Abstract
People empathize with fictional displays of behaviour, including those of cartoons and computer animations, even though the stimuli are obviously artificial. However, the extent to which other animals also may respond empathetically to animations has yet to be determined. Animations provide a potentially useful tool for exploring non-human behaviour, cognition and empathy because computer-generated stimuli offer complete control over variables and the ability to program stimuli that could not be captured on video. Establishing computer animations as a viable tool requires that non-human subjects identify with and respond to animations in a way similar to the way they do to images of actual conspecifics. Contagious yawning has been linked to empathy and poses a good test of involuntary identification and motor mimicry. We presented 24 chimpanzees with three-dimensional computer-animated chimpanzees yawning or displaying control mouth movements. The apes yawned significantly more in response to the yawn animations than to the controls, implying identification with the animations. These results support the phenomenon of contagious yawning in chimpanzees and suggest an empathic response to animations. Understanding how chimpanzees connect with animations, to both empathize and imitate, may help us to understand how humans do the same.
Keywords: contagious yawning, chimpanzees, computer animations, empathy
1. Introduction
Empathy in humans (Homo sapiens) is so highly developed that humans empathize with fictitious depictions of human behaviour (e.g. theatre in its many live and recorded forms), and even non-living representations of humans and animals, such as puppets, cartoons and computer animations (Paiva et al. 2005). Our emotional engagement with the characters in the various media is why we experience suspense at their predicaments and happiness, sadness or other emotions that ensue. The perception–action model (PAM) proposes that our emotional connection derives from an activation of neural representations associated with our own experiences (Preston & de Waal 2002). Recently, imitation of computer-generated animations has been put to clinical use in children with autism spectrum disorder (ASD; Shane & Albert 2008) and has also been a cause for concern over violent video games (Bartholow et al. 2006).
Three-dimensional computer animation is of potential interest for studying the cognition, emotion and behaviour of non-human animals. Presentation of video images of real behaviour has several limitations. Different examples of the same behaviour may be highly variable owing to factors outside of the experimenter's control (e.g. individuals present/absent, intensity and duration of behaviours, lighting, background composition etc.). Rare behaviours pose additional challenges, since recording multiple examples requires either extraordinary luck or a large and uncertain time investment. Videos of ‘impossible’ behaviours (i.e. behaviours not in the repertoire of the subjects or species) are by definition impossible to obtain. All of these obstacles can be overcome using computer animation, and the creation of impossible behaviours is one application of animation that has already been exploited with pigeons (Columba livia; Watanabe & Troje 2006). The disadvantage to computer animation is that the stimuli may not look like real conspecifics; they are inherently artificial. Before the advantages of animations can be exploited, two critical questions need to be answered. (i) Do non-human animals view or process animated images the same way they do real images of conspecifics? (ii) Will non-human animals identify or empathize with animations? We know humans both process and empathize with animations in a way similar to the way they do real humans, and if other animals do as well, then computer animations represent a new and flexible tool in the study of animal behaviour.
The first question above was recently answered by Parr et al. (2008), who tested how chimpanzees (Pan troglodytes) categorize facial expressions using virtual chimpanzees created with Poser 6.0 (Smith Micro, Inc.). Chimpanzee facial expressions are graded signals, and the computer program allowed for a precise, standardized library of images impossible to collect through photography. Chimpanzees discriminated between different expressions, and inversion of the animated faces disrupted performance, as it does with photographs of actual chimpanzee faces (Parr et al. 1998, 2008). The inversion effect demonstrates configurative processing of animations, the same way chimpanzees process faces, rather than feature-based processing. If the animations were not processed as whole faces but rather as a collection of shapes and colours, no inversion effect would have been seen.
The next step is to determine whether chimpanzees identify with animations, thus addressing the second question above. We tested whether chimpanzees show contagious yawning in response to animated chimpanzee yawns. There are both theoretical and empirical links between contagious yawning and empathy. Lehmann (1979) considered yawning an ‘affective expression’ dependent upon empathy. According to the PAM, contagious yawning is controlled by the same mechanism that makes emotions contagious (Preston & de Waal 2002). Empirical evidence comes from the findings that individuals who possessed more schizotypal personality traits performed less contagious yawning (Platek et al. 2003), and contagious yawning was greatly reduced, and may even have been absent, in children with ASD (Senju et al. 2007; Giganti & Esposito Ziello 2009). In both schizotypy and ASD, empathy may be impaired, although Senju et al. (in press) suggest that attention may also be an issue for children with ASD.
Contagious yawning is well suited for this type of test for several reasons. Because yawning is involuntary, contagion would indicate subconscious identification with the animations rather than deliberate imitation (which may result in opening of the mouth but not an actual yawn). Physiological measurements of emotional arousal might also indicate identification, but these methods are not currently feasible with awake, behaving, adult chimpanzees. Hence, there is a need for purely behavioural measures. Whether considered a part of affect or not, contagious yawning and emotional responses are both involuntary psychophysiological responses. Hence, they provide complementary measures of an empathic connection to a stimulus. Although the methods, results and conclusion vary, evidence for contagious yawning has been observed in chimpanzees (Anderson et al. 2004), stumptail macaques (Macaca arctoides; Paukner & Anderson 2006) and domestic dogs (Canis familiaris; Joly-Mascheroni et al. 2008; Harr et al. in press), so our experiment may generalize to other species.
Anderson et al. (2004) found that two of six chimpanzees yawned more in response to videos of chimpanzees yawning than to control videos. The population-level statistic was non-significant, which is not surprising given the small sample size. We presented 24 chimpanzees with three-dimensional computer-animated chimpanzees yawning or displaying control mouth movements. We hypothesized that if the chimpanzees identified with the animations, then they would yawn more in response to the yawn animations than the control animations.
2. Material and Methods
Subjects were 24 chimpanzees (P. troglodytes) housed in two groups of 12 at the Yerkes National Primate Research Center Field Station. The 4 males and 20 females ranged from 9 to 43 years of age (electronic supplementary material S1). Both groups of chimpanzees lived in large outdoor enclosures (group FS 1, 711 m2; group FS 2, 528 m2) with indoor sleeping quarters. Group FS 1 had an additional indoor testing building. We tested subjects from FS 1 in one room of the testing building (5.78 m3) and from FS 2 in one room of the sleeping quarters (26.1 m3). There was one test per day between 10.30 and 14.00.
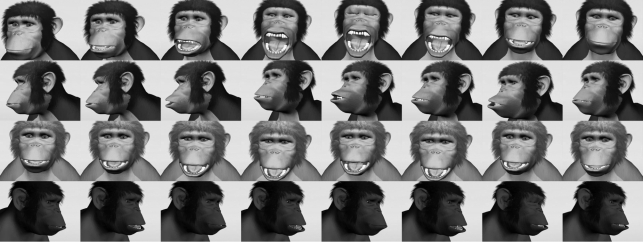
The stimuli were three-dimensional computer animations of chimpanzee facial movements created with Lightwave 3D (NewTek, Inc.; figure 1; see electronic supplementary material S2 for a brief description of the animation process and electronic supplementary material S3–S6 for example animations). Three distinct chimpanzee heads were rendered, and each face was given two different movements: a yawn and a control expression. The control expressions resembled a relaxed open-mouth display (or play face) with head-bobbing, hooting (without the vocalization) and tooth-clacking (often made during grooming). There were no sounds with any of the videos. Each movement was presented with the animation facing in one of three directions, to the left or right in the three-quarter profile or centred. This yielded nine distinct yawn animations (three different heads from three angles; one example is found in electronic supplementary material S3) and nine distinct control animations (three different heads—each performing a different expression—from three angles; see electronic supplementary material S4–S6). Each animation lasted 9 s with 1 s of blue screen for a total clip length of 10 s. Each clip was presented 10 times, and the full set of clips was shown before repeating in pseudo-random order. These clips were assembled into a yawn video and a control video, each comprising 90 clips and lasting 15 min.
Figure 1.
A sample animation of each movement, from top: yawn, hoot, relaxed open-mouth and tooth-clack.
The animations were presented on a 48 cm LCD monitor sitting on a small cart, bringing the monitor close to the eye level for a seated chimpanzee. Two experimenters operated two digital video cameras. Testing began with bringing chimpanzees inside, giving a small reward and closing the pre-determined test subjects into one room used throughout the experiment. The chimpanzees were tested in 12 mutually exclusive pairs, chosen for compatibility, as social contact put the chimpanzees in a relaxed or baseline state. Video presentation began immediately after the subjects were closed into the test room, and each experimenter video-taped one of the subjects. The animations lasted 15 min, and there was an additional 5 min observation period in case of a build-up effect. After the 20 min session ended, the subjects were given a reward and released back to the group. Subjects were tested twice with the order of stimulus presentation counterbalanced: half of the subjects saw the yawn video first and half saw the control video first.
Two independent observers coded the video-taped sessions for the number of yawns. One observer coded the amount of attention towards the monitor, distinguishing three levels: level 3, the subject was close (within 2 m), directly looking at the screen; level 2, any possible attention to the screen, including from further away and when body orientation would allow peripheral vision of the screen; and level 1, no possible attention towards the screen, as when body or head orientation would make seeing the screen impossible. On a 5 s interval, the observer recorded the highest level of attention attained by each individual. In the analysis, we examined two measures of attention: level 3 (close attention) and the combined data of levels 3 and 2 (total attention). Statistics were conducted using SPSS 16.0 for Macintosh (SPSS, Inc.). All statistics were two-tailed.
3. Results
The two independent coders agreed on 189 yawns, with eight disagreements discarded. In the control sessions, the rate of yawning by one subject was a statistical outlier (33 yawns, greater than 4 s.d. above the mean; Grubb's test: Z = −4.44, p < 0.01). This individual was removed from all further analyses, yielding a final n of 23.
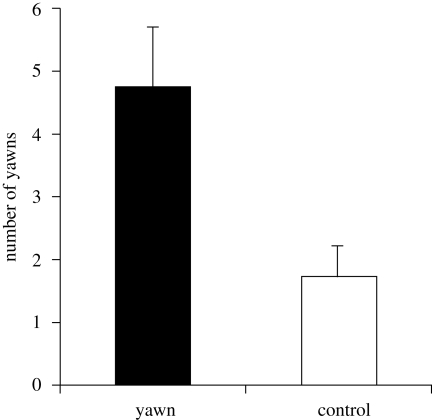
The chimpanzees yawned more frequently in response to the yawn video than the control video (paired t-test: t22 = 3.19, p = 0.003, d = 0.82; figure 2; electronic supplementary material S1). This difference remained significant when we used the combined yawn rate per pair as independent data points (paired t-test: t10 = 2.43, p = 0.035, d = 0.87). There was no difference between males (mean 6.50 ± 2.50 s.e.m.) and females (mean 4.37 ± 1.06 s.e.m.; independent samples t-test, t21 < 1.0, n.s.). A table with the raw data on yawns and individual binomials can be found in electronic supplementary material S1, and a video demonstrating the experiment can be found in electronic supplementary material S7.
Figure 2.
Mean (+s.e.m.) number of yawns per subject during the yawn and the control sessions. The difference is significant.
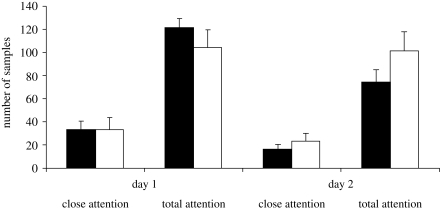
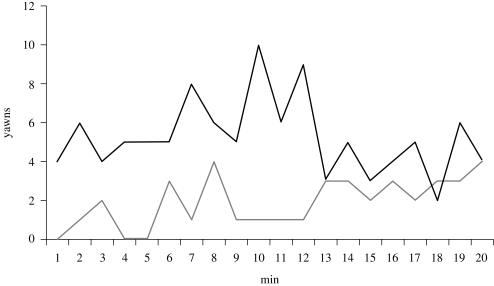
Subjects paid similar amounts of attention to the yawn and control videos (figure 3). Thus, one stimulus set was not more salient than the other. Neither measure of attention correlated with the individual rate of yawning in the yawn sessions (Spearman's, n = 23, close attention: ρ = 0.119, R2 = 0.014, n.s.; total attention: ρ = −0.064, R2 = 0.004, n.s.). We also investigated the relationship between yawns and attention over time while the animations were playing (i.e. 1–15 min), but there were no significant correlations here on data pooled across individuals (Spearman's, n = 15, close attention: ρ = −0.051, R2 = 0.003, n.s.; total attention: ρ = 0.426, R2 = 0.181, p = 0.11). The rate of yawning over time during the entire 20 min session showed a slight, non-significant decrease to the yawn video (Spearman's, n = 20, ρ = −0.224, R2 = 0.050, n.s.), but the few yawns given to the control videos increased significantly over time (Spearman's, n = 20, ρ = 0.636, R2=0.404, p = 0.003; figure 4).
Figure 3.
Mean amounts of ‘close’ and ‘total’ attention paid to the screen (+s.e.m.) on the two testing days. Half of the chimpanzees saw the yawn stimulus and half saw the control on day 1, and the order was reversed on day 2. There are no significant differences for any of the comparisons (independent samples t-tests: day 1, close attention t21 < 1.0 n.s.; day 1, total attention t21 < 1.0 n.s.; day 2, close attention t21 < 1.0 n.s.; day 2, total attention t21 = 1.35, p = 0.19). Black bars, yawn; white bars, control.
Figure 4.
Rate of yawning over the 20 min session in response to the two stimuli. Most of the effect took place in the first 12 min, but there was still a large difference between the yawn and control conditions when we analysed the entire 20 min session. Yawning showed a non-significant decrease over time to the yawn video, whereas the rate showed a significant increase over time to the control video. Black lines, yawn video, ρ = −0.224; grey lines, control video, ρ = 0.636.
4. Discussion
Chimpanzees showed contagious yawning in response to animated chimpanzee yawns as demonstrated by a significant population-level effect. The population-level effect tells us that contagious yawning is a common trait in chimpanzees and that the results of Anderson et al. (2004) are representative. The ideal way to compare the potency of animated yawns and video-taped yawns is to test the same subjects with both sets of stimuli, and that is something we are presently working on. Whereas at first sight the tendency for yawn contagion may seem lower for chimpanzees than what has been reported for humans (Provine 1986; Platek et al. 2003), a direct comparison is hampered by the differing methods in calculating yawn contagion. Because we tested our subjects in pairs, we cannot be certain in all cases whether an individual yawned in response to the yawn animations or a partner's yawn. Either could produce contagious yawning. However, the large difference in yawning to the yawn versus control animations, using either the individual or the pair as the unit of analysis, demonstrates that the yawn animations did stimulate contagious yawning.
Importantly, the chimpanzees attended similarly to the yawn and the control videos, so we can rule out that the control video inhibited yawning because it was more interesting. Furthermore, if yawning was induced by boredom, we would expect the rate of yawning to have increased over time. As the novelty of the animations wore off, yawning should have gone up as a product of boredom. This was the case for the control video, but not the yawn video, which actually had a slight decrease in yawning over time. Therefore, we can safely conclude that it was the yawns themselves, and not boredom, that produced greater yawning in response to the yawn video.
Our measures of attention did not correlate with the rate of yawning. It is possible that our sampling method was not precise enough. However, there may not be much of a relationship between total attention and the amount of contagious yawning. A small amount of attention could stimulate multiple yawns by a highly susceptible individual, and a large amount of attention by an individual less susceptible to contagious yawning could produce few or no yawns. Approximately half of human subjects show contagious yawning under experimental conditions (Provine 1986; Platek et al. 2003). All of the human subjects watched numerous yawns but around half showed no yawn response (Provine 1986; Platek et al. 2003), so there appears to be no correlation between attention and contagious yawning in humans either.
For chimpanzees to display a contagious behaviour in response to three-dimensional computer animations, they probably identified on some level with the animations. We think that simple stimulus generalization is an unlikely explanation because Parr et al. (2008) demonstrated that chimpanzees processed three-dimensional animated chimpanzee faces in a way similar to the way they processed actual chimpanzee faces. To test stimulus generalization versus identification, we plan to enhance and degrade the quality of the animations to see if realism affects the rate of contagious yawning. In the meantime, the combined results of our study and Parr et al. (2008) strongly suggest that chimpanzees view, process, identify with and empathize with animated chimpanzees similarly to photographs and video of actual chimpanzees.
This opens the possibility to exploit animations in the study of chimpanzee behaviour and cognition. Future testing will determine how widely among non-human animals this resource can be applied. Animation presents the possibility to display stimuli with a new level of control, and the ability to custom-design behaviours allows for new questions to be asked. Furthermore, understanding the propensity of non-humans to imitate animated, fictitious displays may shed light on the conditions under which humans do the same.
Acknowledgements
We thank Victoria Horner and Jennifer J. Pokorny for helpful suggestions and the animal care and veterinary staff of the Yerkes National Primate Research Center's Field Station. The DAVE School (Digital Animation and Video Effects, Universal Studios Florida) provided training to J.D.C. on Lightwave 3D. We also thank three anonymous reviewers for helpful and constructive comments that improved this article. This work was supported by Emory's College of Arts and Sciences, the Living Links Center, as well as the base grant of the National Institutes of Health (USA) no. RR-00165 to the Yerkes NPRC. The Yerkes NPRC is fully accredited by the American Association for Accreditation for Laboratory Animal Care.
References
- Anderson J. R., Myowa-Yamakoshi M., Matsuzawa T.2004Contagious yawning in chimpanzees. Proc. R. Soc. Lond. B 271, S468–S470 (doi:10.1098/rsbl.2004.0224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholow B. D., Bushman B. J., Sestir M. A.2006Chronic violent video game exposure and desensitization to violence: behavioral and event-related brain potential data. J. Exp. Social Psychol. 42, 532–539 (doi:10.1016/j.jesp.2005.08.006) [Google Scholar]
- Giganti F., Esposito Ziello M.2009Contagious and spontaneous yawning in autistic and typically developing children. Curr. Psychol. Lett. 25, 1–11 [Google Scholar]
- Harr A. L., Gilbert V. R., Philips K. A. Do dogs (Canis familiaris) show contagious yawning? Anim. Cogn. doi: 10.1007/s10071-009-0233-0. In press. ( doi:10.1007/s10071-009-0233-0) [DOI] [PubMed] [Google Scholar]
- Joly-Mascheroni R. M., Senju A., Shepherd A. J.2008Dogs catch human yawns. Biol. Lett. 4, 446–448 (doi:10.1098/rsbl.2008.0333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann H. E.1979Yawning: a homeostatic reflex and its psychological significance. Bull. Menninger Clin. 43, 123–136 [PubMed] [Google Scholar]
- Paiva A., Dias J. O., Sobral D., Aylett R., Woods S., Hall L., Zoll C.2005Learning by feeling: evoking empathy with synthetic characters. Appl. Artif. Intell. 19, 235–266 (doi:10.1080/08839510590910165) [Google Scholar]
- Parr L. A., Dove T., Hopkins W. D.1998Why faces may be special: evidence of the inversion effect in chimpanzees. J. Cogn. Neurosci. 10, 615–622 (doi:10.1162/089892998563013) [DOI] [PubMed] [Google Scholar]
- Parr L. A., Waller B. M., Heintz M.2008Facial expression categorization by chimpanzees using standardized stimuli. Emotion 8, 216–231 (doi:10.1037/1528-3542.8.2.216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukner A., Anderson J. R.2006Video-induced yawning in stumptail macaques (Macaca arctoides). Biol. Lett. 2, 36–38 (doi:10.1098/rsbl.2005.0411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek S. M., Critton S. R., Myers T. E., Gallup G. G.2003Contagious yawning: the role of self-awareness and mental state attribution. Cogn. Brain Res. 17, 223–227 (doi:10.1016/S0926-6410(03)00109-5) [DOI] [PubMed] [Google Scholar]
- Preston S. D., de Waal F. B. M.2002Empathy: its ultimate and proximate bases. Behav. Brain Sci. 25, 1–72 [DOI] [PubMed] [Google Scholar]
- Provine R. R.1986Yawning as a stereotyped action pattern and releasing stimulus. Etholoy 72, 109–122 [Google Scholar]
- Senju A., Maeda M., Kikuchi Y., Hasegawa T., Tojo Y., Osanai H.2007Absence of contagious yawning in children with autism spectrum disorder. Biol. Lett. 3, 706–708 (doi:10.1098/rsbl.2007.0337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A., Kikuchi Y., Akechi H., Hasegawa T., Tojo Y., Osanai H. Does eye contact induce contagious yawning in children with autism spectrum disorder? J. Autism Dev. Disord. doi: 10.1007/s10803-009-0785-5. In press. ( doi:10.1007/s10803-009-0785-5) [DOI] [PubMed] [Google Scholar]
- Shane H., Albert P.2008Electronic screen media for persons with autism spectrum disorders: results of a survey. J. Autism Dev. Disord. 38, 1499–1508 (doi:10.1007/s10803-007-0527-5) [DOI] [PubMed] [Google Scholar]
- Watanabe S., Troje N. F.2006Towards a ‘virtual pigeon’: a new technique for investigating avian social perception. Anim. Cogn. 9, 271–279 (doi:10.1007/s10071-006-0048-1) [DOI] [PubMed] [Google Scholar]