Abstract
The presynaptic scaffold molecule RIM1α is important for regulating neurotransmitter release. In this issue, Yao et al. (2007) show in mice that an E3 ubiquitin ligase, SCRAPPER, targets a set of presynaptic proteins including RIM1α for degradation by the ubiquitin-proteasome system. Their results identify protein degradation as a mechanism for holding rapid synaptic communication in check.
In the nervous system, the chemical synapse is the major site of communication between cells. Synapses are highly specialized structures composed of a presynaptic site containing machinery for release of neurotransmitter from vesicles, which is directly apposed to a postsynaptic site enriched in neurotransmitter receptors and signaling molecules. Synapses are crucial not only for mediating basal neurotransmission but also for ensuring malleability in response to stimuli—the latter being a cellular correlate of learning and memory termed synaptic plasticity. Synaptic plasticity is often thought of as a selective “strengthening” or “weakening” of synapses and can arise in several ways, including alterations in neurotransmitter release or in the number or biophysical properties of receptors. Ubiquitination is emerging as an important mechanism in modulating synapse formation and function (Yi and Ehlers, 2005). Monoubiquitination can serve as a regulatory posttranslational modification like phosphorylation, whereas polyubiquitination targets proteins for degradation through the ubiquitin-proteasome system (UPS). In this issue, Yao et al. (2007) describe a new mechanism for UPS-mediated presynaptic modulation that sheds light on how neurotransmitter release is held in check.
The protein RIM1α is a key player in the presynaptic terminal forming a scaffold that links synaptic vesicles with the fusion machinery as well as priming vesicles for release (Schoch et al., 2002). Setou and colleagues (Yao et al., 2007) now show that the amount of RIM1α protein is tightly controlled by a new E3 ubiquitin ligase F box protein, SCRAPPER. (F box subunits like SCRAPPER determine target specificity). SCRAPPER is selectively expressed in the brain, enriched in the presynaptic compartment, and binds to and polyubiquitinates RIM1α when in a complex with accessory E3 ubiquitin ligase subunits. Mice lacking SCRAPPER show an increase in half-life and steady state levels of RIM1α. These mice also show enhanced neurotransmission, consistent with the effect of RIM1α deletion, which decreases evoked transmission by reducing the probability of neurotransmitter release. Both RIM1α and SCRAPPER mutant mice exhibit altered paired pulse facilitation, a form of short-term plasticity. The enhanced rate of spontaneous neurotransmission in the SCRAPPER-deficient animals is mimicked by overexpression of RIM1α, and these effects arise by a presynaptic rather than a postsynaptic mechanism (as revealed in elegant coculture experiments). Similar to previous findings (Willeumier et al., 2006), Yao et al. also determined that proteasome inhibitors increase the frequency of vesicle release in wild-type neurons. However, this effect of proteasome inhibitors is occluded in neurons from SCRAPPER-deficient animals. Taken together the findings of Yao et al. (2007) reveal that SCRAPPER is a major presynaptic E3 ubiquitin ligase that acts through RIM1α and the UPS pathway to critically regulate synaptic transmission (Figure 1).
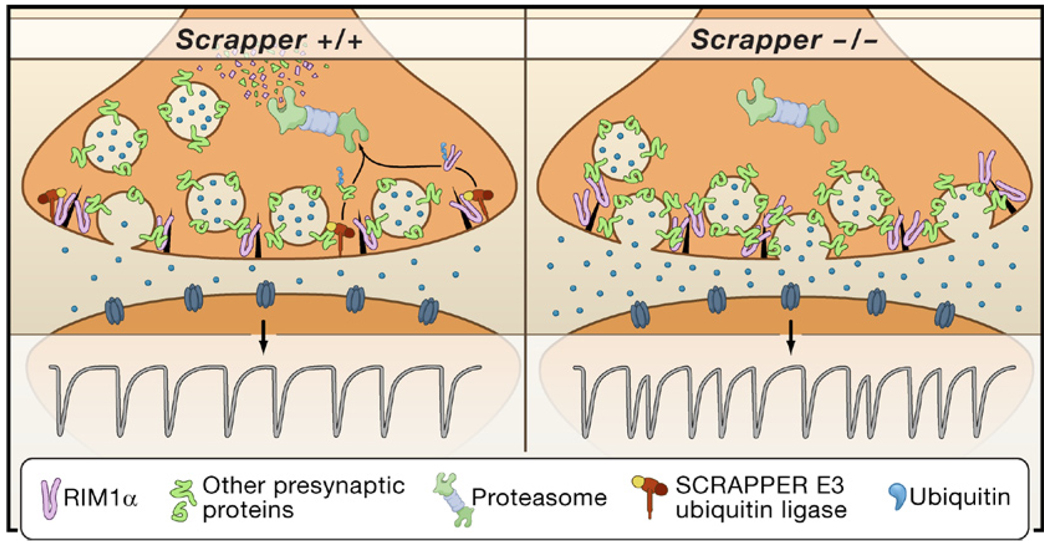
Figure 1. SCRAPPER and the Proteasome in Neurotransmission.
At a typical synapse in the central nervous system, regulated fusion of synaptic vesicles releases neurotransmitter from one cell to activate postsynaptic receptors and other signaling molecules on a target cell. Synaptic vesicles are studded with a complement of proteins that function in vesicle biogenesis, transport, docking, priming, fusion, and recycling. RIM1α acts as a scaffold to connect synaptic vesicles with active zone fusion machinery (black triangles), priming vesicles for release. In wild-type animals (left), the F box protein SCRAPPER is enriched in the presynaptic terminal and anchored to the plasma membrane via prenylation. In concert with Skr/Cullin subunits, SCRAPPER acts as an E3 ubiquitin ligase to conjugate multiple ubiquitin moieties to target proteins. These targets include RIM1α and numerous other presynaptic proteins. Upon polyubiquitination by SCRAPPER E3 ubiquitin ligase, proteins are degraded by the proteasome. In SCRAPPER-deficient mice (right), RIM1α and other important synaptic proteins accumulate. The result is a greater number of vesicles near the active zone, an increased frequency of synaptic vesicle release (trace, bottom right versus bottom left), and altered short-term synaptic plasticity. Note that the figure represents events occurring over a span of time.
In addition to its effects on RIM1α, might SCRAPPER regulate presynaptic function via other targets? Remarkably, the levels of most of the presynaptic proteins assayed, including synaptophysin, synaptogyrin, synaptotagmin, CASK, Munc13, and Munc18, are elevated in SCRAPPER knockout mice and/or reduced upon SCRAPPER overexpression. Furthermore, there are phenotypes observed in the SCRAPPER knockout animals that are not paralleled in RIM1α knockout mice, including altered synaptic vesicle distribution and altered calcium ion dependence of vesicle fusion. In addition, whereas overexpression of SCRAPPER reduces spontaneous vesicle fusion rates, loss of RIM1α does not. SCRAPPER- mediated alterations in degradation of the calcium sensor synaptotagmin and the active zone protein Munc18 might be involved in these effects. Interestingly, prior work in Drosophila (Speese et al., 2003) also indicated negative regulatory control of synaptic transmission by the UPS, at least in part through regulation of the Munc13 ortholog, but the ubiquitin ligase involved was not identified.
The exciting results of Yao et al. (2007) raise a number of additional questions. Is changing the absolute amount of specific presynaptic proteins, or rather their relative stoichiometries, important? Like many presynaptic proteins, the RIM protein family itself is composed of six main isoforms that have partially redundant roles but are expressed differentially in the brain (Kaeser and Sudhof, 2005). Is SCRAPPER’s mode of action the same for all RIM family members, or is its activity limited to only certain family members and specific synapses? Are there other substrates of SCRAPPER, including postsynaptic targets? Apart from its membrane-targeting sequence, what directs SCRAPPER to the synapse? Yao et al. (2007) report that cAMP modulates Scrapper mRNA levels; what else may regulate level, activity, or localization of SCRAPPER? How does SCRAPPER contribute to the functional plasticity of specific neural circuits? What is the behavioral phenotype of mice lacking SCRAPPER?
SCRAPPER does not play a major role in synapse development, unlike the previously described synaptic ubiquitin ligases Pam/Highwire/RPM-1 and anaphase promoting complex (APC) (DiAntonio et al., 2001; van Roessel et al., 2004). In both cases, at least in Drosophila, loss of the ubiquitin ligase leads to overgrowth of synapses along with alterations in function. Key presynaptic targets of UPS-mediated degradation include a MAP kinase kinase kinase for High-wire/RPM-1 and the active zone scaffolding and tyrosine phosphatase linked protein Liprin-α for APC.
In a new twist on the idea of ubiquitin ligases keeping synapses in check, recent findings published in Science (Ding et al., 2007) indicate a requirement for another E3 ubiquitin ligase in stereotyped synapse elimination. Using a well-characterized set of synapses in the worm Caenorhabditis elegans as their model, Ding et al. (2007) showed that the ubiquitin ligase SKR-1 is needed to eliminate secondary synapses. Primary synapses are protected by the local presence of the synaptic adhesion molecule SYG-1, which binds to SKR-1 and inhibits assembly of the ubiquitin ligase complex. These data suggest that synapses are built to fall apart, that is, unless they are protected from doing so. It will be interesting to see if similar mechanisms operate for activity-dependent synapse elimination in more complex systems.
Might SCRAPPER or a related E3 ubiquitin ligase be responsible for the activity-dependent coregulation of postsynaptic protein levels by the UPS? Unlike C. elegans, where the major excitatory neurotransmitter receptor GLR-1 is ubiquitinated (Burbea et al., 2002), in mammals evidence for direct ubiquitination of AMPA receptors is lacking. However, a host of postsynaptic scaffolding and signaling molecules including NMDA receptors are coordinately upregulated or down-regulated by the UPS in response to long-term changes in activity (Yi and Ehlers, 2005). Coordinated regulation of pre- and postsynaptic protein levels via the UPS may function in activity-dependent homeostatic responses. In addition, activity can regulate the trafficking of proteasomes and their amounts in spine synapses (Bingol and Schuman, 2006).
The results presented by Yao et al. (2007) are exceptional in that they provide evidence that a rapid kinetic process, synaptic vesicle release, can be regulated over a short timescale using the permanent process of protein degradation. The work presented in Science shows how a seemingly passive process, synapse elimination, is surprisingly dynamic. It is also exciting to think that SCRAPPER activity could be localized to or excluded from an individual synapse, reminiscent of Ding et al. (2007). This spatial regulation of UPS-mediated degradation would allow for precise tuning of neuronal networks. We now have to look beyond transcription, translation, and posttranslation modifications and look toward UPS-mediated protein degradation as a means of fine tuning already intricate systems of neurotransmission.
ACKNOWLEDGMENTS
Support was provided by the Canadian Institutes of Health Research and Michael Smith Foundation for Health Research (F.D. and A.M.C.) and the National Institutes for Health and Canada Research Chair program (A.M.C.).
REFERENCES
- DiAntonio A, Haghighi AP, Portman SL, Lee JD, Amaranto AM, Goodman CS. Nature. 2001;412:449–452. doi: 10.1038/35086595. [DOI] [PubMed] [Google Scholar]
- Bingol B, Schuman EM. Nature. 2006;441:1144–1148. doi: 10.1038/nature04769. [DOI] [PubMed] [Google Scholar]
- Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Neuron. 2002;35:107–120. doi: 10.1016/s0896-6273(02)00749-3. [DOI] [PubMed] [Google Scholar]
- Ding M, Chao D, Wang G, Shen K. Science. 2007;317:947–951. doi: 10.1126/science.1145727. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Sudhof TC. Biochem. Soc. Trans. 2005;33:1345–1349. doi: 10.1042/BST0331345. [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Speese SD, Trotta N, Rodesch CK, Aravamudan B, Broadie K. Curr. Biol. 2003;13:899–910. doi: 10.1016/s0960-9822(03)00338-5. [DOI] [PubMed] [Google Scholar]
- van Roessel P, Elliott DA, Robinson IM, Prokop A, Brand AH. Cell. 2004;119:707–718. doi: 10.1016/j.cell.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Willeumier K, Pulst SM, Schweizer FE. J. Neurosci. 2006;26:11333–11341. doi: 10.1523/JNEUROSCI.1684-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao I, Takagi H, Ageta H, Kahyo T, Sato S, Hatanaka K, Fukuda Y, Chiba T, Morone N, Yuasa S, et al. Cell. 2007 doi: 10.1016/j.cell.2007.06.052. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JJ, Ehlers MD. Neuron. 2005;47:629–632. doi: 10.1016/j.neuron.2005.07.008. [DOI] [PubMed] [Google Scholar]