Abstract
The form of Darwinian selection has important ecological and management implications. Negative effects of harvesting are often ascribed to size truncation (i.e. strictly directional selection against large individuals) and resultant decrease in trait variability, which depresses capacity to buffer environmental change, hinders evolutionary rebound and ultimately impairs population recovery. However, the exact form of harvest-induced selection is generally unknown and the effects of harvest on trait variability remain unexplored. Here we use unique data from the Windermere (UK) long-term ecological experiment to show in a top predator (pike, Esox lucius) that the fishery does not induce size truncation but disruptive (diversifying) selection, and does not decrease but rather increases variability in pike somatic growth rate and size at age. This result is supported by complementary modelling approaches removing the effects of catch selectivity, selection prior to the catch and environmental variation. Therefore, fishing most likely increased genetic variability for somatic growth in pike and presumably favoured an observed rapid evolutionary rebound after fishery relaxation. Inference about the mechanisms through which harvesting negatively affects population numbers and recovery should systematically be based on a measure of the exact form of selection. From a management perspective, disruptive harvesting necessitates combining a preservation of large individuals with moderate exploitation rates, and thus provides a comprehensive tool for sustainable exploitation of natural resources.
Keywords: adaptive landscape, conservation, contemporary life-history evolution, evolvability, fisheries management, nonlinear selection
1. Introduction
Humans are tremendously strong agents of Darwinian selection. Evolutionary change in response to human activities is best documented in harvested systems where selective removal of large individuals by fishers, hunters and plant harvesters rapidly reduces mean body size and mean age at maturity (Haugen & Vøllestad 2001; Conover & Munch 2002; Coltman et al. 2003; Law & Salick 2005; Edeline et al. 2007). Maybe because of a focus on mean trait values, harvest-induced selection is almost systematically equated to strict size truncation, i.e. purely directional selection against large body size, and many of the negative effects of harvesting to population persistence and recovery are ascribed to size truncation (Conover & Munch 2002; Berkeley et al. 2004; Hutchings 2005; Hsieh et al. 2006; Lewin et al. 2006; Hutchings & Fraser 2008). Indeed, truncation selection against large individuals is particularly detrimental to exploited populations because older and larger individuals often produce more and higher quality offspring than younger, smaller individuals (Berkeley et al. 2004; Hutchings 2005). Hence, selective harvesting of large individuals affects population rate of increase more negatively than would do random harvesting. Additionally, truncation selection erodes genetic variance (Roff 1997) and could thus cause irreversible trait changes because genetic variability is necessary for selection to act on (Hutchings & Fraser 2008). In particular, artificial size truncation acting in directional opposition with natural selection (Edeline et al. 2007) might induce a more severe reduction in body size variance compared with size truncation acting alone (Conover et al. 2009). However, most often, the exact form of harvest-induced selection is unknown and the effect of harvesting on trait variability remains unexplored.
Recent studies in Gulf of Saint Lawrence cod, Gadus morhua (Sinclair et al. 2002) and Windermere pike, Esox lucius (Carlson et al. 2007) show that fishery-induced selection is not necessarily truncation selection but can instead be disruptive selection. Disruptive selection occurs when individuals with intermediate trait values (here body size) have lower fitness than individuals with extreme trait values (Roff 1997; Carlson et al. 2007), and disruptive fishing arises from gear selectivity and/or segregation of fishes on fishing grounds by size and age. Theory predicts that disruptive selection, in contrast to truncation selection, should increase phenotypic variance, genetic variance and population adaptability. Here we use unique data from the Windermere long-term ecological experiment (Le Cren 2001; Winfield et al. 2008) to link changes in the strength of disruptive selection from a gillnet scientific fishery to changes in phenotypic variability of Windermere pike. We show that, as predicted by the theory, disruptive fishery selection is associated with increased phenotypic variance in pike somatic growth and size at age.
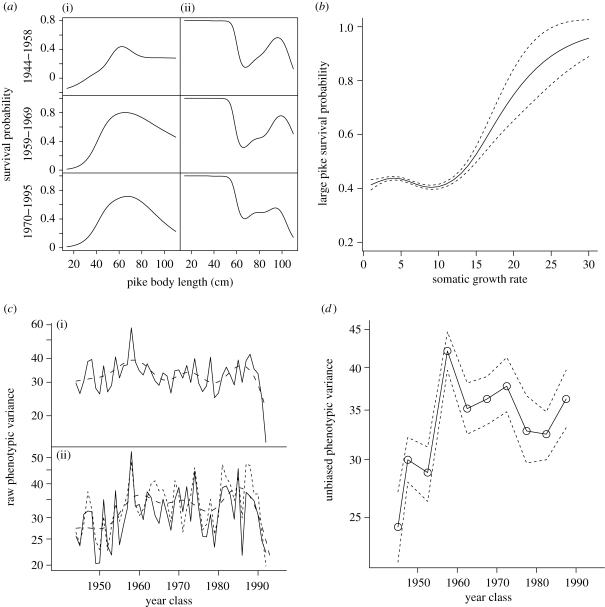
Windermere is a glacial valley lake of the English Lake District (northwest UK) that has been under extensive scientific monitoring for decades (see §2). Natural and fishery-induced selection act in direct opposition on Windermere pike body size (Carlson et al. 2007). We illustrate this antagonism between natural and fishery selection in table 1 and figure 1a, which were built using the same data and methods as Carlson et al. (2007) but with a different grouping structure for data (see §2). Natural selection acting on Windermere pike body size is stabilizing (intermediate-sized pike have the highest survival probability; table 1 and figure 1a), became increasingly stabilizing over time (figure 1a) and the naturally selected body size optimum is larger than the population mean so that the directional selection gradient is positive (table 1). The stabilizing form of natural selection presumably reflects the combined effects of cannibalism (Le Cren 1987; Haugen et al. 2007) and senescence (note that owing to fish asymptotic growth, natural selection acting on age is less stabilizing than natural selection acting on body length (Edeline et al. submitted)). By contrast, scientific fishery selection is disruptive (table 1 and figure 1a), became less disruptive over time (figure 1a), and the fitness valley is also larger than the population mean so that the resulting selection gradient is negative (table 1). Changes in mean pike somatic growth were driven by the directional antagonism between the two selective forces (Edeline et al. 2007). From 1944 to 1963, the strength of fishery selection dominated over the strength of natural selection, resulting in decreased mean individual somatic growth. During this period, exploitation rate increased and the fishery annually removed from 1.1 to 7.3 per cent (mean 3.3%) of the pike population. From 1963 to 1993, the exploitation rate decreased (from 4.95 to 0.13%, mean 1.1%) and fishery selection was overridden by natural selection, triggering a rapid increase in mean pike somatic growth. These results have shown that the dominant selective force determined the position of the phenotypic optimum (i.e. drove movements of the adaptive peak on the adaptive landscape). Here, our aim was to investigate whether the dominant selective force also drove peak sharpness around the phenotypic optimum (i.e. drove the curvature of the adaptive landscape). We predicted that the dominance of fishery selection over natural selection upto the early 1960s should have imposed a concave adaptive landscape and thus favoured an increase in pike phenotypic variance. By contrast, the dominance of natural selection over fishery selection after the early 1960s should have imposed a convex adaptive landscape and thus favoured a decrease in pike phenotypic variance. Our results support these predictions.
Table 1.
Directional (linear) and nonlinear (quadratic) selection gradients acting on Windermere pike body size from age 1 to age 6 years. (Positive directional gradients indicate that large fish were favoured, while negative directional gradients indicate that small fish were favoured. Positive nonlinear gradients indicate disruptive selection (i.e. that intermediate-sized fish had the lowest survival probability), while negative nonlinear gradients indicate stabilizing selection (i.e. that intermediate-sized fish had the highest survival probability).)
| fishery selection |
natural selection |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| phenotypic trait | directional (linear) gradients |
nonlinear gradients |
n | directional (linear) gradients |
nonlinear gradients |
n | ||||||||
| coefficient | s.e. | p-value | coefficienta | s.e.a | p-value | coefficient | s.e. | p-value | coefficienta | s.e.a | p-value | |||
| length at age 1 | 0.47 | 0.15 | 0.0019 | −0.22 | 0.30 | 0.4597 | 443 | |||||||
| length at age 2 | −1.27 | 0.17 | <0.0001 | −0.12 | 0.25 | 0.6183 | 13 942 | 0.72 | 0.04 | <0.0001 | −0.06 | 0.06 | 0.3514 | 4331 |
| length at age 3 | −1.75 | 0.04 | <0.0001 | 1.33 | 0.10 | <0.0001 | 13 898 | 0.68 | 0.03 | <0.0001 | −0.40 | 0.06 | <0.0001 | 4870 |
| length at age 4 | −0.77 | 0.02 | <0.0001 | 1.21 | 0.05 | <0.0001 | 11 584 | 0.59 | 0.03 | <0.0001 | −0.41 | 0.05 | <0.0001 | 4497 |
| length at age 5 | −0.20 | 0.03 | <0.0001 | 0.76 | 0.05 | <0.0001 | 6316 | 0.45 | 0.04 | <0.0001 | −0.38 | 0.05 | <0.0001 | 3830 |
| length at age 6 | 0.03 | 0.04 | 0.3979 | 0.42 | 0.07 | <0.0001 | 2822 | 0.29 | 0.04 | <0.0001 | −0.32 | 0.05 | <0.0001 | 3155 |
aCalculated by doubling regression coefficients (Stinchcombe et al. 2008).
Figure 1.
Context for selection in Windermere pike (E. lucius) and associated temporal changes in pike phenotypic variance. (a) Nonlinear relationship between pike body length and an individual's survival probability through (i) natural selection and (ii) fishery selection, separated into three time periods covering the entire time series. Note that natural selection tended to be increasingly stabilizing over time while fishery selection tended to be less disruptive. (b) Nonlinear relationship between large pike (55 cm body length and longer) somatic growth rate (cm yr−1) and an individual's survival probability through fishery selection with 95 per cent confidence intervals. (c) Observed time series of natural log-transformed variance for (i) lifetime somatic growth rate (von Bertalanffy asymptotic length) and (ii) length at age 3 years (solid line) and age 4 years (thin dashed line) (both in cm2, y-axis on a log scale). Bold dashed line is a smoother of the effect of year class. (d) Unbiased change in phenotypic variance with 95 per cent confidence intervals for pike lifetime somatic growth rate (von Bertalanffy asymptotic length in cm2, data pooled by groups of 5 year classes, y-axis on a log scale).
2. Material and methods
(a). Sampling site and data collection
Pike in Windermere have been sampled each year since 1944 as part of a long-term scientific monitoring programme (Le Cren 2001; Winfield et al. 2008). A spring (March–April) component of this sampling was designed to capture a large size range of pike using shore seines, perch traps and 38 and 64 mm gillnets (Kipling & Le Cren 1984). Captured pike were all measured for total body length (to the nearest cm), tagged and released. Resulting catch–mark–recapture (CMR) data have been extensively described in two recent papers (Haugen et al. 2006, 2007). As part of the scientific programme, pike were also sampled in winter (October–February) in a gillnet fishery (64 mm mesh size) which targeted pike longer than 54 cm (Frost & Kipling 1967). All pike captured in the winter fishery were killed, sexed, measured for total body length (to the nearest centimetre) and the opercular bones were removed for age and length backcalculation following a validated method (Frost & Kipling 1959). Bone density differs between summer and winter, producing narrow bands (‘checks’) that are deposited on the opercular bones during the slow winter growth period. These checks then serve as an annual mark and allow the ageing of individual fish (Frost & Kipling 1959). An individual's length is backcalculated at each age using a relationship between the radius of the opercular bone at each check and body length (Frost & Kipling 1959). Windermere surface water temperatures (in °C) were recorded on a near daily basis and were averaged for each year. Finally, the abundance of pike and perch (Perca fluvialitis, the main food for pike) have been estimated annually for the 1944–1995 period, separately for each basin as well as separately for young (age = 2 years) and old individuals (age > 2 years) (des Clers et al. 1994).
(b). The form and strength of selection
We estimated the form of selection from survival instead of total fitness (survival*reproductive success) because (i) no data exist for pike reproductive success, (ii) an approximation of reproductive success by fecundity can be made only for female pike because no data exist for fecundity in male pike and (iii) female survival is by far more critical to population growth than female fecundity in Windermere pike (Edeline et al. submitted). We quantified the age-specific strength and form of natural and fishery-induced selection acting on Windermere pike body size (table 1, figure 1a,b) using previously described procedures based on logistic regressions (Carlson et al. 2007). In the CMR data, an individual pike tagged in spring of year t was considered to have survived through the summer of year t (survival = 1) if recaptured at any point in time after the summer of year t. This assumption has been validated (Carlson et al. 2007). By contrast, a fish that was never recaptured after the summer of year t was attributed a survival of 0 for this summer. In the fishery data, fish were given a survival of 0 for age and size at capture and a survival of 1 for previous backcalculated ages and sizes (Sinclair et al. 2002). In table 1, estimates of directional selection gradients acting on body length were obtained using logistic regressions of survival on standardized body length (zero mean and s.d. of unity), and quadratic selection gradients were obtained using logistic regressions of survival on standardized body length plus its squared term (Janzen & Stern 1998). We estimated age-specific selection gradients in table 1 by breaking both datasets into age classes. In the CMR dataset, because only recaptured fish were aged, we produced age classes based on the minimum and maximum body lengths of each age class in the fishery data. For instance, backcalculated length at age 1 ranged from 14 to 38 cm, and all fish of length at capture ranging from 14 to 38 cm were attributed age 1 in the CMR data. In figure 1a,b, we visualized the form of the pike adaptive landscape using natural cubic splines with 9 knots in logistic generalized additive models (mgcv library of the R software; Wood 2006; Carlson et al. 2007; R Development Core Team 2008), i.e. there was no a priori assumption about the form of selection. Note that reduced catchability of larger fish in 64 mm gillnets might have influenced our estimation of the shape of natural mortality in figure 1a. Finally, to model survival as a function of somatic growth in figure 1b, survival at age t was considered relative to length increase between age t − 1 and age t, and only fish of length at-age t − 1 >54 cm were included in the analysis.
(c). Maximum-likelihood estimates of unbiased means and variances
Observed somatic growth distributions in each year class might be distorted pictures of distributions at birth owing to natural selection prior to the catch and owing to the selectivity of the catch itself. We modelled such possible sampling biases using selection functions from figure 1a as filters to backward estimate unbiased somatic growth rate distributions from sampled distributions. Individual pike lifetime somatic growth rate gi was equated to von Bertalanffy's asymptotic length (Edeline et al. 2007), which was computed using a nonlinear restricted maximum-likelihood (REML) mixed-effects model with a random individual effect in the nlme library of the R software (Pinheiro & Bates 2000; R Development Core Team 2008). Briefly, Windermere pike growth is best described by the three-parameter von Bertalanffy growth curve (VBGC) (Ratkowsky 1990)
| 2.1 |
where lgit is the length at age t of fish i of asymptotic length gi and −2.93 and 0.698 are constants estimated by nonlinear least squares fitting of the VBGC on the whole population. To compute gi, equation (2.1) was incorporated into an REML linear mixed effects model in which gi was associated to a random individual effect bi so that gi = β0 + bi, where β0 was model intercept (i.e. population's g). The survival probability of fish i at age t through natural selection (probability s(lgit)) and through fishery selection (probability f(lgit)) is known from survival functions (figure 1a). To be caught at age ci, a given fish i has to survive selection (probability product s(lgit)f(lgit)) for ci − 1 years, and then to survive natural selection 1 more year before being caught (with probability s(lgit) × (1 - f(lgit))). The likelihood of the particular life history of fish i can thus be written as
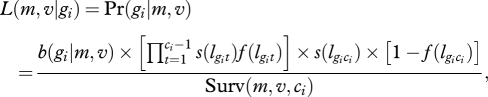 |
2.2 |
where m and v are the estimated mean and variance of the normally distributed asymptotic lengths in the population before selection and b(gi|m, v) is the probability for an individual of asymptotic length gi to be born in a population of mean m and variance v for g. Surv (m, v, ci) is the expected survival rate until age of capture ci in the whole population such that
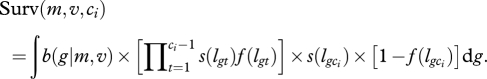 |
2.3 |
The full model, including all N sampled fish, allows the mean and the variance of the population to be independently estimated for each year class. There are thus two vectors of parameters to estimate: M = (m1944, m1945, … , m1993) and V = (v1944, v1941, … , v1993). The form and strength of natural selection and fishery selection were allowed to vary according to time (three periods from 1944 to 1958, from 1959 to 1969 and from 1970 to 1993; see figure 1a). The general likelihood function is
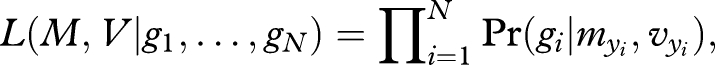 |
2.4 |
where yi is the year class of fish i. Maximum-likelihood (ML) estimates of reconstructed means and variances, as well as their standard errors, were obtained using the software AD Model Builder (Otto Research Ltd, http://admb-project.org/). Reconstructed means were not directly relevant to the present study and are thus provided as electronic supplementary material (figure S1). We validated the method using the simulated data (see below). The simulations stressed that bias removal necessitates large sample sizes (at least a couple of hundred individuals). For this reason, the analysis of real data has been performed by pooling the measures in 5 year bins (figure 1d and electronic supplementary material, figure S1).
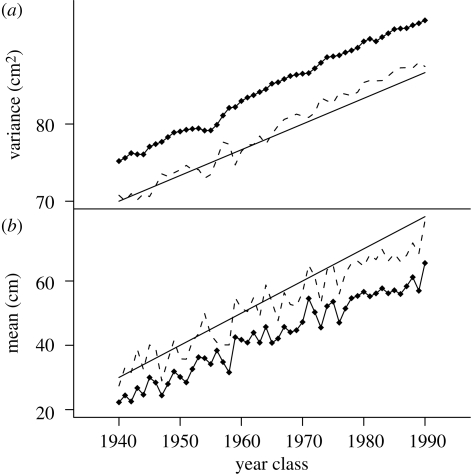
The model has been checked using the simulated data. Simulations were performed with the R software. Fifty cohorts of N = 100 000 individuals have been simulated during the 1940–1990 period. The growth rate of each individual was sampled from a normal distribution of given mean and variance. To make sure that the simulations explored the whole range of realistic means and variances, a trend has been simulated (‘theoretical’ lines in figure 2). Each individual of the population then went through a series of survival events; the probability to survive natural selection and fishery depended on the length of the fish (calculated from its age and its asymptotic length gi) and the selection strengths at that time (figure 1a). If the fish was caught (failure to survive the fishery event), it was added to the simulated dataset (‘uncorrected’ lines in figure 2). Overall, owing to strong juvenile selection, between 500 and 1000 fishes were ‘caught’ every year, which is the same order of magnitude as the real dataset. The simulated dataset was analysed with the same model as the real data to produce ‘unbiased’ lines in figure 2. Proximity between ‘theoretical’ and ‘unbiased’ lines in figure 2 indicates that our model successfully reconstructed somatic growth rate distributions at birth from sampled distributions.
Figure 2.
Model validation for ML estimation of unbiased (a) phenotypic variances and (b) means in Windermere pike. Solid line, theoretical; dashed line, unbiased estimates; line with filled diamonds, uncorrected data.
(d). Trends in residual phenotypic variance
We modelled changes in residual variance for pike lifetime somatic growth rate and length at age while statistically accounting for (i) the plastic effects of temperature and food availability on pike growth (Kipling 1983; Winfield et al. 2008), (ii) changes in average trait values owing to directional selection (Edeline et al. 2007) and (iii) temporal trends in residual variance owing to nonlinear (i.e. disruptive or stabilizing) selection. We used linear REML mixed-effects models in the nlme library of the R software, which provides built-in functions to explicitly model variance structure of within-group residuals using covariates (Pinheiro & Bates 2000). Model details are provided in table 2. To estimate changes in variance for pike lifetime somatic growth rate, we used gi as the response in a REML linear mixed-effects model
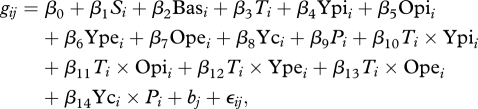 |
2.5 |
where βs are model coefficients, gij is somatic growth rate of individual i in group j and fixed-effects covariates are as follows: S, sex; Bas, basin; T, temperature; Ypi, density of young pike (age = 2 years); Opi, density of old pike (age > 2 years); Ype, density of young perch; Ope, density of old perch; Yc, year class (i.e. cohort); P, period factor (P1: Period 1 of dominating fishery selection from 1944 to 1963, P2: Period 2 of dominating natural selection from 1964 to 1993). Finally, bj is a normally distributed random Yc effect, nested into S, nested into Bas (table 2) and ϵij represents normally distributed within-group residuals. Environmental covariates (T, Ypi, Opi, Ype, Ope) were averaged for each individual i from birth to capture. Interaction between temperature and each biological covariate accounted for the thermal dependence of food conversion efficiency and predator–prey overlap and generated a drop of model Akaike's Information Criterion (AIC) compared with a simple additive formulation (from 80 054 to 79 820, p < 0.0001, comparison based on ML parameter estimation). The Yc × P interaction accounted for the effects of directional selection on mean growth rate (Edeline et al. 2007). We modelled temporal changes in the variance of ϵij with two different variance functions. First, we modelled the residual variance ratio between P1 and P2
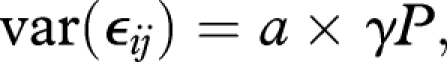 |
2.6 |
where a is within Yc, within S, within Bas residual variance during P1, and γ is the estimated ratio parameter. Second, we modelled changes in the variance of ϵij as a power function of Yc inside each P, while accounting for the variance ratio between P1 and P2
| 2.7 |
where b is within Yc, within S, within Bas residual variance in year class 1944 (i.e. for the first Yc of P1), γ′ is the estimated variance ratio between the first Yc of P1 and the first Yc of P2, and δ is the estimated within-P power parameter. Equation (2.7) yielded a significantly better fit of ϵij in equation (2.5) than equation (2.6) (p < 0.05). We modelled temporal changes in variance for natural log-transformed length at age 1 to age 6 using six age-specific linear REML mixed-effects models of the form
| 2.8 |
where ln(Lik) is natural log-transformed body length at age x of individual i in group k, βs are model coefficients, and ϵij represents normally distributed within-group residuals. Environmental growth conditions (T, Ypi, Opi, Ype, Ope) were averaged from birth to age x. This simpler formulation of fixed effects (compared with equation (2.5)) yielded a lower AIC for the majority of ages and was thus retained for all ages for parsimony and consistency. Each model had a different structure of normally distributed random effect bk, which was selected among a set of candidate structures based on model AIC (details provided in table 2). We modelled changes in the variance of ϵik in equation (2.8) with equations (2.6) and (2.7), which yielded a significantly better model fit than equation (2.6) (p < 0.05) for all lengths at age except for length at age 2 years.
Table 2.
Details of the random effects structure for each linear REML mixed-effects model used to estimate changes in Windermere pike somatic growth and length at age.
| response | random effects, model parameters allowed to vary randomly | number of groups N, number of observations n | variance–covariance matrix for the random effects |
|---|---|---|---|
| lifetime somatic growth rate | year class nested into sex, nested into basin, random intercept | N = 197, n = 13 942 | identity |
| Ln (body length) | |||
| age 1 year | year class nested into basin, random intercept | N = 99, n = 13 941 | identity |
| age 2 years | year class nested into basin, random intercept | N = 99, n = 13 941 | identity |
| age 3 years | year class, random intercept and random sex*basin effect | N = 50, n = 13 898 | symmetric |
| age 4 years | year class nested into sex nested into basin, random intercept | N = 196, n = 11 584 | identity |
| age 5 years | year class, random intercept and random sex*basin effect | N = 48, n = 6316 | symmetric |
| age 6 years | year class, random intercept and random basin effect | N = 47, n = 2822 | symmetric |
Finally, estimated parameters γ, γ′ and δ from equations (2.6) and (2.7) inserted in the above-listed models allowed us to quantify per cent changes in residuals phenotypic variance for each trait (lifetime somatic growth and six lengths at age; table 3). For instance, for gi, γ = 0.96 in equation (2.6) indicating that residual variance for gi overall decreased by 4 per cent between P1 and P2. In equation (2.7), γ′ = 1.41, δ = 0.01 during P1 (19 year classes) and δ = −0.1 during P2 (30 year classes), indicating that residual variance in gi increased by 3.8 per cent during P1 and decreased by 29.6 per cent during P2 (see table 3 for full results).
Table 3.
Estimated per cent changes in Windermere pike residual phenotypic variance with respect to periods of dominating fishery selection (P1) and dominating natural selection (P2).
| phenotypic trait | change in variance from P1 to P2 (in % with 95% CI) | change in variance within P1 (in % with 95% CI) | change in variance within P2 (in % with 95% CI) |
|---|---|---|---|
| lifetime somatic growth rate | −4 (−6.5, −1.5) | +3.8 (−3.4, +11.5) | −29.6 (−44, −11.5) |
| length at age 1 year | −9.5 (−11.7, −7.4) | +3.2 (−3.9, +10.8) | −28.1 (−44.2, −7.4) |
| length at age 2 years | −11.5 (−13.5, −9.3) | +21.2 (+11.2, +32) | +2.7 (−3.9, +9.8) |
| length at age 3 years | −7 (−9.2, −4.8) | +53.6 (+40, +68.4) | −33.9 (−47.4, −17) |
| length at age 4 years | −5.8 (−8.2, −3.3) | +48.2 (+33, +65.2) | −45.5 (−58.2, −29) |
| length at age 5 years | −6.4 (−9.7, .3) | +21.2 (+3.6, +41.7) | −48.1 (−64.1, −24.9) |
| length at age 6 years | −8 (−12.8, −2.9) | +0.59 (−25.1, +35) | −52.6 (−74.8, −10.5) |
3. Results
(a). Raw trends in pike phenotypic variance
Raw variance in individual pike somatic growth rate among sampled fish increased up to the early 1960s (figure 1c(i)), in accordance with the prediction that disruptive fishery selection on body size simultaneously favoured both slow and fast growers. Directional fishery selection for slow growth and delayed entry to the fishery is self-evident and has already been demonstrated (Carlson et al. 2007; Edeline et al. 2007). However, concurrent selection for fast growth is less intuitive. Therefore, we inspected fishery survival probability as a function of somatic growth rate in pike larger than 54 cm body length, i.e. pike already recruited into the fishery (see §2). Once recruited into the fishery, faster growers had increased survival probability (figure 1b; n = 15 972; p < 0.001), confirming that the fishery was disruptive not only on pike body size, but also on pike somatic growth rate. After the early 1960s, sampled pike had a decreased variance in somatic growth (figure 1c(i)), in accordance with the prediction that stabilizing natural selection on body size selected against both slow and fast growers.
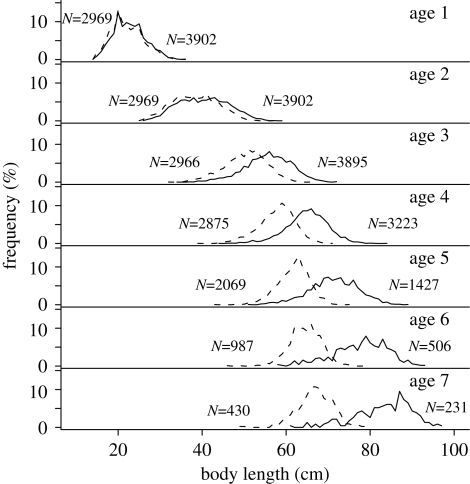
Raw trends in somatic growth rate were transmitted into corresponding trends in length at age. Variance in length at age 3 years and age 4 years (the two age classes most strongly affected by the fishery; table 1) increased at the start of the time series and decreased at its end (figure 1c(ii)). However, the intensity of disruptive fishery selection was not strong enough to generate bimodality in length-at-age distributions (figure 3), probably owing to weak fishing mortality (see §1). Temporal fluctuations in somatic growth rate and length at age 3 years and age 4 years were statistically significant when tested with generalized additive models in which natural log-transformed variance was the response and a smoothed Year class term was the predictor (somatic growth rate: n = 49; p < 0.001; length at age 3 and age 4: n = 98; p < 0.001). Taken together, raw trends are remarkably consistent with the prediction that disruptive fishery selection opposed the effects of stabilizing natural selection in increasing pike phenotypic variance. As a second step of our analysis, we applied analytical methods aiming at testing for a possible sampling bias and at removing environmental noise into raw trends.
Figure 3.
Sex-specific distributions of backcalculated length at age 1 year to age 7 years of Windermere pike born between 1944 and 1963 (period of dominating fishery selection) showing the absence of biomodality. Numbers on the left of the curves indicate male sample size and numbers on the right of the curves indicate female sample size. Dashed line, males; solid line, females.
(b). Modelled trends in pike phenotypic variance
The reconstructed unbiased trend in somatic growth variance (figure 1d) is in close agreement with the trend observed in raw data (i.e. variance increased up to the early 1960s and decreased afterwards), indicating that patterns observed in the raw data reflect true patterns that occurred in the population. After having validated that our data were not biased, we accounted for environmental noise into pike phenotypic variance using statistical models in which we explicitly modelled temporal changes in residual variance (see §2). Results are shown in table 3 (statistical significance is considered when both confidence limits have the same sign). Residual variance significantly decreased for all analysed traits from Period 1 of dominating fishery selection (year classes from 1944 to 1963) to Period 2 of dominating natural selection (year classes from 1964 to 1993). Moreover, across Period 1, residual variance increased in all traits, although statistical significance was not reached for somatic growth rate, length at age 1 year and length at age 6 years (table 3). Residual variance in length at age 1 year and length at age 2 years increased, despite the fact that age 1 year pike were not caught by the fishery and that nonlinear fishery selection was not statistically significant on age 2 pike (table 1), a result reflecting disruptive fishery selection on lifetime somatic growth rate. Interestingly, residual variance in length at age 3 to age 6 pike increased in parallel with the amplitude of nonlinear fishery selection gradients acting on these age classes (table 1), further supporting the view that the fishery was the primary driver of increased pike phenotypic variance across Period 1. Across Period 2 of dominating natural selection, natural selection became increasingly stabilizing and fishery selection tended to be less disruptive (figure 1a). In parallel, variance in body length decreased significantly in all analysed traits except length at age 2 years (table 3). Lack of change in variance for length at age 2 years might be linked to exceptionally weak stabilizing natural selection combined with relaxation of strongly directional fishery selection on this age class (table 1). To sum up, our results support the view that pike phenotypic variance tracked the curvature of the adaptive landscape, which was imposed by the dominating selective force.
4. Discussion
The negative effects of harvesting on population persistence are often ascribed to harvest-induced size truncation and resultant decrease in the variance of fitness related traits. However, our results empirically demonstrate that harvesting does not necessarily cause size truncation and decreased trait variability. In Windermere, gillnet fishing instead caused disruptive selection and increased trait variability. Increased trait variability arises not only from disruptive selection (when fitness minimum occurs between phenotypic extremes), but also from concave selection (when fitness minimum occurs at one of the phenotypic extremes). Therefore, careful consideration of the exact form of selection should systematically precede inference about the mechanisms through which populations are negatively affected by harvesting. In our study, we might have overestimated natural mortality of large fish owing to a reduced catchability at a large size. However, this does not influence the main conclusion of our paper that disruptive fishing increases variance in somatic growth and size at age, and our field-based findings are consistent with laboratory-based experiments in fruitflies Drosophila melanogaster and mice Mus musculus, demonstrating that disruptive selection increases genetic variability and capacity to respond to selection (Roff 1997). Accordingly, in Windermere pike, a fishery-driven increase in capacity to respond to selection might have favoured the observed rapid evolutionary rebound after relaxation of the fishing pressure (Conover 2007; Edeline et al. 2007; Coltman 2008). Interestingly, stabilizing natural selection decreased Windermere pike trait variance as observed in other systems (Roff 1997; Haugen et al. 2008), and disruptive fishery selection might thus have paradoxically increased pike capacity to respond to selection compared with a pristine pike population.
In contrast to Windermere pike, several fish stocks have been found to be unable to rebound after relaxation of fishing (Hutchings 2000; Sinclair et al. 2002; Berkeley et al. 2004; Hutchings & Fraser 2008). For instance, cod in the southern Gulf of St Lawrence collapsed in the early 1990s and has still failed to recover since the fishery was closed in 1993 (Sinclair et al. 2002). To our knowledge, the southern Gulf of St Lawrence cod is the only population beside Windermere pike for which the exact form of fishery selection has been investigated. Fishery selection acting on cod body size changed from disruptive in the 1970s, indicating that disruptive fishing can also occur at commercial exploitation rates, to truncation selection in the 1980s and early 1990s (i.e. before and during stock collapse). The change in the form of fishery selection was owing to a parallel increase in both the fishing effort (that reduced survival in medium-sized cod) and the minimum mesh size of fishing gears (that reduced survival in large cod) (Sinclair et al. 2002). Increasing minimum size limits is generally intended as a conservative measure allowing intensification of exploitation rates, but it might instead favour erosion of genetic variance for somatic growth and correlated traits, promote population collapse and impair recovery. By contrast, maintaining disruptive harvesting necessitates combining slot size regulations (to preserve large individuals) with moderate exploitation rates (so that enough medium-sized individuals survive to a large size). Therefore, managed disruptive harvesting might represent a comprehensive and efficient tool for sustainable exploitation of living resources.
Acknowledgments
All fish sampling was carried out under appropriate UK licensing from the Environment Agency or its predecessors.
We are grateful to the many individuals who have participated in the Windermere data collection over the years. We also thank the Freshwater Biological Association for their joint stewardship of these invaluable data. We thank Leif Chr. Stige (CEES) for invaluable advice concerning variance functions in mixed-effects models. Thomas F. Hansen (CEES) provided helpful suggestions at an early stage of the study. We thank Stephanie M. Carlson (University of California, Berkeley) for commenting on our manuscript. E.E. received support from the Research Council of Norway and A.L.R. received support from a Marie Curie Post Doctoral fellowship. Support from the Natural Environment Research Council to the Centre for Ecology and Hydrology is also acknowledged.
References
- Berkeley S. A., Hixon M. A., Larson R. J., Love M. S.2004Sustainability via protection of age structure and spatial distribution of fish populations. Fisheries 29, 23–32 (doi:10.1577/1548-8446(2004)29[23:FSVPOA]2.0.CO;2) [Google Scholar]
- Carlson S. M., Edeline E., Vøllestad L. A., Haugen T. O., Winfield I. J., Fletcher J. M., James J. B., Stenseth N. C.2007Four decades of opposing natural and human-induced artificial selection acting on Windermere pike (Esox lucius). Ecol. Lett. 10, 512–521 (doi:10.1111/j.1461-0248.2007.01046.x) [DOI] [PubMed] [Google Scholar]
- Coltman D. W.2008Evolutionary rebound from selective harvesting. Trends Ecol. Evol. (Amst.) 23, 117–118 (doi:10.1016/j.tree.2007.12.002) [DOI] [PubMed] [Google Scholar]
- Coltman D. W., O'Donoghue P., Jorgenson J. T., Hogg J. T., Strobeck C., Festa-Bianchet M.2003Undesirable evolutionary consequences of trophy hunting. Nature 426, 655–658 (doi:10.1038/nature02177) [DOI] [PubMed] [Google Scholar]
- Conover D. O.2007Fisheries: nets versus nature. Nature 450, 179–180 (doi:10.1038/450179a) [DOI] [PubMed] [Google Scholar]
- Conover D. O., Munch S. B.2002Sustaining fisheries yields over evolutionary time scales. Science 297, 94–96 (doi:10.1098/rspb.2009.0003) [DOI] [PubMed] [Google Scholar]
- Conover D. O., Munch S. B., Arnott S. A.2009Reversal of evolutionary downsizing caused by selective harvest of large individuals. Proc. R. Soc. B 276, 2015. –2020. (doi:10.1098/rspb.2009.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Clers S., Fletcher J. M., Winfield I. J., Kirkwood G. P., Cubby P. R., Beddington J. R.1994Long-term changes in Windermere perch and pike populations at the basin level. WI/T11050d5/4, Ministry of Agriculture, Fisheries and Food. [Google Scholar]
- Edeline E., Carlson S. M., Stige L. C., Winfield I. J., Fletcher J. M., James J. B., Haugen T. O., Vøllestad L. A., Stenseth N. C.2007Trait changes in a harvested population are driven by a dynamic tug-of-war between natural and harvest selection. Proc. Natl Acad. Sci. USA 104, 15 799–15 804 (doi:10.1073/pnas.0705908104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline E., Haugen T. O., Weltzien F. A., Claessen D., Winfield I. J., Stenseth N. C., Vøllestad L. A.Submitted The demographic cost of social stress in a top predator. Proc. R. Soc. B. [Google Scholar]
- Frost W. E., Kipling C.1959The determination of the age and growth of pike (Esox lucius) from scales and opercular bones. J. Cons. Int. Explor. Mer. 24, 314–341 [Google Scholar]
- Frost W. E., Kipling C.1967A study of reproduction, early life weight–length relationship and growth of pike Esox lucius in Windermere. J. Anim. Ecol. 36, 651–693 (doi:10.2307/2820) [Google Scholar]
- Haugen T. O., Vøllestad L. A.2001A century of life history evolution in grayling. Genetica 112–113, 475–491 (doi:10.1023/A:1013315116795) [PubMed] [Google Scholar]
- Haugen T. O., Winfield I. J., Vøllestad L. A., Fletcher J. M., James J. B., Stenseth N. C.2006The ideal free pike: 50 years of fitness-maximizing dispersal in Windermere. Proc. R. Soc. B 273, 2917–2924 (doi:10.1098/rspb.2006.3659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen T. O., Winfield I. J., Vøllestad L. A., Fletcher J. M., James J. B., Stenseth N. C.2007Density dependence and density independence in the demography and dispersal of pike over four decades. Ecol. Monogr. 77, 483–502 (doi:10.1890/06-0163.1) [Google Scholar]
- Haugen T. O., Aass P., Stenseth N. C., Vøllestad L. A.2008Changes in selection and evolutionary responses in migratory brown trout following the construction of a fish ladder. Evol. Appl. 1, 319–335 (doi:10.1111/j.1752-4571.2008.00031.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C., Reiss C. S., Hunter J. R., Beddington J. R., May R. M., Sugihara G.2006Fishing elevates variability in the abundance of exploited species. Nature 443, 859–862 (doi:10.1038/nature05232) [DOI] [PubMed] [Google Scholar]
- Hutchings J. A.2000Collapse and recovery of marine fishes. Nature 406, 882–885 (doi:10.1038/35022565) [DOI] [PubMed] [Google Scholar]
- Hutchings J. A.2005Life history consequences of overexploitation to population recovery in Northwest Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 62, 824–832 (doi:10.1139/f05-081) [Google Scholar]
- Hutchings J. A., Fraser D. J.2008The nature of fisheries- and farming-induced evolution. Mol. Ecol. 17, 294–313 (doi:10.1111/j.1365-294X.2007.03485.x) [DOI] [PubMed] [Google Scholar]
- Janzen F. J., Stern H. S.1998Logistic regression for empirical studies of multivariate selection. Evolution 52, 1564–1571 (doi:10.2307/2411330) [DOI] [PubMed] [Google Scholar]
- Kipling C.1983Changes in the growth of pike (Esox lucius) in Windermere. J. Anim. Ecol. 52, 647–657 (doi:10.2307/4578) [Google Scholar]
- Kipling C., Le Cren E. D.1984Mark–recapture experiments on fish in Windermere, 1943–1982. J. Fish Biol. 24, 395–414 (doi:10.1111/j.1095-8649.1984.tb04811.x) [Google Scholar]
- Law W., Salick J.2005Human-induced dwarfing of Himalayan snow lotus, Saussurea laniceps (Asteraceae) Proc. Natl Acad. Sci. USA 102, 10 218–10 220 (doi:10.1073/pnas.0502931102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cren E. D.1987Perch (Perca fluviatilis) and pike (Esox lucius) in Windermere from 1945 to 1985: studies in population-dynamics. Can. J. Fish. Aquat. Sci. 44Suppl 2), 216–228 (doi:10.1139/f87-324) [Google Scholar]
- Le Cren E. D.2001The Windermere perch and pike project: an historical review. Freshw. Forum 15, 3–34 [Google Scholar]
- Lewin W. C., Arlinghaus R., Mehner T.2006Documented and potential biological impacts of recreational fishing: insights for management and conservation. Rev. Fish. Sci. 14, 305–367 (doi:10.1080/10641260600886455) [Google Scholar]
- Pinheiro J. C., Bates D. M.2000Mixed-effects models in S and S-PLUS, 1st edn. New York, NY: Springer [Google Scholar]
- R Development Core Team. 2008R: a language environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- Ratkowsky D. A.1990Handbook of nonlinear regression models, 1st edn. New York, NY: Marcel Dekker [Google Scholar]
- Roff D. A.1997Evolutionary quantitative genetics, 1st edn. New York, NY: Chapman & Hall [Google Scholar]
- Sinclair A. F., Swain D. P., Hanson J. M.2002Measuring changes in the direction and magnitude of size-selective mortality in a commercial fish population. Can. J. Fish. Aquat. Sci. 59, 361–371 (doi:10.1139/f02-015) [Google Scholar]
- Stinchcombe J. R., Agrawal A. F., Hohenlohe P. A., Arnold S. J., Blows M. W.2008Estimating nonlinear selection gradients using quadratic regression coefficients: double or nothing? Evolution 62, 2435–2440 (doi:10.1111/j.1558-5646.2008.00449.x) [DOI] [PubMed] [Google Scholar]
- Winfield I. J., James J. B., Fletcher J. M.2008Northern pike (Esox lucius) in a warming lake: changes in population size and individual condition in relation to prey abundance. Hydrobiologia 601, 29–40 (doi:10.1007/s10750-007-9264-1) [Google Scholar]
- Wood S. N.2006Generalized additive models, 1st edn. Boca Raton, FL: Chapman & Hall [Google Scholar]