Abstract
Background:
Systemic inflammation has been associated with reduced lung function. Adhesion molecules, such as intercellular adhesion molecule (ICAM)-1 and P-selectin, figure importantly in initiating the inflammatory response. We studied the association between ICAM-1 and P-selectin concentrations and lung function in the Coronary Artery Risk Development in Young Adults study.
Methods:
Spirometry testing was conducted at years 5, 10, and 20. ICAM-1 and P-selectin were assayed at year 15.
Results:
Complete data were obtained from 2,455 participants. We first predicted year-20 lung function from year-15 ICAM-1 concentration data. After controlling for race, gender, height, age, physical activity, smoking status, alcohol intake, BMI, and asthma status, all taken at year 15, the year-20 FVC was 164 mL higher (p < 0.0001) and FEV1 was 164 mL higher (p = 0.0003) in the lowest ICAM-1 concentration quartile than the highest ICAM-1 quartile, whereas the FEV1/FVC ratio showed no association (p = 0.25). We then predicted the year-15 ICAM-1 concentration from year-5 lung function and change in lung function (year 10 − year 5). The year-15 ICAM-1 concentration was about 13 ng/mL higher in the lowest vs highest quartile of either the year-5 FVC (p = 0.01) or year-5 FEV1 (p = 0.005). Year-15 ICAM-1 concentration was unrelated to year-5 FEV1/FVC ratio. Greater loss in FVC and FEV1 (year 10 − year 5) also was associated with higher year-15 ICAM-1 concentrations. Associations between P-selectin and lung function followed a similar but weaker pattern to that observed for ICAM-1.
Conclusions:
These data suggest a bidirectional association between circulating adhesion molecules, such as ICAM-1 and P-selectin, and pattern of lung function change in adults.
Increased levels of systemic inflammatory markers have been associated with decreased FVC and FEV1 in longitudinal studies1–3 and with COPD in cross-sectional studies.4,5 The pulmonary endothelium is an integral part of the alveolar capillary unit. Noxious agents that initiate an inflammatory response in endothelial cells may damage not only the endothelium, but also the entire alveolus.6 Intercellular adhesion molecule (ICAM)-1 is expressed on the surface of pulmonary vascular endothelial cells and in type II pneumocytes.7 Soluble P-selectin, another adhesion molecule, is derived at least partly from proteolytic cleavage of the membrane-bound form of P-selectin and reflects endothelial cell activation.8 P-selectin on the surface of activated platelets reportedly9 plays an important role in the pathogenesis of lung inflammation by recruiting neutrophils to the lung. Circulating adhesion molecules, such as P-selectin and ICAM-1, play a key role in the recruitment of neutrophils to activated endothelial cells and initiation of the inflammatory response.6,8,10,11 Further substantiating an important role of ICAM-1 in the lung inflammatory response, knockout mice with no ICAM-1 activity do not develop an inflammatory response in the lung after radiation injury.12
Consistent with the idea that circulating adhesion molecules are involved in the inflammatory response, P-selectin and ICAM-1 have been associated13–15 with an increased risk of cardiovascular diseases, such as myocardial infarction and stroke. Data supporting an association between P-selectin and ICAM-1 and lung disease are more limited, but the levels of circulating ICAM-1 are increased among patients with emphysema and chronic bronchitis, diseases in which inflammation is thought to play an important role.16 In addition, increased ICAM-1 concentration is associated with decreased lung function,17 and cross-sectional data from the Framingham cohort study18 have revealed higher P-selectin levels with decreased FEV1 in participants with an average age of 60 years. However, these observations have not been entirely consistent, as another study19 found no association of serum ICAM-1 levels with stable asthma or COPD.
Little information exists on the role of circulating adhesion molecules in the decline in lung function in apparently healthy young adults. In the present study, we have evaluated the possibly bidirectional associations of circulating levels of ICAM-1 and P-selectin with lung function. We hypothesized that higher levels of circulating ICAM-1 and P-selectin measured at year 15 of our study predicted lower lung function at year 20 and, in turn, were predicted by lower lung function at year 5 and by a more rapid loss in lung function from year 5 to year 10.
Materials and Methods
The detailed methods, instruments, and quality control procedures for the Coronary Artery Risk Development in Young Adults (CARDIA) study have been described in other published reports.20,21 The CARDIA study is reviewed annually by the internal review boards at each participating institution, and participants sign a new informed consent form at every examination. A description of participants' demographic information, lifestyle habits, medical history (previously described),20 spirometry measurements, and ICAM-1 and P-selectin measurements are provided in the online supplemental data.
After excluding observations from participants with missing covariates and one observation from a pregnant woman, 2,455 persons were included in the analyses of year-20 lung function in relation to year-15 ICAM-1 and P-selectin concentrations. Attendance at year 20 was higher among white participants (77%) than among black participants (63%; p < 0.0001). After adjustment for race, year-5 FVC was significantly lower among participants who were lost to follow-up after the year-5 examination than among those who attended the year-20 examination (FVC, 4,462 vs 4,297 mL, respectively). There were no other significant differences in lung function between participants who attended vs those who did not attend the year-20 examination. In addition, current smokers at the year-10 examination (72%) were less likely to attend the year-20 examination than were never-smokers (85%) and former smokers (83%).
Statistical Analysis
We used a statistical software linear regression program (PROC GLM in SAS, version 9; SAS Institute; Cary, NC) to evaluate the associations of year-15 ICAM-1 and P-selectin levels with lung function at years 5, 10, and 20. Initially, ICAM-1 and P-selectin concentrations at year 15 were used to estimate FVC, FEV1 and the FEV1/FVC ratio at year 20. These analyses were adjusted for race, gender, age, amount of physical activity, asthma status (asthma diagnosed before study beginning, during the study, or never), smoking status (never, former, or current), alcohol intake, and body mass index (BMI) at year 15 as well as for height and height squared measured at year 0. Physical activity and alcohol intake were analyzed as continuous variables. In addition to these analyses, the prediction equations of Hankinson et al22 were used to analyze the association between year-15 ICAM-1 and P-selectin levels and year-20 lung function values. Because ICAM-1 concentrations have been associated with both smoking status23–26 and acute exacerbations of asthma,27,28 we also evaluated the association between year-20 lung function as the dependent variable and concentrations of circulating adhesion molecules stratified by smoking status (smokers vs never-smokers) and asthma status using the models described previously.
Subsequently, we reversed the role of dependent and independent variables to evaluate the role of early lung function in predicting adhesion molecule concentrations. We restricted the analyses to lung function measured from year 5 onward to include only adults who had attained peak lung function. This method allowed us to use lung function (FVC, FEV1, and FEV1/FVC ratio) at year 5 and the change in FVC, FEV1, and FEV1/FVC ratio of > 5 years (year 10 − year 5) to estimate ICAM-1 and P-selectin concentrations at year 15 after adjustment for all the previously mentioned covariates measured at year 5 and height and height squared measured at year 0.
Results
The mean (± SD) serum ICAM-1 concentration was 153.5 ± 44.1 ng/mL, whereas the mean plasma P-selectin concentration was 36.7 ± 11.1 ng/mL. At year 15, participants in the highest vs lowest ICAM-1 quartiles tended to be black; had lower educational attainment; were more likely to be current smokers; had higher BMI, lower physical activity scores, and higher C-reactive protein and P-selectin concentrations (p < 0.0001); and had received a diagnosis of asthma more often during the study period (p = 0.003) [Table 1]. At year 10, the FVC and FEV1 values for participants in the highest ICAM-1 quartiles were lower vs those in the lowest ICAM-1 quartiles (p < 0.0001) [Table 1]. Year-15 ICAM-1 and P-selectin concentrations had a correlation coefficient of 0.25 (p < 0.0001). At year 15, participants in the highest vs lowest P-selectin quartile also tended to be black and men, had lower educational attainment, were more likely to be current smokers, and had higher BMI, C-reactive protein, and ICAM-1 concentrations (data not shown).
Table 1.
Clinical Characteristics According to Quartile Serum ICAM-1 at Year 15*
| Characteristic | Q1† | Q2‡ | Q3§ | Q4‖ | p Value |
|---|---|---|---|---|---|
| Age, yr | 40.5 (3.4) | 40.3 (3.6) | 40.3 (3.5) | 40.4 (3.7) | 0.76 |
| White race | 71.6 | 65.3 | 52.6 | 40.9 | < 0.0001 |
| Female gender | 59.7 | 53.9 | 52.1 | 53.6 | 0.04 |
| Education level (completed high school or lower), % | 12.4 | 16.8 | 22.2 | 30.8 | < 0.0001 |
| Former-smoker, % | 22.4 | 18.7 | 16.9 | 16.1 | < 0.0001 |
| Current-smoker, % | 8.5 | 10.9 | 19.2 | 38.9 | < 0.0001 |
| Prevalent asthma at baseline, % | 4.6 | 5.4 | 6.7 | 4.2 | 0.003 |
| Cumulative year-15 incident asthma, % | 7.7 | 7.2 | 6.4 | 12.2 | 0.003 |
| BMI, kg/m2 | 25.9 (4.9) | 27.7 (5.5) | 29.1 (5.9) | 31.0 (7.0) | < 0.0001 |
| Physical activity score, exercise units | 396 (297) | 376 (276) | 352 (294) | 293 (250) | < 0.0001 |
| Alcohol consumption, g/d | 10.4 (15.6) | 9.3 (18.0) | 11.8 (35.8) | 11.9 (27.1) | 0.21 |
| CRP, g/mL | 1.6 (3.1) | 2.5 (5.2) | 3.0 (4.0) | 5.2 (7.5) | < 0.0001 |
| P-selectin, ng/mL | 32.6 (9.6) | 35.2 (9.9) | 38.1 (11.6) | 40.7 (11.6) | < 0.0001 |
| FVC at year 10, mL | 4,465 (999) | 4,490 (1005) | 4,313 (1069) | 4,081 (1034) | < 0.0001 |
| FEV1 at year 10, mL | 3,536 (744) | 3,561 (776) | 3,451 (833) | 3,246 (794) | < 0.0001 |
| FEV1/FVC ratio at year 10 | 79.6% (6.3%) | 79.6% (6.3%) | 80.3% (5.9%) | 80.0% (6.5%) | 0.22 |
*Values are given as the mean (SD) or %, unless otherwise indicated, and are based on 2,455 participants at the year-15 examination in the CARDIA Study (2000 to 2001). All variables were measured at year 15, except FVC, FEV1, and FEV1/FVC, which were measured at year 10. CRP = C-reactive protein; Q = quartile.
†ICAM-1 concentration ≤ 125.47 ng/mL (n = 613).
‡ICAM-1 concentration 125.48 to 145.20 ng/mL (n = 614).
§ICAM-1 concentration 145.21 to 171.08 ng/mL (n = 614).
‖ICAM-1 concentration ≥ 171.09 ng/mL (n = 614).
Estimation of Year-20 Lung Function From Year-15 Adhesion Molecules
Year-20 FVC was 164 mL higher in the lowest vs highest quartiles of year-15 ICAM-1 (99.0% vs 94.3% predicted, respectively; p < 0.001 [for trend]) [Table 2]. FEV1 followed a similar pattern, being 114 mL higher in the lowest vs highest ICAM-1 quartile (96.6% vs 92.5% predicted, respectively; p = 0.0003 [for trend]) [Table 2]. The FEV1/FVC ratio was not associated with year-15 ICAM-1 levels (97.0% vs 97.55% predicted, respectively; p = 0.25 [for trend]). There were similar but smaller differences in year-20 lung function measurements across year-15 P-selectin quartiles (Table 2). There was no association between year-15 ICAM-1 or P-selectin levels and change in FVC, FEV1, or FEV1/FVC ratio over 10 years (year 20 − year 10; data not shown).
Table 2.
Associations of Year-15 ICAM-1 and P-selectin Concentrations With Year-20 Lung Function*
| Year-20 Absolute Values |
|||
|---|---|---|---|
| Year-15 Qs | FVC, mL | FEV1, mL | FEV1/FVC Ratio, % |
| ICAM-1 concentrations | |||
| Q1 (≤ 125.57 ng/mL) | 4,016 | 3,128 | 78.38 |
| Q2 (125.58–145.52 ng/mL) | 3,976 | 3,113 | 78.55 |
| Q3 (145.53–171.31 ng/mL) | 3,932 | 3,098 | 79.07 |
| Q4 (≥ 171.32 ng/mL) | 3,852 | 3,014 | 78.76 |
| Q1–Q4† | 164 (97 to 231) | 114 (58 to 172) | − 0.38 (− 1.13 to 0.37) |
| p Value for trend | < 0.0001 | 0.0003 | 0.025 |
| P-selectin concentrations | |||
| Q1 (≤ 29.49 ng/mL) | 3,988 | 3,122 | 78.64 |
| Q2 (29.50–35.63 ng/mL) | 3,951 | 3,103 | 78.99 |
| Q3 (36.64–42.51 ng/mL) | 3,952 | 3,088 | 78.55 |
| Q4 (≥ 42.52 ng/mL) | 3,885 | 3,041 | 78.58 |
| Q1–Q4,† mL | 102 (38 to 166) | 81 (27 to 135) | 0.06 (− 0.65 to 0.77) |
| p Value for trend | 0.02 | 0.07 | 0.55 |
*Tabulated values are estimates from models adjusting for race, gender, age, amount of physical activity, asthma status, smoking status, alcohol intake, and BMI, all at year 15, and for height, height2 measured at year 0. See Table 1 for abbreviation not used in the text.
†Values in parentheses are 95% confidence intervals.
Estimation of Year-15 Adhesion Molecules From Year-5 Lung Function and 5-Year Change in Lung Function
Both year-5 FVC and FEV1 were significant predictors of year-15 ICAM-1 concentrations. Serum ICAM-1 concentration was 12.6 ng/mL higher in the lowest vs highest year-5 FVC quartiles (p for trend, 0.01) and higher by 13.7 ng/mL in the lowest vs highest year-5 FEV1 quartile (p = 0.005 [for trend]) [Table 3]. The year-5 FEV1/FVC ratio did not predict year-15 ICAM-1 concentration (p = 0.33 [for trend]) [Table 3]. Year-5 FVC, FEV1, and FEV1/FVC ratio did not predict year-15 P-selectin concentration (Table 3).
Table 3.
Associations Between Absolute Values of Year-5 FVC, FEV1, and FEV1/FVC Ratio and Year-15 Levels of Circulating Adhesion Molecules*
| Variables | Year-15 ICAM-1, ng/mL | Year-15 P-Selectin, ng/mL |
|---|---|---|
| Year 5 FVC | ||
| Q1 (≤ 3,609 mL) | 160.73 | 36.88 |
| Q2 (3,610–4,249 mL) | 152.62 | 37.18 |
| Q3 (4,250–5,089 mL) | 149.84 | 36.46 |
| Q4 (≥ 5,090 mL) | 148.09 | 36.26 |
| Q1–Q4,† ng/mL | 12.63 (3.92 to 21.35) | 0.63 (− 1.72 to 2.97) |
| p Value for trend | 0.01 | 0.76 |
| Year-5 FEV1 | ||
| Q1 (≤ 2,979 mL) | 159.98 | 37.69 |
| Q2 (2,980–3,499 mL) | 152.39 | 36.57 |
| Q3 (3,500–4,109 mL) | 152.67 | 36.71 |
| Q4 (≥ 4,110 mL) | 146.26 | 35.81 |
| Q1–Q4† | 13.72 (5.67 to 21.78) | 1.88 (− 0.29 to 4.05) |
| p Value for trend | 0.005 | 0.32 |
| Year-5 FEV1/FVC ratio | ||
| Q1 (≤ 77.9%) | 150.61 | 36.76 |
| Q2 (78.0–81.9%) | 151.58 | 36.52 |
| Q3 (82.0–85.9%) | 154.03 | 36.49 |
| Q4 (≥ 86.0%) | 155 | 37 |
| Q1–Q4,† ng/mL | − 4.39 (− 9.74 to 0.96) | − 0.24 (− 1.68 to 1.20) |
| p Value for trend | 0.33 | 0.88 |
*Tabulated values are estimates from models adjusting for race, gender, age, amount of physical activity, asthma status, smoking status, alcohol intake, and BMI, all at year 15, and height, height2 measured at year-0 and 5-year change (year 10 − year 5) in lung function (FVC, FEV1, and FEV1/FVC ratio). See Table 1 for abbreviation not used in the text.
†Values in parentheses are 95% confidence intervals.
After adjustment for year-5 FVC and FEV1, the corresponding 5-year changes in FVC and FEV1 (year 10 − year 5) were significant predictors of year-15 ICAM-1 concentrations. Serum ICAM-1 concentration was 6.6 ng/mL higher in the lowest vs highest quartiles of 5-year change in FVC (p = 0.02 [for trend]), and it was correspondingly higher by 5.0 ng/mL in the lowest vs highest quartiles of 5-year change in FEV1 (p = 0.01 [for trend]) [Table 4]. Year-15 P-selectin concentration also was significantly associated with 5-year change in FVC, with a 1.1 ng/mL difference between lowest vs highest P-selectin quartiles (p = 0.01 [for trend]) [Table 4]. However, changes in both FEV1 and FEV1/FVC ratio from year 5 to year 10 were not significantly associated with year-15 P-selectin concentration (Table 4).
Table 4.
Association Between 5-Year Change (Year 10 − Year 5) in FVC, FEV1, and FEV1/FVC Ratio, and Year-15 Circulating Adhesion Molecules*
| Variable | Year-15 ICAM-1, ng/mL | Year-15 P-selectin, ng/mL |
|---|---|---|
| 5-year change in FVC | ||
| Q1 (decline of at least 160 mL) | 157.48 | 37.9 |
| Q2 (decline of 159 mL to decline of 30 mL) | 152.35 | 36.25 |
| Q3 (decline of 29 mL to increase of 89 mL) | 150.35 | 35.8 |
| Q4 (increase of at least 90 mL) | 150.87 | 36.81 |
| Q1–Q4,† ng/mL | 6.61 (1.54–11.68) | 1.09 (− 0.27–2.46) |
| p Value for trend | 0.02 | 0.01 |
| 5-year change in FEV1 | ||
| Q1 (decline of at least 220 mL) | 157.6 | 37.59 |
| Q2 (decline of 219 mL to decline of 110 mL) | 150.56 | 36.36 |
| Q3 (decline of 109 mL to decline of 10 mL) | 150.38 | 36.21 |
| Q4 (increase or decline of ≤ 9 mL) | 152.62 | 36.6 |
| Q1–Q4,† ng/mL | 4.98 (0.07–9.89) | 0.99 (− 0.33–2.31) |
| p Value for trend | 0.01 | 0.18 |
| 5-year change in FEV1/FVC ratio | ||
| Q1 (decline of at least 4%) | 153.44 | 36.49 |
| Q2 (decline of 3.9% to decline of 2.0%) | 150.88 | 36.2 |
| Q3 (decline of 1.9% to decline of 0.1%) | 151.41 | 36.92 |
| Q4 (no change or any increase) | 155.43 | 37.16 |
| Q1–Q4,† ng/mL | − 1.98 (− 7.04–3.08) | − 0.67 (− 2.03–0.69) |
| p Value for trend | 0.26 | 0.50 |
*Tabulated values are estimates from models adjusting for race, gender, age, amount of physical activity, asthma status, smoking status, alcohol intake, and BMI, all at year 15, and height, height2 measured at year 0 and lung function at year 5 (FVC, FEV1, and FEV1/FVC ratio). See Table 1 for abbreviation not used in the text.
†Values in parentheses are 95% confidence intervals.
Subgroup Analysis Estimating Year-20 Lung Function From Year-15 Adhesion Molecules Among Smokers and Participants With Asthma
The estimated difference for lowest to highest ICAM-1 quartile in FVC and FEV1 was greater in smokers than in nonsmokers and in participants with asthma than in those without asthma, although the interaction terms between smoking and asthma and ICAM levels on lung function were not significant. The FEV1/FVC ratio was nonsignificantly higher in the lowest ICAM-1 quartile by 0.57% in participants without asthma and nonsignificantly lower by 0.59% in participants with asthma; this difference in relationship was significant (p = 0.03). P-selectin was not associated with lung function in any subgroup analyses. Details are shown in the online supplemental data.
Discussion
This study shows that year-5 FVC and FEV1, 5-year decline in FVC and FEV1, and year-20 FVC and FEV1 are inversely associated with year-15 ICAM-1 concentrations, with similar but weaker associations with P-selectin. These findings are statistically independent of smoking, asthma status, and BMI; nevertheless, there appears to be stronger associations in smokers and participants with asthma than in participants without these inflammatory conditions.
This study demonstrates mutually predictive relationships between ICAM-1 and lung function as follows: year-15 ICAM-1 concentrations are predictive of year-20 FVC and FEV1 and year-5 FVC and FEV1, and the subsequent 5-year change is predictive of year 15 ICAM-1 concentrations. These findings are consistent with a bidirectional nature for the association between systemic inflammation and lung function.
The inverse association between year-15 serum ICAM-1 concentration and year-20 lung function is consistent with the hypothesis that factors that increase concentrations of circulating adhesion molecules can induce local lung inflammation and subsequently result in lower FVC and FEV1. The associations between circulating adhesion molecules and lung function have been previously found16,17 in subgroups, such as workers in occupational settings with exposure to ceramic dust, smokers, or persons with asthma. One other study18 has evaluated the associations among P-selectin, ICAM-1, and lung function in the general population, and showed a cross-sectional association among increased ICAM-1 levels (when analyzed as a sole biomarker), increased P-selectin levels, and reduced FEV1. In the present study, the association between P-selectin and lung function followed a similar, but weaker pattern than that observed with ICAM-1. P-selectin is expressed to a greater extent on activated platelets than on activated endothelial cells, whereas ICAM-1 is expressed predominantly on the surface of activated endothelial cells.29–31 The intimate relationship between endothelial cells in the pulmonary vasculature and alveolar epithelial cells and the increased expression of ICAM-1 by pulmonary endothelial cells during an inflammatory response compared to expression of P-selectin may explain the stronger association of ICAM-1 with lung function.
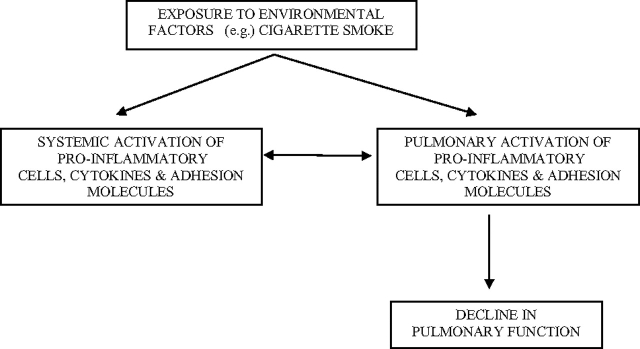
This study also showed that lower year-5 FVC and FEV1 and greater loss of lung function between years 5 and 10 were predictive of higher year-15 serum ICAM-1 concentrations. This study is the first to show that lower lung function at baseline (year-5 examination) and increased loss of lung function over the subsequent 5 years are predictive of increased concentrations of circulating adhesion molecules. This finding supports the existence of a positive feedback loop between circulating (systemic) adhesion molecule concentrations and lung function (Fig 1). Ongoing lung injury and inflammation (reflected by a decline in FVC and FEV1) may result in increased secretion of proinflammatory cytokines.32 These cytokines and the associated recruitment of activated T and B lymphocytes to the site of injury increase local and systemic expression of adhesion molecules, which may provide a biological basis for the increased concentrations of circulating adhesion molecules following decrease in FVC and FEV1.
Figure 1.
Environmental factors increase expression of adhesion molecules and induce both systemic inflammation and local airway inflammation. Local airway inflammation results in lung tissue injury and lower FEV1 and FVC. Local lung injury results in the secretion of proinflammatory cytokines and results in the recruitment of proinflammatory cells and cytokines to the site of airway inflammation, resulting in increased concentrations of circulating adhesion molecules.
Numerous studies17,18,23–26 have shown serum levels of ICAM-1 and P-selectin to be higher among smokers than among nonsmokers and levels of circulating adhesion molecules to be associated with decreased FEV1 in smokers than in never-smokers. In addition, both ICAM-1 and P-selectin play an important role in lung inflammation and may contribute to the pathogenesis of asthma.27,28 Our estimates tend to agree in that the association between lung function and circulating adhesion molecules was stronger among smokers than among never-smokers and in participants with asthma than in those without asthma. However, this tendency did not achieve statistical significance (see the online supplemental data).
We further noted that both FVC and FEV1 were significantly reduced at year 20 across ICAM-1 quartiles without any significant change in the FEV1/FVC ratio, which is most consistent with a restrictive pattern of lung function abnormality. The cause of this possible restriction is not known. Possible explanations include reduced compliance of the lungs and chest wall and inflammation-related neuromuscular weakness. Additionally, the FEV1/FVC ratio may sometimes be normal in those with airway obstruction as a result of an increase in the expiratory reserve volume at which airway closure occurs (airtrapping).
The study design was limited by the available measurements, which did not coincide temporally for lung function (years 5, 10, and 20) and endothelial markers (year 15). The lack of simultaneous measurements of circulating adhesion molecule and lung function in this study complicates interpretation; that is, the direction of the associations cannot be determined conclusively. Nearly 31% of the participants were lost to follow-up of > 20 years, with this loss being greater in black participants and among current smokers. Although this observational study has a bias in retaining never-smokers and former smokers with higher FVC, the analyses included large numbers of participants in the groups that had lower retention. Even though we have adjusted for all known confounders when evaluating the association between circulating adhesion molecules and lung function, residual confounding due to unknown factors remains a potential bias in all observational studies. On the other hand, the present study has several strengths, including the large number of generally healthy participants, the inclusion of black and white participants and men and women, follow-up soon after peak lung function was achieved, high-quality spirometry data collection, and excellent retention of the original cohort.
Conclusions
The findings from this study support the idea that circulating ICAM-1 and P-selectin may contribute to or be affected by lung function in adults. Future longitudinal studies that measure concentrations of circulating adhesion molecules during young adulthood would help us to better understand the temporal association between adhesion molecules and lung function.
Supplementary Material
Abbreviations:
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- ICAM
intercellular adhesion molecule
Footnotes
This study was supported by National Heart, Lung, and Blood Institute contracts N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050 (CARDIA field centers); N01-HC-95095 (CARDIA Coordinating Center); and PF-HC95095 Reading Center (CARDIA Pulmonary Reading Center, subcontract to CARDIA Coordinating Center).
The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Fogarty AW, Jones S, Britton JR, et al. Systemic inflammation and decline in lung function in a general population: a prospective study. Thorax. 2007;62:515–520. doi: 10.1136/thx.2006.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thyagarajan B, Jacobs DR, Apostol GG, et al. Plasma fibrinogen and lung function: the CARDIA Study. Int J Epidemiol. 2006;35:1001–1008. doi: 10.1093/ije/dyl049. [DOI] [PubMed] [Google Scholar]
- 3.Hancox RJ, Poulton R, Greene JM, et al. Systemic inflammation and lung function in young adults. Thorax. 2007;62:1064–1068. doi: 10.1136/thx.2006.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahl M, Tybjaerg-Hansen A, Vestbo J, et al. Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1008–1011. doi: 10.1164/ajrccm.164.6.2010067. [DOI] [PubMed] [Google Scholar]
- 5.Kony S, Zureik M, Driss F, et al. Association of bronchial hyperresponsiveness and lung function with C-reactive protein (CRP): a population based study. Thorax. 2004;59:892–896. doi: 10.1136/thx.2003.015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orfanos SE, Mavrommati I, Korovesi I, et al. Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive Care Med. 2004;30:1702–1714. doi: 10.1007/s00134-004-2370-x. [DOI] [PubMed] [Google Scholar]
- 7.Guzman J, Izumi T, Nagai S, et al. ICAM-1 and integrin expression on isolated human alveolar type II pneumocytes. Eur Respir J. 1994;7:736–739. doi: 10.1183/09031936.94.07040736. [DOI] [PubMed] [Google Scholar]
- 8.Woollard KJ. Soluble bio-markers in vascular disease: much more than gauges of disease? Clin Exp Pharmacol Physiol. 2005;32:233–240. doi: 10.1111/j.1440-1681.2005.04178.x. [DOI] [PubMed] [Google Scholar]
- 9.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 11.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol. 2005;77:4874–4895. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallahan DE, Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc Natl Acad Sci U S A. 1997;94:6432–6437. doi: 10.1073/pnas.94.12.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frijns CJ, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002;33:2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- 14.Shimomura H, Ogawa H, Arai H, et al. Serial changes in plasma levels of soluble P-selectin in patients with acute myocardial infarction. Am J Cardiol. 1998;81:397–400. doi: 10.1016/s0002-9149(97)00945-4. [DOI] [PubMed] [Google Scholar]
- 15.Luc G, Arveiler D, Evans A, et al. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: the PRIME Study. Atherosclerosis. 2003;170:169–176. doi: 10.1016/s0021-9150(03)00280-6. [DOI] [PubMed] [Google Scholar]
- 16.Riise GC, Larsson S, Lofdahl CG, et al. Circulating cell adhesion molecules in bronchial lavage and serum in COPD patients with chronic bronchitis. Eur Respir J. 1994;17:1673–1677. doi: 10.1183/09031936.94.07091673. [DOI] [PubMed] [Google Scholar]
- 17.Backe EM, Lotz G, Tittelbach U, et al. Soluble intercellular adhesion molecules in the serum of subjects exposed to dust at different workplaces: correlation to airway symptoms, lung function, tobacco and dust exposure. Int J Hyg Environ Health. 2002;204:377–379. doi: 10.1078/1438-4639-00111. [DOI] [PubMed] [Google Scholar]
- 18.Walter RE, Wilk JB, Larson MG, et al. Systemic inflammation and COPD: the Framingham Heart Study. Chest. 2008;133:19–25. doi: 10.1378/chest.07-0058. [DOI] [PubMed] [Google Scholar]
- 19.Riise GC, Larsson S, Lowhagen O, et al. Circulating leukocyte adhesion molecules in stable asthma and nonobstructive chronic bronchitis. Allergy. 1995;50:693–698. doi: 10.1111/j.1398-9995.1995.tb02588.x. [DOI] [PubMed] [Google Scholar]
- 20.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 21.Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) Study. Control Clin Trials. 1987;8:68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23.Takizawa H, Tanaka M, Takami K, et al. Increased expression of inflammatory mediators in small-airway epithelium from tobacco smokers. Am J Physiol Lung Cell Mol Physiol. 2000;278:L906–L913. doi: 10.1152/ajplung.2000.278.5.L906. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez S, Hards J, van Eeden S, et al. The expression of adhesion molecules in cigarette smoke-induced airways obstruction. Eur Respir J. 1996;9:1995–2001. doi: 10.1183/09031936.96.09101995. [DOI] [PubMed] [Google Scholar]
- 25.Hozawa A, Jacobs DR, Jr, Steffes MW, et al. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clin Chem. 2007;53:447–455. doi: 10.1373/clinchem.2006.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rezavandi K, Palmer RM, Odell EW, et al. Expression of ICAM-1 and E-selectin in gingival tissues of smokers and non-smokers with periodontitis. J Oral Pathol Med. 2002;31:59–64. doi: 10.1046/j.0904-2512.2001.joptest.doc.x. [DOI] [PubMed] [Google Scholar]
- 27.Stanciu LA, Djukanovic R. The role of ICAM-1 on T-cells in the pathogenesis of asthma. Eur Respir J. 1998;11:949–957. doi: 10.1183/09031936.98.11040949. [DOI] [PubMed] [Google Scholar]
- 28.Tang ML, Fiscus LC. Important roles for L-selectin and ICAM-1 in the development of allergic airway inflammation in asthma. Pulm Pharmacol Ther. 2001;14:203–210. doi: 10.1006/pupt.2001.0293. [DOI] [PubMed] [Google Scholar]
- 29.Asimakopoulos G, Taylor KM. Effects of cardiopulmonary bypass on leukocyte and endothelial adhesion molecules. Ann Thorac Surg. 1998;66:2135–2144. doi: 10.1016/s0003-4975(98)00727-9. [DOI] [PubMed] [Google Scholar]
- 30.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24:2166–2179. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Kuebler WM. Selectins revisited: the emerging role of platelets in inflammatory lung disease. J Clin Invest. 2006;116:3106–3108. doi: 10.1172/JCI30664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roth M. Pathogenesis of COPD: part III. Inflammation in COPD. Int J Tuberc Lung Dis. 2008;12:375–380. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.