Growing awareness of selective publication of research studies (“publication bias”) and the selective reporting of outcomes in publications (“outcome reporting bias”),1 has led policymakers to call for increased “clinical trial transparency” through the public disclosure of key information about clinical trials.2 A US federal law3 enacted in 2007 mandates registration and results reporting for certain clinical trials of drugs, biological products, and devices, regardless of study funding source, at ClinicalTrials.gov, an online registry and results database operated by the National Library of Medicine of the National Institutes of Health (NIH).
Table 1 summarizes the goals of the results database. The theoretical benefits and limitations of non-peer-reviewed public results databases have been discussed elsewhere.4–6 This article summarizes the current requirements for reporting results and describes how to submit results data elements to the ClinicalTrials.gov results database. These tips for creating clear, understandable entries may be kept as a resource for use when preparing to enter results data for a clinical trial. A previous “Medical Writing Tips” article focused on trial registration.7
Table 1.
Results Database Purposes for Various Groups
| Results Database Purpose | Group That Benefits |
|---|---|
| Provide public record of basic study results in a standardized format | Researchers, journal editors, institutional review boards, and ethicists |
| Promote fulfilling of ethical responsibility to participants; use of research results to contribute to medical knowledge | Patients, general public, and research community |
| Mitigate “publication” and “outcome reporting” biases | Users of medical literature |
| Facilitate systematic reviews and other analyses of the research literature | Researchers and policymakers |
Background
Section 801 of the Food and Drug Administration Amendments Act (FDAAA 801) expands the scope of mandatory clinical trial registration and adds new requirements for reporting results at ClinicalTrials.gov (http://clinicaltrials.gov/).8 The ClinicalTrials.gov “basic results” database was launched in September 2008. Both registration and results reporting are accomplished through the Web-based Protocol Registration System (PRS) [http://prsinfo.clinicaltrials.gov/].
In general, the law requires study sponsors or designated principal investigators (PIs) [called “responsible parties” in FDAAA 801] to report summary results information for interventional studies of drugs, biological products, and devices within 1 year of completing data collection for the prespecified primary outcome, regardless of sponsor or funding source. Results data are submitted online through the PRS and are displayed with the corresponding registered summary protocol information at ClinicalTrials.gov.9 Results submission may be delayed under certain circumstances described in the law. Noncompliance could result in penalties specified by the law, such as the withholding of NIH grant funding and civil monetary penalties of up to $10,000 a day. (See http://prsinfo.clinicaltrials.gov/fdaaa.html for more information on reporting requirements).
Results reporting in the database will commonly occur prior to journal publication. Unlike journal articles that have been reviewed by both scientific colleagues and editors, results submitted to ClinicalTrials.gov are not peer reviewed prior to posting, although minimal quality standards must be met. The public display of results in stand-alone tables needs to be self-explanatory and meaningful to a range of users. Results entries do not contain discussions or conclusions. However, as in writing a manuscript for a journal, an individual familiar with the study design and data analysis (eg, a clinical investigator) will need to carefully consider ways to organize and annotate the results in order to optimize data presentation, especially for complex clinical study designs and results. Several leading medical journals have stated that reporting results to ClinicalTrials.gov in compliance with FDAAA 801 will not be considered “prior publication.”10–12
Reporting Requirements
For Which Trials Should Results Be Reported?
Under FDAAA 801, basic results must be reported for applicable clinical trials involving drugs or devices that have been approved by the US Food and Drug Administration (FDA). The term applicable clinical trial includes studies that meet the following criteria:
Phase 2 to 4 interventional studies;
Studies involving drugs, biological products, and medical devices regulated by the FDA; and
Studies having at least one site in the United States or are conducted under an investigational new drug application or investigational device exemption; and
Studies initiated or ongoing as of September 27, 2007, or later.
Note that the following types of studies are excluded from the mandates of the law: phase 1 drug trials including “studies in which investigational drugs are used as research tools to explore biological phenomena or disease processes13;” small feasibility/pilot studies of devices; and noninterventional (observational) clinical research, such as case series and cohort studies.
When Should Results Be Reported?
In general, FDAAA 801 specifies that results need to be reported no later than 12 months after the date of final data collection for the prespecified primary outcome measure (called “Primary Completion Date” in ClinicalTrials.gov). Results submissions can be delayed for the following applicable clinical trials:
Trials of a drug or device that has not been initially approved for marketing (if the drug or device is approved, submission is required within 30 days after approval);
Trials of a drug or device for which the manufacturer has filed or will file an application seeking approval of the new use studied in the trial; or
Trials for which a request that “demonstrates good cause” has been granted by the Director of the NIH.
Who Should Report Results?
Under FDAAA 801, the “responsible party” is required to report results information. The term responsible party is defined as follows:
The study sponsor (eg, the holder of an investigational new drug application/investigational device exemption or grantee); or
The PI responsible for conducting the study, analyzing the data, and publishing the results, if designated by the sponsor.
Note that the responsible party (ie, the sponsor or designated PI) needs to be specified on initial registration of the study.
What Results Information Should Be Reported?
The following two types of results information are to be reported (see draft results data element definitions at http://prsinfo.clinicaltrials.gov/results_definitions.html):
Scientific information, organized as four “modules” (ie, participant flow, baseline characteristics, outcome measures and statistical analyses, and adverse events) [Table 2]; and
Administrative information (eg, a point of contact to obtain more information about the reported results).
Table 2.
Summary Description of the ClinicalTrials.gov Results Database Modules
| Basic Results Module | Summary Description | Overview of Minimum Required Information |
|---|---|---|
| Participant flow | Description of the No. of research participants starting and completing the study, including exclusions and dropouts, for each arm or comparison group (frequently reported as a CONSORT diagram in a journal article) | No. of participants who entered study; and No. of participants who completed study |
| Baseline characteristics | Demographic and baseline data for the study population and each arm or comparison group (frequently reported as “Table 1” in a journal article) | Overall No. of participants analyzed; age; gender; for all other measures reported: name (and description); unit of measurement; and summary data, total and by arm |
| Outcome measures and statistical analyses | Table of outcome measure values for each arm/comparison group, including appropriate statistical analyses | For all prespecified primary and secondary outcome measures: name and description; unit of measurement; time frame; analysis population; and summary data, total and by arm |
| Adverse events (optional prior to September 2009) | Number and frequency of all serious adverse events and other adverse events exceeding a specified frequency threshold in each arm/group, grouped by organ system | For all adverse events reported: adverse event term; organ system; type of assessment (spontaneous vs systematic); and No. of participants affected, No. of participants at risk, and total No. affected, by arm |
How Do I Report Basic Results?
There are two methods for reporting basic results in the PRS, as follows: (1) interactive data entry using online forms; and (2) automated batch upload of results data files. Both types of submissions require that data providers have PRS accounts. Investigators should check with their institutions to determine their account status. If no account exists, a new account can be established by completing the “Account Application Process” at http://prsinfo.clinicaltrials.gov/. The “User's Guide”, available from the PRS main menu (under “Help”), includes a section on “Procedures for Results.” Note that the submission of basic results requires a completed registration record in ClinicalTrials.gov. Inquiries regarding the identification or use of PRS accounts should be directed to register@clinicaltrials.gov.
Overall, each ClinicalTrials.gov record represents a single study regardless of the number of study sites (ie, a multisite clinical study is reported as a single record), and consists of a protocol section (the “registration”) and a results section. (Note that substudies may be registered as a single, integrated record with sections describing each substudy or as separate records that refer to previous studies [eg, participation in the “main” study as an inclusion criterion], as appropriate). Data providers create the protocol section and register summary protocol information when their studies are initiated.7 Subsequently, data providers create the results section for these study records when data collection for at least one primary outcome measure is complete.
Data providers must complete the required fields with meaningful entries and are encouraged to complete all optional data elements where appropriate to their study. Prior to submitting results data, we recommend reviewing the “Helpful Hints” and “Common Errors” documents (available at http://prsinfo.clinicaltrials.gov/fdaaa.html). The “Helpful Hints” document contains sample results reports for common study models, including a parallel design, crossover design, diagnostic accuracy, and bioequivalence study. The “Common Errors” document illustrates types of errors frequently identified in results submissions and provides tips for avoiding such errors. Both documents are updated as required. Once results data have been entered, but prior to submission, it may be helpful to ask a colleague who is familiar with the overall research area but has not been involved in that particular trial to review the tables for comprehension and clarity.
After the results data are submitted, the ClinicalTrials.gov staff reviews the submissions before public posting. These reviews focus on apparent validity (when possible), meaningful entries, logic and internal consistency of data tables, and formatting. (Review criteria are available at http://prsinfo.clinicaltrials.gov/fdaaa.html.) Data providers may be asked to clarify items or make corrections to the protocol or results section of the study record. Following the initial posting of the results section, the data provider may make updates and edits at any time. The most recent version of the record is displayed at ClinicalTrials.gov. A history of all posted changes is publicly available at the archive site (http://clinicaltrials.gov/archive/).
Using Modules to Report Results
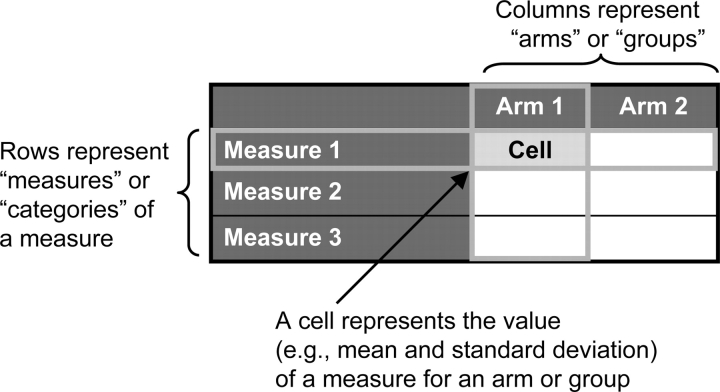
The results section consists of the following: (1) scientific information, consisting of discrete modules that represent information in a series of data tables with supporting notes (Table 2); and (2) administrative information, consisting of semistructured fields. The scientific information requires the sponsor or PI to define the rows (ie, “measures”) and columns (ie, “arms/groups”) of tables before populating the cells with results data (Fig 1). For each table, the measures and arms/groups need to be labeled with meaningful titles and descriptions to allow viewers to understand the data. Considerations for creating tables for each of the four modules are described below. Additional user documentation for reporting results is available at http://prsinfo.clinicaltrials.gov/fdaaa.html.
Figure 1.
General structure of a results data table.
Participant Flow
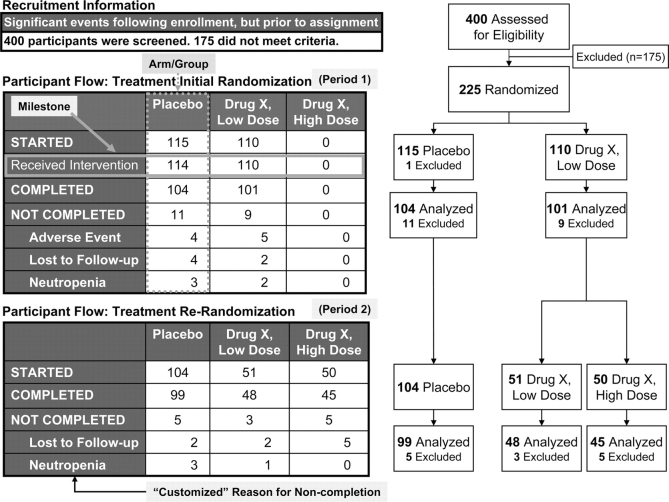
ClinicalTrials.gov uses a tabular format to represent the number of participants involved in a study. This information is typically depicted in journal articles as a Consolidated Standards of Reporting Trials (or CONSORT) flow diagram.14 The participant flow module accommodates a range of study designs, from simple to complex, and allows for the description of key events following study enrollment, but prior to group assignment. At a minimum, the total number of participants starting and completing the overall study needs to be reported for each study arm. Additional detail can be provided by defining more than one study period (eg, for washout, drug initiation, or follow up). Within each period, any number of milestones, representing specific events or time points, may be defined (eg, “received intervention”). Reasons for noncompletion (from a pull-down menu) may be provided.
Figure 2 illustrates a participant flow with two significant intervals of trial activity or periods (ie, “Initial Randomization” and “Re-Randomization”). Note that Figures 2–7 are intended to illustrate concepts related to the ClinicalTrials.gov basic results database modules and do not represent a single study. “Drug X,” “Low dose,” and “High dose” are used for illustrative purposes only. In an actual submission, the drug name and dosages would be provided. In Figure 2, note that period 1 contains a study-specific milestone (“Received Intervention”). While three arms are defined for both periods, only two arms are used during period 1. Reasons for noncompletion are reported for each period.
Figure 2.
Participant flow display in ClinicalTrials.gov (left) and corresponding CONSORT diagram (right).
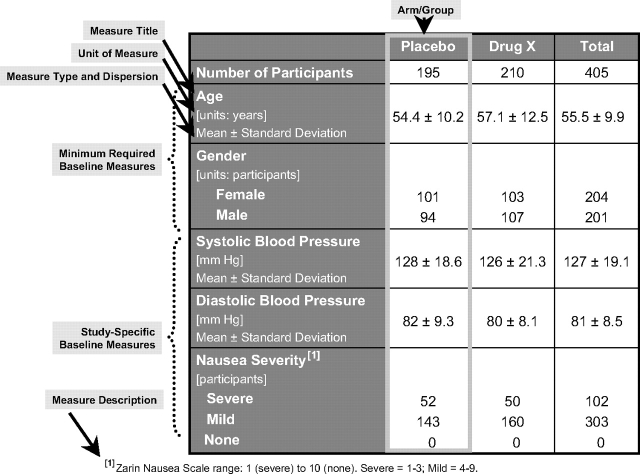
Figure 3.
Baseline characteristics display.
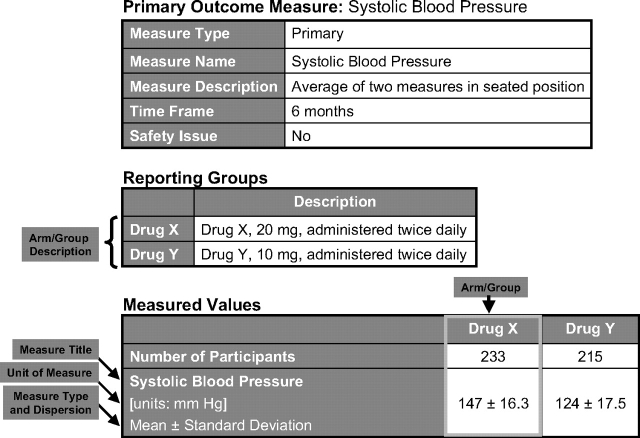
Figure 4.
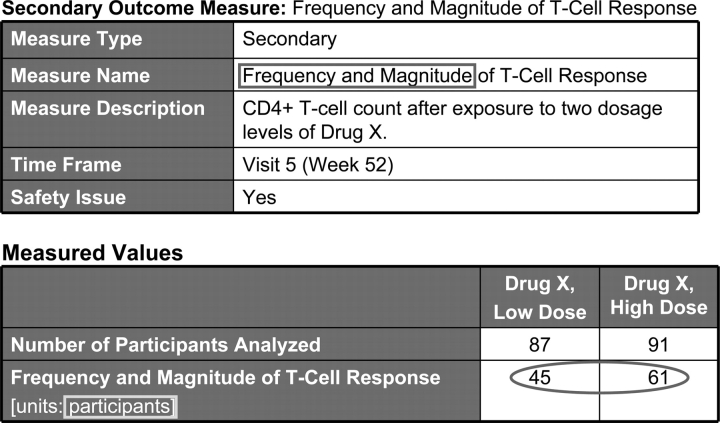
Outcome measure display.
Figure 5.
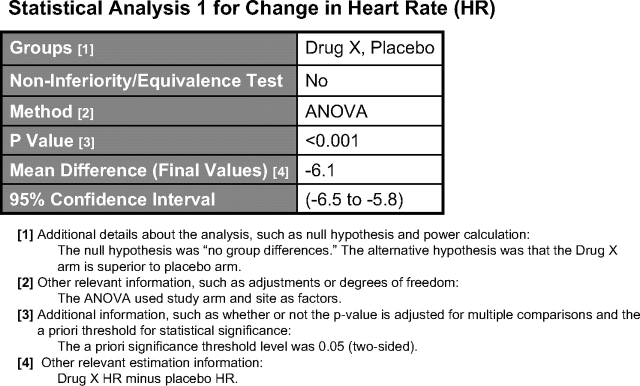
Statistical analysis display.
Figure 6.
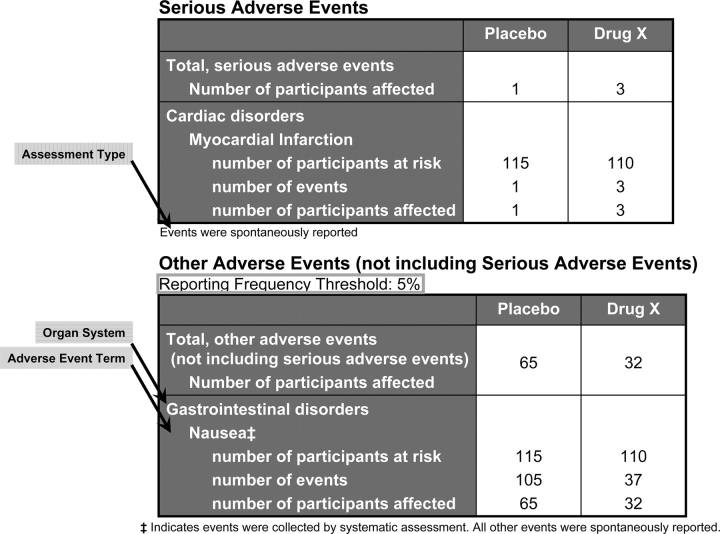
Reported adverse events display.
Figure 7.
Example of mismatch among outcome measure name, unit of measure, and data.
When multiple periods are specified, the number of participants completing a period generally equals the number starting the subsequent period. If milestones are defined, the number of participants achieving each milestone must be less than or equal to the number starting the period and achieving previous milestones. Conversely, the number of participants in each milestone must be greater than or equal to the number completing the period or achieving any subsequent milestones.
Baseline Characteristics
Data entry for baseline characteristics and outcome measures (described below) consists primarily of building and populating data tables, which include the following steps:
Specifying the table columns (eg, arm/group title and description) and rows (eg, measure title and unit of measure);
Stating what type of data will be entered in the cells, as indicated by measure type (eg, number [of participants], mean, median) and measure of dispersion (eg, SD, interquartile range), as appropriate;
Entering values in the cells of the data tables; and
Providing brief comments in measure description to clarify aspects of the data tables, as necessary.
The baseline characteristics table (Fig 3) is used to describe demographic and baseline characteristics of all participants in the study sample and within each arm or comparison group. A number of default demographic characteristics are provided (ie, age, gender, race, ethnicity, and region of enrollment). At a minimum, the age and gender of the participants need to be reported. Age may be reported using either continuous data (eg, mean age and SD) or categorical data (eg, customized or prespecified categories of “≤ 18 years,” “between 18 and 65 years,” and “≥ 65 years”). In addition, any number of study-specific baseline measures can be reported. Each study-specific characteristic should include a specific title that describes the baseline measure (eg, “prior antihypertensive treatment”) and, if necessary, a more complete baseline measure description (eg, what was measured and how was it measured). Each measure must also include the measure type (eg, number, mean, median) and a measure of dispersion for continuous variables (eg, SD, full range, interquartile range). Unit of measure must be given for the values reported (eg, “mm Hg” for BP).
Particular attention should be paid to ensuring that the baseline measure title, description, and unit are consistent. For example, even though “nausea severity” was measured using a point scale at baseline (Fig 3), the categories refer to the number of participants with levels of nausea (“severe,” “mild,” or “none”) at baseline; therefore, the unit of measure is “participants” (for categorical data) rather than “units on a scale” (for continuous data). Note that the baseline measure description data element, used to define the categories, is displayed as a footnote.
Outcome Measures and Statistical Analyses
FDAAA 801 requires the reporting of data for all prespecified primary and secondary outcomes. Other prespecified (ie, not primary or secondary) and post hoc outcome measures may also be reported. Each outcome is represented by a data table (Fig 4). At a minimum, the following outcome information is required: an informative outcome measure title (what was measured); a specific description (how it was measured); the time frame for assessment (when it was measured); measure type (eg, number, median); and a measure of dispersion or precision for continuous measures (eg, SD, 95% confidence interval, interquartile range); and the unit of measure (what the reported values represent). Precise descriptions of outcome measures are necessary to interpret the data. Additional detail may be provided, such as how the number of participants for analysis was determined (ie, analysis population description) and statistical analyses associated with an outcome.
If the outcome data are categorical, the categories should be fully described. Unit of measure should directly reflect the values being reported (ie, what the numbers represent). The measure title, measure type, measure of dispersion (if appropriate), and unit of measure should be consistent with the value. For example, a measure title of “average hours of sleep per day,” a measure type of “mean,” and a unit of measure of “hours per day” are inconsistent with any value greater than 24.
Statistical analyses (Fig 5) are tied to specific outcomes data tables; any number of statistical analyses may be reported for an outcome measure. p Values, confidence intervals, or both, may be reported. For each analysis, the arms/groups compared and whether the analysis was a noninferiority or equivalence analysis must be reported. If a noninferiority analyses is reported, the definition of the noninferiority margin must also be provided. If a p value is reported, the name of the statistical test must be selected from a pull-down menu (eg, “χ2,” “t test, two-sided,” “other” specified tests). If a confidence interval is reported, the estimated value, level (eg, 95%), and upper and lower limits (if two sided), as well as the name of the estimation parameter (eg, “odds ratio,” “mean difference,” “other”) must be provided. Further descriptive information about the analysis may be provided in free-text fields (eg, information about the null hypothesis and power calculation).
Adverse Events
This module, which is not required by law prior to September 2009, contains the following two tables: (1) all serious adverse events (including death); and (2) other adverse events, not including serious adverse events, that are above a specified frequency threshold (Fig 6). At a minimum, for each adverse event, the adverse event term, organ system (from a pull-down menu), assessment type (ie, “systematic” or “spontaneous report”), number of participants at risk, and number of participants affected must be reported. The use of a controlled vocabulary, such as the Medical Dictionary for Regulatory Activities (MedDRa), is encouraged. If one is used, the source vocabulary name data element should be completed. In addition, each table must include the total number affected by serious or other nonserious adverse events in each arm. Note that the default reporting frequency threshold for reporting other nonserious adverse events is 5%. That is, any adverse event for which the number affected divided by the number at risk is greater than the designated reporting frequency threshold in any arm (eg, >5%) must be reported. All serious adverse events are to be reported in the serious adverse event table. Note that while a single type of event (eg, asthma) may appear in both tables, they necessarily represent different sets of participants based on the severity of the adverse event.
Reporting Different Study Designs and Measure Types
The “Helpful Hints” document at http://prsinfo.clinicaltrials.gov/fdaaa.html shows how to use the system to report different study types. For example, to report results for a study with a crossover design, a column could be used to represent each randomization group in participant flow (eg, drug A, then drug B and drug B, then drug A); a single column could be used for all participants in baseline characteristics (eg, the overall group); and columns could be used for each intervention in outcome measures and statistical analyses (eg, drug A and drug B). The numbers of participants should be consistent throughout the results section (ie, across modules), with any discrepancies explained using the appropriate data elements.
Categorical measures consist of two or more defined categories and are generally reported as counts or numbers (eg, “number” for measure type and “participants” for unit). Category titles should be descriptive and meaningful. Categories are typically nonoverlapping and comprehensive, covering all possible results appropriate for a measure. When there are only two (dichotomous) categories, it is preferable to provide both categories explicitly for greater clarity (eg, “with disease” and “without disease” for disease status). Note that outcomes such as “improved” or “responders” are actually dichotomous categories representing change over time. Both possible outcomes (eg, “improved” and “not improved”) should be reported, and the Outcome Measure Description should specify how the change was assessed, a threshold for improvement, and the time period of assessment (eg, improved: participants with threefold or greater decrease in serum concentration of antibody x at 6 weeks compared to baseline).
Continuous measures require a measure of central tendency (ie, measure type) and a measure of dispersion or precision (from pull-down menus). When continuous data are reported as categories, the conversion algorithm should be provided. For example, BP, measured as millimeters of mercury (or mm Hg) may be categorized as hypotensive, normal, and hypertensive. Each of the three categories should include ranges for the systolic and diastolic BPs, reported as millimeters of mercury.
Time-to-event measures are presented in a tabular format, even though they are frequently displayed in publications as figures (eg, Kaplan-Meier plot). Either categorical measures (dichotomous: eg, 5-year progression-free survival; or multichotomous: eg, progression-free survival at 1 year, 2 years, or 3 years) or continuous measures (eg, median time to progression) may be used to represent time-to-event measures.
Reporting a Scale
At a minimum, scale information must include the following:
Specific name of the scale, spelling out acronyms (eg, National Institutes of Health (NIH) Pain Severity Scale [outcome measure title]);
Description of the construct or domain that the scale measures if not clear from the title (eg, pain severity); and
The range and direction of the scores (eg, 0 is no pain, 10 is most severe pain); and
Unit of measure (generally “participants” for categorical data and “units on a scale,” if there are no other units, for continuous data).
Avoiding Common Errors
During the first several months of the operation of the basic results database, several types of results reporting errors were observed. For example, tables in the outcome measure module had mismatches among the measure title, unit of measure, and data (Fig 7). Other frequent errors included the following:
Participant flow numbers that do not make sense;
Reporting scales without sufficient information;
Logical errors in table construction; and
Inadequate titles and descriptions for arm/group and outcome measure data elements.
This article and the documents available at http://prsinfo.clinicaltrials.gov/fdaaa.html are intended to help data providers understand the results reporting process and avoid common errors. We have found that investigators can quickly learn to navigate the system and have successfully used it to report complicated studies.
Conclusion
Systematic reporting of the results of clinical studies in a structured, public results database is a relatively new development designed to increase transparency in clinical research and is expected to serve many different purposes. Under FDAAA 801, the results of certain clinical studies of FDA-approved drugs, biological products, and devices are required to be reported to the ClinicalTrials.gov basic results database. Given that these summary data are displayed in a tabular format with minimal narrative, it is critical that the labels for the rows (representing measures and their units) and columns (representing arms or comparison groups) be specified in a meaningful and precise manner to allow people not familiar with a study to interpret the data. Tables must be completed by an individual who is familiar with the study design and data analysis, and sufficient care must be taken to create and label data tables so that they are informative and easily interpreted. Knowledge, experience, and preplanning by the data provider will help the process of organizing a table and presenting results data in a tabular format that is meaningful and self-explanatory to people viewing basic results in a ClinicalTrials.gov record.
Acknowledgment:
The authors thank Alison Robbins, MA, National Library of Medicine, for her editorial assistance.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health, National Library of Medicine.
Dr. Tse is Senior Analyst at ClinicalTrials.gov, National Library of Medicine (NLM), and has no other financial conflicts to report. Dr. Williams is the Assistant Director of ClinicalTrials.gov, NLM, and has no other financial conflicts to report. Dr. Zarin is the Director of ClinicalTrials.gov, NLM, and has no other financial conflicts to report.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Hotz RL. What you don't know about a drug can hurt you: untold numbers of clinical-trial results go unpublished; those that are made public can't always be believed. [Accessed May 3, 2009]. Available at: http://online.wsj.com/article/SB122903390105599607.html.
- 2.Steinbrook R. Registration of clinical trials: voluntary or mandatory? N Engl J Med. 2004;351:1820–1822. doi: 10.1056/NEJMp048264. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. Amendments Act of 2007. Public Law No. 110–85. [Google Scholar]
- 4.Fisher CB. Public health: clinical trials results databases: unanswered questions. Science. 2006;311:180–181. doi: 10.1126/science.1119685. [DOI] [PubMed] [Google Scholar]
- 5.Zarin DA, Tse T. Medicine: moving toward transparency of clinical trials. Science. 2008;319:1340–1342. doi: 10.1126/science.1153632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch L. Trial registration and results disclosure: impact of US legislation on sponsors, investigators, and medical journal editors. Curr Med Res Opin. 2008;24:1683–1689. doi: 10.1185/03007990802114849. [DOI] [PubMed] [Google Scholar]
- 7.Zarin DA, Keselman A. Registering a clinical trial in ClinicalTrials.gov. Chest. 2007;131:909–912. doi: 10.1378/chest.06-2450. [DOI] [PubMed] [Google Scholar]
- 8.Wood AJJ. Progress and deficiencies in the registration of clinical trials. N Engl J Med. 2009;360:824–830. doi: 10.1056/NEJMsr0806582. [DOI] [PubMed] [Google Scholar]
- 9.Tse T, Williams RJ. ClinicalTrials.gov to include basic results data. [Accessed on May 3, 2009]. Available at: http://www.nlm.nih.gov/pubs/techbull/so08/so08_clinicaltrials.html.
- 10.Laine C, De Angelis C, Delamothe T, et al. Clinical trial registration: looking back and moving ahead. N Engl J Med. 2007;356:2734–2736. doi: 10.1056/NEJMe078110. [DOI] [PubMed] [Google Scholar]
- 11.Marusić A, Huić M. Registration of clinical trials still moving ahead: September 2008 update to uniform requirements for manuscripts submitted to biomedical journals. Croat Med J. 2008;49:582–585. doi: 10.3325/cmj.2008.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groves T. Mandatory disclosure of trial results for drugs and devices. BMJ. 2008;336:170. doi: 10.1136/bmj.39469.465139.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. US code of federal regulations: phases of an investigation; title 21, part 312, section 21. [Accessed on May 3, 2009]. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=312.21.
- 14.Moher D, Schulz KF, Altman DG, et al. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657–662. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]