Abstract
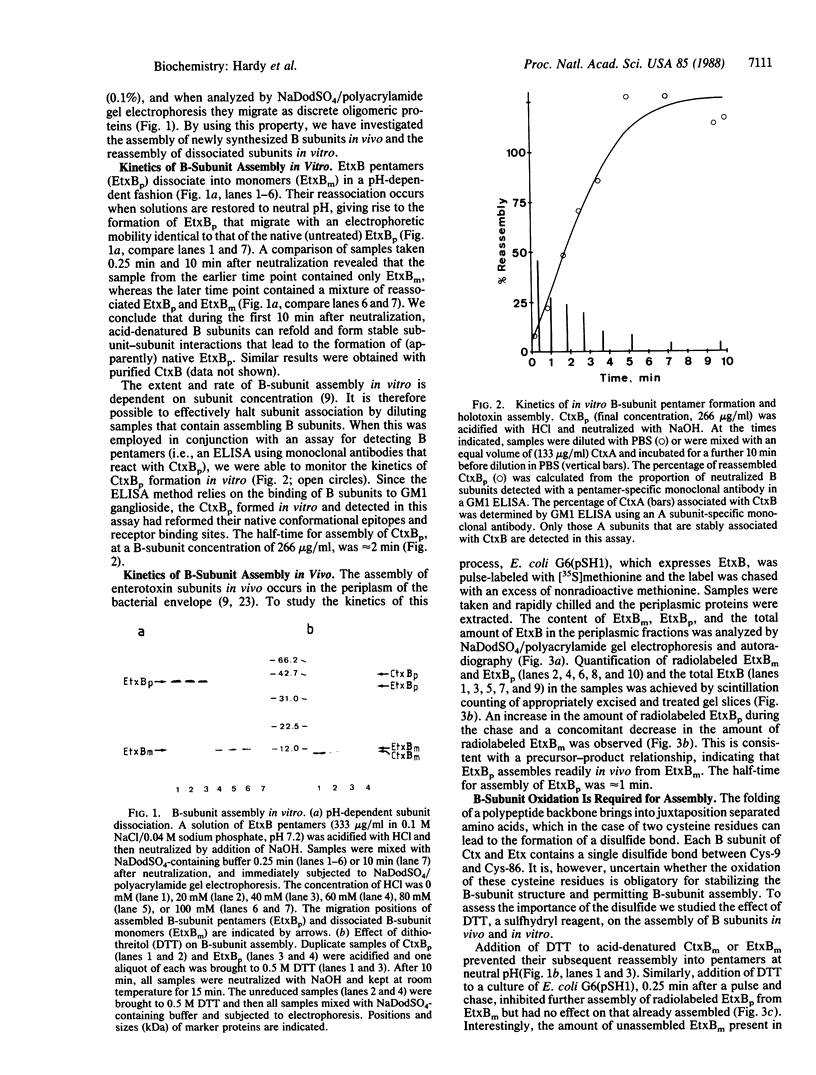
In this paper we study the assembly, in vivo and in vitro, of a family of hexameric, heat-labile enterotoxins produced by diarrheagenic bacteria. The toxins, which consist of an A subunit and five B subunits, are assembled by a highly coordinated process that ensures secretion of the holotoxin complex. We show that (i) oxidation of cysteine residues in the B subunits is a prerequisite step for in vivo formation of B-subunit pentamers, (ii) reduction of dissociated B subunits in vitro abolishes their ability to reassemble, (iii) the kinetics of B-pentamer assembly in vivo can be mimicked under defined conditions in vitro, (iv) A subunits cannot associate with fully assembled B pentamers in vitro, and (v) A subunits cause an approximately 3-fold acceleration in the rate of B-subunit pentamerization in vivo, implying that A subunits play a coordinating role in the pathway of holotoxin assembly. The last finding is likely to be of general significance, since it provides a mechanism for preferentially excluding or favoring certain intermediates in the assembly of multisubunit proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creighton T. E. Disulfide bonds as probes of protein folding pathways. Methods Enzymol. 1986;131:83–106. doi: 10.1016/0076-6879(86)31036-x. [DOI] [PubMed] [Google Scholar]
- Dallas W. S. Conformity between heat-labile toxin genes from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1983 May;40(2):647–652. doi: 10.1128/iai.40.2.647-652.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature. 1980 Dec 4;288(5790):499–501. doi: 10.1038/288499a0. [DOI] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Protein unfolding and the energetics of protein translocation across biological membranes. Cell. 1988 Feb 26;52(4):481–483. doi: 10.1016/0092-8674(88)90458-8. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Clements J. D., Robertson D. C., Finkelstein R. A. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Sep;33(3):677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. S., Brinton C. C., Jr Identification and characterization of E. coli type-1 pilus tip adhesion protein. Nature. 1988 Mar 17;332(6161):265–268. doi: 10.1038/332265a0. [DOI] [PubMed] [Google Scholar]
- Hermann R., Jaenicke R., Price N. C. Evidence for active intermediates during the reconstitution of yeast phosphoglycerate mutase. Biochemistry. 1985 Apr 9;24(8):1817–1821. doi: 10.1021/bi00329a002. [DOI] [PubMed] [Google Scholar]
- Hirst T. R., Hardy S. J., Randall L. L. Assembly in vivo of enterotoxin from Escherichia coli: formation of the B subunit oligomer. J Bacteriol. 1983 Jan;153(1):21–26. doi: 10.1128/jb.153.1.21-26.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Holmgren J. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7418–7422. doi: 10.1073/pnas.84.21.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Holmgren J. Transient entry of enterotoxin subunits into the periplasm occurs during their secretion from Vibrio cholerae. J Bacteriol. 1987 Mar;169(3):1037–1045. doi: 10.1128/jb.169.3.1037-1045.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Randall L. L., Hardy S. J. Cellular location of heat-labile enterotoxin in Escherichia coli. J Bacteriol. 1984 Feb;157(2):637–642. doi: 10.1128/jb.157.2.637-642.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Sanchez J., Kaper J. B., Hardy S. J., Holmgren J. Mechanism of toxin secretion by Vibrio cholerae investigated in strains harboring plasmids that encode heat-labile enterotoxins of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7752–7756. doi: 10.1073/pnas.81.24.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra H., Witholt B. Kinetics of synthesis, processing, and membrane transport of heat-labile enterotoxin, a periplasmic protein in Escherichia coli. J Biol Chem. 1984 Dec 25;259(24):15182–15187. [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973 Dec;8(6):851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R. Folding and association of proteins. Prog Biophys Mol Biol. 1987;49(2-3):117–237. doi: 10.1016/0079-6107(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Jaenicke R., Rudolph R., Heider I. Quaternary structure, subunit activity, and in vitro association of porcine mitochondrial malic dehydrogenase. Biochemistry. 1979 Apr 3;18(7):1217–1223. doi: 10.1021/bi00574a016. [DOI] [PubMed] [Google Scholar]
- Jaenicke R., Rudolph R. Refolding and association of oligomeric proteins. Methods Enzymol. 1986;131:218–250. doi: 10.1016/0076-6879(86)31043-7. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Moss J., Richardson S. H. Activation of adenylate cyclase by heat-labile Escherichia coli enterotoxin. Evidence for ADP-ribosyltransferase activity similar to that of choleragen. J Clin Invest. 1978 Aug;62(2):281–285. doi: 10.1172/JCI109127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Rappuoli R. Promoter of the pertussis toxin operon and production of pertussis toxin. J Bacteriol. 1987 Jun;169(6):2843–2846. doi: 10.1128/jb.169.6.2843-2846.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. D., Mekalanos J. J. Molecular cloning of Vibrio cholerae enterotoxin genes in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 May;79(9):2976–2980. doi: 10.1073/pnas.79.9.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell. 1986 Sep 12;46(6):921–928. doi: 10.1016/0092-8674(86)90074-7. [DOI] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Inducible high level synthesis of mature human fibroblast interferon in Escherichia coli. Nucleic Acids Res. 1983 Jul 25;11(14):4677–4688. doi: 10.1093/nar/11.14.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Tsao H., Fiers W. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene. 1983 Apr;22(1):103–113. doi: 10.1016/0378-1119(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Sandkvist M., Hirst T. R., Bagdasarian M. Alterations at the carboxyl terminus change assembly and secretion properties of the B subunit of Escherichia coli heat-labile enterotoxin. J Bacteriol. 1987 Oct;169(10):4570–4576. doi: 10.1128/jb.169.10.4570-4576.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Wikström M., Lindblad M., Holmgren J. Monoclonal antibodies to Escherichia coli heat-labile enterotoxins: neutralising activity and differentiation of human and porcine LTs and cholera toxin. Med Biol. 1986;64(1):23–30. [PubMed] [Google Scholar]
- Uhlin B. E., Båga M., Göransson M., Lindberg F. P., Lund B., Norgren M., Normark S. Genes determining adhesin formation in uropathogenic Escherichia coli. Curr Top Microbiol Immunol. 1985;118:163–178. doi: 10.1007/978-3-642-70586-1_9. [DOI] [PubMed] [Google Scholar]





