/dL | 52 ± 2 | 50 ± 2 | 0.510 |
| FPG, mg/dL | 101 ± 4 | 94 ± 4 | 0.297 |
| Smoking, % | 22.0 | 26.7 | 0.746 |
| Hypertension, % | 45.0 | 40.0 | 0.739 |
| Diabetes mellitus, % | 17.5 | 20.0 | 0.831 |
Values are given as the mean ± SEM, unless otherwise indicated. AHI = apnea hypopnea index; FPG = fasting plasma glucose; HDL = high-density lipoprotein; Spo2 = pulse oximetric saturation; TG = triglyceride.
Figure 2.
Effect of nCPAP therapy on CRP levels in the good compliance group (n = 40) and the poor compliance group (n = 15). CRP levels decreased significantly only in the good compliance group. Data are given as the mean ± SEM.
Figure 3.
Comparison of CRP changes both in patients with high CRP levels (≥ 0.2 mg/dL) [A] and in OSA patients with normal CRP levels (< 0.2 mg/dL) [B] after nCPAP therapy. Data are given as the mean ± SEM.
Table 2.
Changes in Demographic Data Before and After nCPAP Therapy
| All (n = 55) |
Good Compliance (n = 40) |
Poor Compliance (n = 15) |
||||
|---|---|---|---|---|---|---|
| Data | Before | After | Before | After | Before | After |
| BMI, kg/m2 | 29.9 ± 0.9 | 29.5 ± 0.9 | 29.3 ± 1.1 | 29.0 ± 1.1 | 31.5 ± 1.3 | 31.0 ± 1.2 |
| Systolic BP, mm Hg | 141 ± 2 | 135 ± 3* | 141 ± 3 | 133 ± 3* | 141 ± 5 | 141 ± 6 |
| Diastolic BP, mm Hg | 84 ± 2 | 82 ± 2 | 84 ± 2 | 82 ± 2 | 86 ± 4 | 80 ± 4 |
| Heart rate, beats/min | 73 ± 2 | 72 ± 2 | 72 ± 2 | 73 ± 2 | 77 ± 5 | 70 ± 3 |
| Total cholesterol, mg/dL | 211 ± 5 | 196 ± 4† | 211 ± 6 | 195 ± 5† | 211 ± 8 | 199 ± 7* |
| TG, mg/dL | 154 ± 13 | 127 ± 9† | 138 ± 14 | 116 ± 10* | 208 ± 25 | 160 ± 21† |
| HDL cholesterol, mg/dL | 52 ± 2 | 52 ± 2 | 52 ± 2 | 52 ± 2 | 50 ± 2 | 51 ± 3 |
| FPG, mg/dL | 99 ± 3 | 103 ± 2 | 101 ± 4 | 104 ± 3 | 94 ± 4 | 99 ± 3 |
Values are given as the mean ± SEM. See Table 1 for abbreviations not used in the text.
*p < 0.05 vs before.
†p < 0.01 vs before.
Discussion
In the present study, serum levels of CRP decreased significantly in patients with OSA after a mean duration of 6.1 ± 0.5 months of nCPAP therapy. However, when we divided these patients into those with appropriate vs inadequate use of nCPAP, a significant reduction in CRP levels was seen only in those OSA patients with appropriate use of nCPAP and when initial CRP levels were elevated. Therefore, compliance with nCPAP is important for improving levels of inflammatory markers in patients with OSA.
CRP promotes the secretion of inflammatory mediators by the vascular endothelium13,14 and opsonizes low-density lipoprotein for uptake by macrophages in atherosclerotic plaque.15 These data suggest that CRP may be directly implicated in the development of atherosclerotic lesions. Ridker3 classified cardiovascular risk with high-sensitive CRP, which has a lower detection limit. Lowest (quintile 1) to highest (quintile 5) vascular risk was 0.01 to 0.069 mg/dL to > 0.38 mg/dL. The adjusted relative risk of future myocardial infarction associated with increasing quintiles of high-sensitive CRP among apparently healthy middle-aged men and women was 1.0 to 2.5 in men and 1.0 to 3.0 in women. With this risk estimation, CRP levels in our patients with OSA placed them in the high-risk group. A reduction in serum levels of CRP is thought to be beneficial for cardiovascular risk reduction.16
Yokoe et al10 reported that 1 month of nCPAP therapy decreased CRP and IL-6 levels in patients with OSA. In contrast, Akashiba et al12 described the fact that > 6 months of nCPAP therapy did not decrease CRP levels in patients with OSA. Whether short-term or long-term nCPAP therapy decreases CRP levels in patients with OSA is still controversial, and adherence to nCPAP therapy may be an important determinant. This is especially relevant to studies of the longer-term use of nCPAP when compliance may decrease over time.17 Therefore, we focused on compliance with nCPAP therapy in the present study. We monitored the number of hours per day and the percentage of days (IC card; Respironics, Inc) and conducted a monthly clinical assessment. We further divided these patients into two groups based on compliance. A significant reduction of CRP levels was seen in OSA patients whose CRP concentrations were high (≥ 0.2 mg/dL) only in the good compliance group.
The potential mechanisms for the reduction of CRP with appropriate nCPAP therapy may include the following: first, reduced hypoxic stress with attenuated nocturnal oxygen desaturation; second, reduced sympathetic excitation; and third, improved sleep quality in patients with OSA. Increased plasma levels of IL-6, IL-1 receptor antagonist, and CRP, and synthesis of fibrinogen have been noted during hypoxic conditions at high altitude.18,19 Moreover, nocturnal hypoxia was positively correlated with IL-6 levels in patients with OSA. The apnea-related hypoxia may be involved in the increased levels of IL-6, which induces the synthesis of acute-phase proteins, including CRP.20 Therefore, the appropriate use of nCPAP may decrease systemic inflammation via reducing hypoxic stress. Additionally, leptin, which is elevated in OSA patients,21 may be associated with increased CRP levels.22
Triglyceride levels decreased significantly in both the good and poor compliance groups in the present study. The effect of nCPAP therapy on dyslipidemia has still not been well elucidated. One potential mechanism may be related to lipoprotein lipase (LPL). Iesato et al23 reported decreased LPL levels in OSA patients. LPL might play a major role in lipid metabolism by hydrolyzing triglyceride-rich lipoproteins. After 3 months of nCPAP therapy, LPL levels increased significantly in patients with OSA in the study. Increased LPL levels after nCPAP therapy may decrease triglyceride levels.
Both acute total and acute partial sleep deprivation may also induce elevated high-sensitive CRP concentrations in healthy volunteers.24 Improved sleep quality with effective nCPAP therapy for OSA may also contribute to attenuated systemic inflammation.
A limitation of the present study is that some of our subjects were smokers and were being treated for hypertension and diabetes mellitus. However, we did not change or add any medications in these subjects during the 6-month follow-up period. Furthermore, we excluded patients who were receiving therapy with statins from this analysis because statins alter CRP levels. Another limitation is that although BMI changes were not different in the good vs poor compliance groups, we cannot exclude the effects of changes in body fat or in fat distribution as a potential cause for changes in CRP levels. Finally, these studies were conducted in Japanese patients. It is possible that there may be ethnic or racial differences in the interactions between OSA, CRP, and nCPAP therapy.
In conclusion, long-term nCPAP therapy was associated with decreased CRP levels in patients with OSA and initially elevated CRP levels. The decrease in CRP levels was significant in the good compliance group but not in the poor compliance group. This selective decrease was not explained by other variables such as changes in drug therapy or greater weight loss in the good compliance group. The appropriate use of nCPAP therapy in patients with OSA may be required to decrease serum levels of CRP, with possible implications for decreased cardiovascular morbidity and mortality.
Acknowledgment:
We thank Debra L. Pfeifer for her secretarial assistance.
Abbreviations:
- BMI
body mass index
- CRP
C-reactive protein
- IL
interleukin
- LPL
lipoprotein lipase
- nCPAP
nasal continuous positive airway pressure
- OSA
obstructive sleep apnea
Footnotes
This research was supported by National Institutes of Health grants HL65176, HL 73211, and RR00585 (to Dr. Somers).
Dr. Somers has served as a consultant for ResMed, Respironics, Medtronic, GlaxoSmithKline, Sepracor, and Cardiac Concepts. He has received research grants from the ResMed Foundation, the Respironics Sleep and Breathing Foundation, ELA Medical, and Select Research, Inc. Drs. Ishida, M. Kato, Yanagihara, Kinugasa, Kotani, Igawa, Hisatome, and Shigemasa, and Mr. Y. Kato have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Nieto FJ, Young TB, Lind BK, et al. Association of sleep disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 4.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–2464. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 6.Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–1984. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 7.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep. 2007;30:29–34. doi: 10.1093/sleep/30.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 9.He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea: experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- 10.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 11.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 12.Akashiba T, Akahoshi T, Kawahara S, et al. Effects of long-term nasal continuous positive airway pressure on C-reactive protein in patients with obstructive sleep apnea syndrome. Intern Med. 2005;44:899–900. doi: 10.2169/internalmedicine.44.899. [DOI] [PubMed] [Google Scholar]
- 13.Lagrand WK, Visser CA, Hermens WT, et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100:96–102. doi: 10.1161/01.cir.100.1.96. [DOI] [PubMed] [Google Scholar]
- 14.Pasceri V, Willerson JT, Yeh ETH. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 15.Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103:1194–1197. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 17.Collard PH, Pieters TH, Aubert G, et al. Compliance with nasal CPAP in obstructive sleep apnea patients. Sleep Med. 1997;1:33–44. doi: 10.1016/s1087-0792(97)90004-6. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann G, Tschop M, Fischer R, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12:246–252. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- 19.Imoberdorf R, Garlick PJ, McNurlan MA, et al. Enhanced synthesis of albumin and fibrinogen at high altitude. J Appl Physiol. 2001;90:528–537. doi: 10.1152/jappl.2001.90.2.528. [DOI] [PubMed] [Google Scholar]
- 20.Heinrich PC, Castell JV, Andus T. Interleukin-6 and acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips BG, Kato M, Narkiewicz K, et al. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279:H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 22.Shamsuzzaman ASM, Winnicki M, Wolk R, et al. An independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004;109:2181–2185. doi: 10.1161/01.CIR.0000127960.28627.75. [DOI] [PubMed] [Google Scholar]
- 23.Iesato K, Tatsumi K, Saibara T, et al. Decreased lipoprotein lipase in obstructive sleep apnea syndrome. Circ J. 2007;71:1293–1298. doi: 10.1253/circj.71.1293. [DOI] [PubMed] [Google Scholar]
- 24.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effects of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]