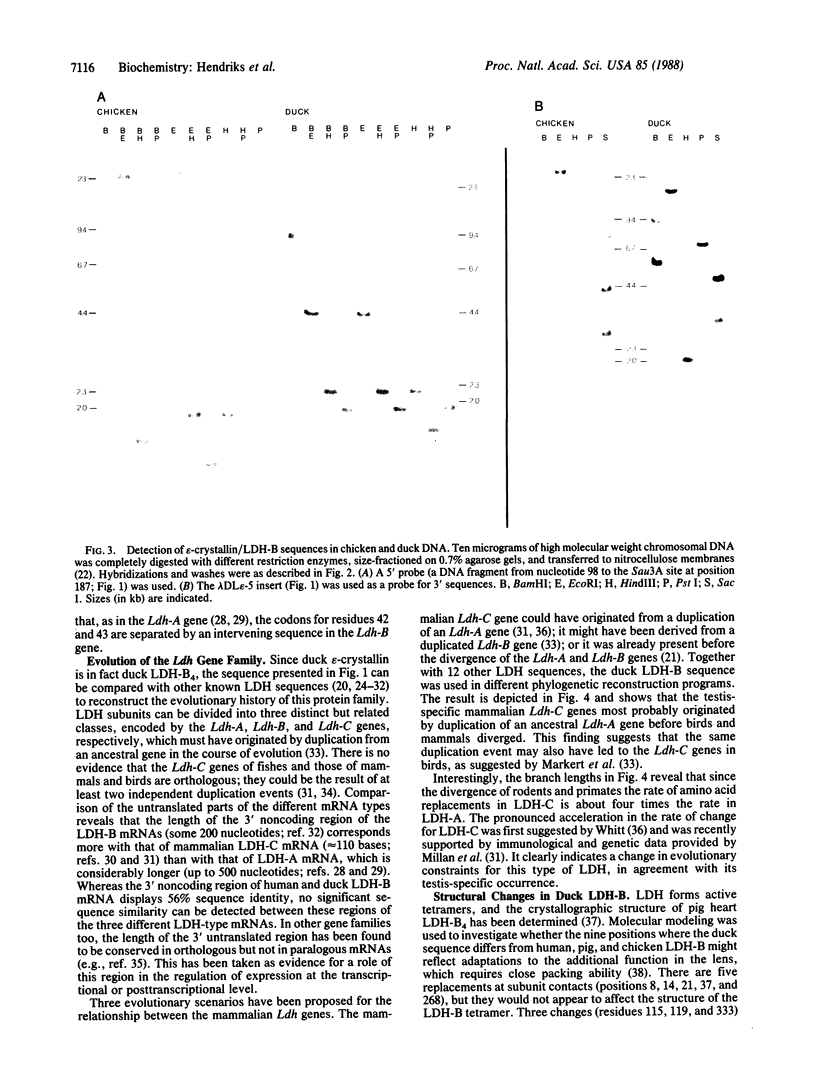
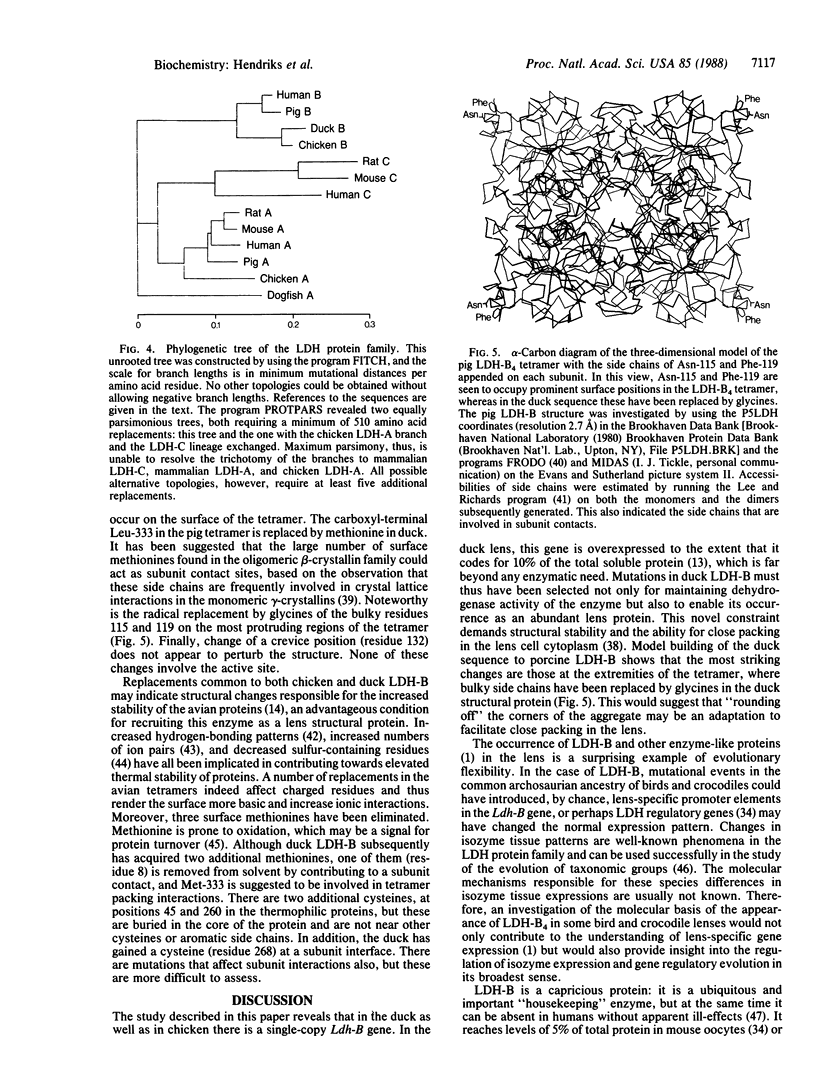
Abstract
To investigate whether or not duck lens epsilon-crystallin and duck heart lactate dehydrogenase (LDH) B4 are the product of the same gene, we have isolated and sequenced cDNA clones of duck epsilon-crystallin. By using these clones we demonstrate that there is a single-copy Ldh-B gene in duck and in chicken. In the duck lens this gene is overexpressed, and its product is subject to posttranslational modification. Reconstruction of the evolutionary history of the LDH protein family reveals that the mammalian Ldh-C gene most probably originated from an ancestral Ldh-A gene and that the amino acid replacement rate in LDH-C is approximately 4 times the rate in LDH-A. Molecular modeling of LDH-B sequences shows that the increased thermostability of the avian tetramer might be explained by mutations that increase the number of ion pairs. Furthermore, the replacement of bulky side chains by glycines on the corners of the duck protein suggests an adaptation to facilitate close packing in the lens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T., Sun D. P., Wilson K., Wozniak J. A., Cook S. P., Matthews B. W. Contributions of hydrogen bonds of Thr 157 to the thermodynamic stability of phage T4 lysozyme. Nature. 1987 Nov 5;330(6143):41–46. doi: 10.1038/330041a0. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Barlow D. J., Thornton J. M. Ion-pairs in proteins. J Mol Biol. 1983 Aug 25;168(4):867–885. doi: 10.1016/s0022-2836(83)80079-5. [DOI] [PubMed] [Google Scholar]
- Carper D., Nishimura C., Shinohara T., Dietzchold B., Wistow G., Craft C., Kador P., Kinoshita J. H. Aldose reductase and p-crystallin belong to the same protein superfamily as aldehyde reductase. FEBS Lett. 1987 Aug 10;220(1):209–213. doi: 10.1016/0014-5793(87)80905-5. [DOI] [PubMed] [Google Scholar]
- Chung F. Z., Tsujibo H., Bhattacharyya U., Sharief F. S., LI S. S. Genomic organization of human lactate dehydrogenase-A gene. Biochem J. 1985 Nov 1;231(3):537–541. doi: 10.1042/bj2310537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska A., Gutowicz J. Interaction of bovine heart lactate dehydrogenase with erythrocyte lipids. Biochim Biophys Acta. 1986 Feb 13;855(1):99–104. doi: 10.1016/0005-2736(86)90193-8. [DOI] [PubMed] [Google Scholar]
- Delaye M., Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. 1983 Mar 31-Apr 6Nature. 302(5907):415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Driessen H. P., Herbrink P., Bloemendal H., de Jong W. W. Primary structure of the bovine beta-crystallin Bp chain. Internal duplication and homology with gamma-crystallin. Eur J Biochem. 1981 Dec;121(1):83–91. doi: 10.1111/j.1432-1033.1981.tb06433.x. [DOI] [PubMed] [Google Scholar]
- Eventoff W., Rossmann M. G., Taylor S. S., Torff H. J., Meyer H., Keil W., Kiltz H. H. Structural adaptations of lactate dehydrogenase isozymes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2677–2681. doi: 10.1073/pnas.74.7.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti F., Marty M. C., Courvalin J. C., Maunoury R., Bornens M. Centrosomal proteins and lactate dehydrogenase possess a common epitope in human cell lines. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1000–1004. doi: 10.1073/pnas.84.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau U. M., Trommer W. E., Rossmann M. G. Structure of the active ternary complex of pig heart lactate dehydrogenase with S-lac-NAD at 2.7 A resolution. J Mol Biol. 1981 Sep 15;151(2):289–307. doi: 10.1016/0022-2836(81)90516-7. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiltz H. H., Keil W., Griesbach M., Petry K., Meyer H. The primary structure of porcine lactate dehydrogenase: isoenzymes M4 and H4. Hoppe Seylers Z Physiol Chem. 1977 Jan;358(1):123–127. doi: 10.1515/bchm2.1977.358.1.123. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Li S. S., Fitch W. M., Pan Y. C., Sharief F. S. Evolutionary relationships of vertebrate lactate dehydrogenase isozymes A4 (muscle), B4 (heart), and C4 (testis). J Biol Chem. 1983 Jun 10;258(11):7029–7032. [PubMed] [Google Scholar]
- Li S. S., Tiano H. F., Fukasawa K. M., Yagi K., Shimizu M., Sharief F. S., Nakashima Y., Pan Y. E. Protein structure and gene organization of mouse lactate dehydrogenase-A isozyme. Eur J Biochem. 1985 Jun 3;149(2):215–225. doi: 10.1111/j.1432-1033.1985.tb08914.x. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Lei S. P., Wilcox G. An improved DNA sequencing strategy. Anal Biochem. 1985 May 15;147(1):114–119. doi: 10.1016/0003-2697(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Markert C. L. Lactate dehydrogenase. Biochemistry and function of lactate dehydrogenase. Cell Biochem Funct. 1984 Jul;2(3):131–134. doi: 10.1002/cbf.290020302. [DOI] [PubMed] [Google Scholar]
- Markert C. L., Shaklee J. B., Whitt G. S. Evolution of a gene. Multiple genes for LDH isozymes provide a model of the evolution of gene structure, function and regulation. Science. 1975 Jul 11;189(4197):102–114. doi: 10.1126/science.1138367. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Rautmann G., Magun B. E., Breathnach R. Epidermal growth factor or serum stimulation of rat fibroblasts induces an elevation in mRNA levels for lactate dehydrogenase and other glycolytic enzymes. Nucleic Acids Res. 1985 Feb 11;13(3):711–726. doi: 10.1093/nar/13.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan J. L., Driscoll C. E., LeVan K. M., Goldberg E. Epitopes of human testis-specific lactate dehydrogenase deduced from a cDNA sequence. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5311–5315. doi: 10.1073/pnas.84.15.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V., Dunne D. W., Johnson K. S., Taylor D. W., Cordingley J. S. Sequence and expression of a major egg antigen from Schistosoma mansoni. Homologies to heat shock proteins and alpha-crystallins. Mol Biochem Parasitol. 1986 Nov;21(2):179–188. doi: 10.1016/0166-6851(86)90021-6. [DOI] [PubMed] [Google Scholar]
- Pan Y. C., Sharief F. S., Okabe M., Huang S., Li S. S. Amino acid sequence studies on lactate dehydrogenase C4 isozymes from mouse and rat testes. J Biol Chem. 1983 Jun 10;258(11):7005–7016. [PubMed] [Google Scholar]
- Perry L. J., Wetzel R. The role of cysteine oxidation in the thermal inactivation of T4 lysozyme. Protein Eng. 1987 Feb-Mar;1(2):101–105. doi: 10.1093/protein/1.2.101. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., O'Brien W. E., Norman B. L., Kalumuck K., Wistow G. J., Borras T., Nickerson J. M., Wawrousek E. F. Gene sharing by delta-crystallin and argininosuccinate lyase. Proc Natl Acad Sci U S A. 1988 May;85(10):3479–3483. doi: 10.1073/pnas.85.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai I., Sharief F. S., Li S. S. Molecular cloning and nucleotide sequence of the cDNA for sperm-specific lactate dehydrogenase-C from mouse. Biochem J. 1987 Mar 1;242(2):619–622. doi: 10.1042/bj2420619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai I., Sharief F. S., Pan Y. C., Li S. S. The cDNA and protein sequences of human lactate dehydrogenase B. Biochem J. 1987 Dec 15;248(3):933–936. doi: 10.1042/bj2480933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Slingsby C., Driessen H. P., Mahadevan D., Bax B., Blundell T. L. Evolutionary and functional relationships between the basic and acidic beta-crystallins. Exp Eye Res. 1988 Mar;46(3):375–403. doi: 10.1016/s0014-4835(88)80027-7. [DOI] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapel S. O., Zweers A., Dodemont H. J., Kan J. H., de Jong W. W. epsilon-Crystallin, a novel avian and reptilian eye lens protein. Eur J Biochem. 1985 Feb 15;147(1):129–136. doi: 10.1111/j.1432-1033.1985.tb08728.x. [DOI] [PubMed] [Google Scholar]
- WILSON A. C., KAPLAN N. O., LEVINE L., PESCE A., REICHLIN M., ALLISON W. S. EVOLUTION OF LACTIC DEHYDROGENASES. Fed Proc. 1964 Nov-Dec;23:1258–1266. [PubMed] [Google Scholar]
- Watanabe K., Fujii Y., Nakayama K., Ohkubo H., Kuramitsu S., Kagamiyama H., Nakanishi S., Hayaishi O. Structural similarity of bovine lung prostaglandin F synthase to lens epsilon-crystallin of the European common frog. Proc Natl Acad Sci U S A. 1988 Jan;85(1):11–15. doi: 10.1073/pnas.85.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt G. S. Genetic, developmental and evolutionary aspects of the lactate dehydrogenase isozyme system. Cell Biochem Funct. 1984 Jul;2(3):134–139. doi: 10.1002/cbf.290020303. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Mulders J. W., de Jong W. W. The enzyme lactate dehydrogenase as a structural protein in avian and crocodilian lenses. Nature. 1987 Apr 9;326(6113):622–624. doi: 10.1038/326622a0. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wistow G., Piatigorsky J. Recruitment of enzymes as lens structural proteins. Science. 1987 Jun 19;236(4808):1554–1556. doi: 10.1126/science.3589669. [DOI] [PubMed] [Google Scholar]
- Wistow G., Summers L., Blundell T. Myxococcus xanthus spore coat protein S may have a similar structure to vertebrate lens beta gamma-crystallins. 1985 Jun 27-Jul 3Nature. 315(6022):771–773. doi: 10.1038/315771a0. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Nudel U., Mayer Y., Neuman S. Highly conserved sequences in the 3' untranslated region of mRNAs coding for homologous proteins in distantly related species. Nucleic Acids Res. 1985 May 24;13(10):3723–3737. doi: 10.1093/nar/13.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong W. W., Leunissen J. A., Leenen P. J., Zweers A., Versteeg M. Dogfish alpha-crystallin sequences. Comparison with small heat shock proteins and Schistosoma egg antigen. J Biol Chem. 1988 Apr 15;263(11):5141–5149. [PubMed] [Google Scholar]
- van den Heuvel R., Hendriks W., Quax W., Bloemendal H. Complete structure of the hamster alpha A crystallin gene. Reflection of an evolutionary history by means of exon shuffling. J Mol Biol. 1985 Sep 20;185(2):273–284. doi: 10.1016/0022-2836(85)90403-6. [DOI] [PubMed] [Google Scholar]