Abstract
Animals often form groups to increase collective vigilance and allow early detection of predators, but this benefit of sociality relies on rapid transfer of information. Among birds, alarm calls are not present in all species, while other proposed mechanisms of information transfer are inefficient. We tested whether wing sounds can encode reliable information on danger. Individuals taking off in alarm fly more quickly or ascend more steeply, so may produce different sounds in alarmed than in routine flight, which then act as reliable cues of alarm, or honest ‘index’ signals in which a signal's meaning is associated with its method of production. We show that crested pigeons, Ocyphaps lophotes, which have modified flight feathers, produce distinct wing ‘whistles’ in alarmed flight, and that individuals take off in alarm only after playback of alarmed whistles. Furthermore, amplitude-manipulated playbacks showed that response depends on whistle structure, such as tempo, not simply amplitude. We believe this is the first demonstration that flight noise can send information about alarm, and suggest that take-off noise could provide a cue of alarm in many flocking species, with feather modification evolving specifically to signal alarm in some. Similar reliable cues or index signals could occur in other animals.
Keywords: alarm call, alarm signal, index signal, signal reliability, grouping, vigilance
1. Introduction
A major benefit of animal grouping in general and bird flocking in particular is that it can increase collective vigilance, and so allow early detection of attacking predators, but this benefit of sociality relies on rapid transfer of information about danger from the individual first detecting the predator (Elgar 1989; Krause & Ruxton 2002). It is often unclear how information is passed to others or whether it is reliable (Lima 1994). One possibility is that individuals flee if they see another flying from a flock without first giving ‘intention movements’ of intended departure (Davis 1975). In other cases, individuals can take a cue from simultaneous flight of multiple individuals, but such a rule of thumb provides only probabilistic information and is vulnerable to both ‘false alarms’ and missed detections (Lima 1995; Cresswell et al. 2000). Acoustic signals or cues of danger are likely to be particularly effective, as information is passed rapidly to all nearby members of a group, and can be detected even if a bird is not currently vigilant or is out of sight. Consistent with this advantage of sound, many species of birds give alarm calls to warn others of danger (Searcy & Nowicki 2005). However, alarm calls are not present in all species, and it is possible that non-vocal flight sounds could provide acoustic signals or cues of danger to other members of a flock.
Conspicuous wing flight sounds are most commonly associated with courtship (Bostwick & Prum 2003), but they might also function as alarm signals, because the sound of taking-off in alarm could warn of immediate danger and incite others to flee (Johnston 1960; Coleman 2008). All birds produce sound during flight, and some species produce sounds that can be louder than their vocalizations, and distinct from the atonal ‘whooshing’ sounds that are an inevitable consequence of flapping, which suggests that they have evolved as acoustic signals through structural modification of flight feathers (Bostwick & Prum 2003; Clark & Feo 2008; Hunter 2008). A problem with their use as alarm signals or cues is that wing flight noises may be produced whenever air is forced over feathers during flapping flight, so it is unclear how they could be modulated to encode alarm. However, alarmed birds fly faster or take off at a steeper angle (Kullberg et al. 1998; Veasey et al. 1998; Van der Veen & Lindström 2000), which affects wingbeat kinematics and therefore acoustic amplitude, sound tempo and potentially acoustic frequency (Tobalske et al. 2004; Clark 2008; Clark & Feo 2008). Therefore, wing sounds might provide a reliable cue of alarmed take-off or even be an index signal, in which the signal's meaning is closely related to its production and therefore costly to fake (Maynard Smith & Harper 2003). If a wing sound does encode accurate information on alarm, it would provide a novel and efficient solution to the problem of how members of a flock benefit from the vigilance of others in the absence of alarm calls (Lima 1994, 1995; Cresswell et al. 2000). We are aware of only one study on the possible alarm function of a non-vocal sound in birds, which considered the wing ‘whistle’ of mourning doves, Zenaida macroura (Coleman 2008). Results were consistent with an alarm function but the small sample of wing whistles did not allow acoustic analyses or appropriately replicated playback experiments (Coleman 2008).
We tested whether wing whistles produced by the crested pigeon, Ocyphaps lophotes, encode acoustic information on alarm and whether others use this information. The wing whistle is a conspicuous, loud metallic sound, sufficient to identify the species in flight and leading to the common name ‘whistle-winged pigeon’. However, it has not been described acoustically and there has been no study of its function. The sound occurs only in flapping flight, not during glides, and is probably produced at least partly by the pigeon's strongly attenuated eighth primary feather (Higgins & Davies 1996, figure 1 inset), a feature associated with conspicuous wing whistles in other species (Bostwick & Zyskowski 2001; Snow 2004). We quantified primary width, compared the acoustic features of whistles produced in alarmed and non-alarmed take-offs, carried out a playback experiment to test whether these alarmed whistles are sufficient to incite flight and tested whether listeners use information on danger encoded in amplitude or acoustic structure. Throughout, we use the term ‘wing whistle’ because of its widespread usage, but do not imply a specific sound-production mechanism; such sounds are likely to be caused by feather vibration rather than being true acoustic whistles (Clark & Feo 2008; C. J. Clark 2009, personal communication).
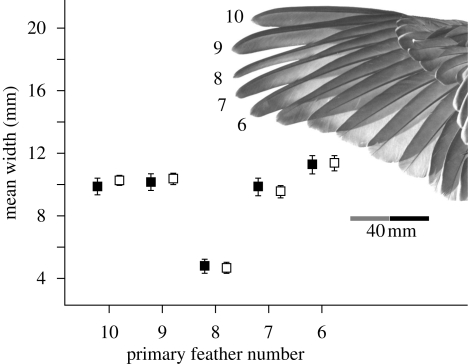
Figure 1.
Primary feather structure in the crested pigeon. Feather width is shown as the mean and 95 per cent confidence interval for 10 birds of each sex, and was measured 2.5 cm from the tip of each feather. The primary number is shown in the inset photograph of a spread wing (adult female from the Australian National Wildlife Collection, number 5653). Black boxes, female; white boxes, male.
2. Material and Methods
(a). Study site, species and feather attenuation
We studied the crested pigeon in Canberra, Australia, where it is an abundant resident in suburban areas with a mix of open areas and trees. It is a medium-sized (150–250 g), granivorous pigeon that breeds in pairs but often forages in small flocks on the ground, and can gather in large flocks at water and food (Frith 1982; Higgins & Davies 1996). When disturbed on the ground, individuals in flocks fly together to cover on elevated vantage points. Crested pigeons are vulnerable to both terrestrial predators and raptors (Marchant & Higgins 1993). The bird produces vocalizations during courtship, and some calls of distress or disturbance at the nest, but is not known to produce an alarm call in a flock (Higgins & Davies 1996). We quantified feather attenuation by measuring the width of the five outer primaries (eighth and adjacent two primaries) of 10 male and 10 female study skins at the Australian National Wildlife Collection, Canberra. Width was measured to the nearest millimetre, 2.5 cm from each feather's tip, avoiding skins where feathers were missing.
(b). Recording and sound analysis
Wing whistles were recorded at 13 feeders baited with wheat seed, and distributed over a distance of 17.4 km, with feeders on average 1.7 km from their nearest neighbour (range 0.2–6.8 km). Birds were not artificially marked, but the distribution of feeders was designed to minimize the risk of re-recording individual birds. Feeders were 30 cm diameter dishes raised 16 cm above the ground, and whistles were recorded using a Sennheiser ME67 directional microphone placed 1 m from the feeder, and connected by a 15 m cable to a Marantz PMD670 digital recorder sampling wave files at 16 bits and 44.1 kHz. Recordings were used for subsequent analysis and playback only if the bird flew from the feeder and away from the microphone.
We recorded 15 alarmed and 15 non-alarmed flight whistles. ‘Alarmed’ flights were prompted with a gliding model accipiter hawk (model description in Magrath et al. 2007), which was thrown from a hide over the feeder when there was a single bird feeding. ‘Non-alarmed’ flights were recorded when a single bird flew unprompted from a flock, which reduced the possibility that a bird may have been startled by an event unseen by the observer. We recorded only one alarm and one non-alarm from a site unless birds had individually distinctive features, in which case a second alarm could be recorded from the same site. Similarly, more than one non-alarmed whistle could be taken from a single site if non-alarmed birds flew in sequence without any birds arriving.
Flight whistles were composed of a sequence of tonal and atonal elements (§3) that rapidly declined in amplitude as the pigeon flew away from the feeder, so we carried out acoustic analysis on the first 0.5 s of each whistle. The short time sample was appropriate for a potential flee signal, to which birds must respond quickly, and ensured a clear recording before the birds flew too far from the microphone. We analysed whistles using Raven Pro 1.3 (Charif et al. 2008) after first filtering out background sound below 300 Hz. For the 0.5 s samples, we measured the (i) number of elements, (ii) mean amplitude (dB sound pressure level (SPL)), (iii) rate of repetition of element types (Hz), which corresponds to the wingbeat rate (§3), and (iv) peak fundamental frequency of the two tonal element types (Hz). In preliminary analyses, we also examined other amplitude and temporal measures of each element, but these measures were intercorrelated and overall measures were better for statistical analyses. Measurements were made on spectrograms with a temporal grid of 2.27 ms with an 82.9 per cent overlap, a frequency grid resolution of 21.5 Hz with a discrete Fourier transform (DFT) size of 2048 samples and a Blackman window function. Amplitude was calibrated against a tone of known amplitude determined from an SPL meter. In addition to acoustic analysis, video collected during the playback experiment (below) was used to compare the rate of repetition of sound elements with the wingbeat rate for 10 non-alarmed flights. Alarmed flights were unsuitable for this analysis because birds took off simultaneously during the playback experiment. Note that all references to frequency, rate, tempo and amplitude refer to the sound produced and not to wingbeats, unless specified otherwise.
(c). Playback experiment
The playback experiment was designed to test whether birds used alarm information encoded in flight whistles, and if so whether amplitude, structural features such as sound tempo, or both provided the information. The experiment was based on a matched design, with 15 groups of birds each exposed to five playback treatments presented in random order: (i) alarmed whistle at original amplitude, (ii) non-alarmed whistle at original amplitude; (iii) alarmed whistle reduced in amplitude to the matching non-alarmed whistle; (iv) non-alarmed whistle increased in amplitude to the matching alarmed whistle; and (v) the bell contact call of the crimson rosella (Platycercus elegans, a harmless parrot) as a control, played at the amplitude of the alarm whistle. Each whistle playback consisted of a single take-off whistle, and each group received unique examples of each whistle type and control. The whistle playbacks at original amplitude tested whether birds used the alarm information in natural whistles. If they did so, birds should flee the alarmed but not the non-alarmed whistle. The whistles at modified amplitude tested whether amplitude or acoustic structure carry information on alarm. If birds fled to both original and reduced-amplitude alarmed whistles, but not to non-alarmed whistles of either amplitude, then structure carries the information. By contrast, if birds fled to both alarmed and non-alarmed whistles at high amplitude but neither at low amplitude, then amplitude carries the information. An intermediate outcome would mean that both structure and amplitude are important.
We broadcast sounds using a broad-frequency dual cone speaker connected by a 15 m cable to an amplifier and a Bose PM-1 CD player. To set the correct amplitude, we put the speaker on top of a feeder with the microphone set at 1 m, the distance of the original recordings, and recorded playbacks using the same recording equipment and settings as the original recordings. We then adjusted playback volume so that the re-recordings had the same amplitude as the original recordings. Our recording and playback protocol meant that the amplitude from the listener's perspective declined in the same way as if they were listening to a bird flying away from the feeder, despite the speaker being stationary. This was because the original recordings were taken from a stationary microphone placed the same distance from a fleeing pigeon as the speaker was from the focal feeding birds.
Playbacks were carried out at 11 of the original feeder sites. The speaker was placed 1 m from the feeder with a directional microphone directly opposite and connected to a Sony Digital video camera that recorded responses of birds. Whistles were never broadcast at their recording site, to avoid any effect of individual recognition. Four sites were assigned two playback sets carried out at least two weeks apart. Each of these sites was visited by a large number of birds, minimizing the risk of repeated playback to the same individuals.
Birds were allowed to feed undisturbed for 15 min after arrival before any playbacks. If there was no flight response to a playback, the next trial was carried out at least 10 min after birds had resumed feeding normally. If birds flew after playback, we left at least 15 min of undisturbed feeding before the next playback. A full playback set at a site usually required visits on more than one day. We used Adobe Soundbooth to measure from video recordings the duration that individual birds held their head upright, and used the mean duration of individual vigilance as the measure of vigilance.
(d). Statistical analyses
We used a repeated-measures general linear model to compare primary widths of male and female pigeons, with sex as a between-subject factor and primary number as a within-subject factor, and t-tests to compare individual feathers. We used independent t-tests to compare individual acoustic measures of non-alarmed and alarmed whistles, and a stepwise discriminant function analysis to assess the overall acoustic difference (Tabachnick & Fidell 2007). The playback experiment was based on a repeated-measures design, so we used Cochran Q tests to examine the change in proportion of flight responses (flee or not) within a group according to playback type, and repeated-measures ANOVA for comparable matched analyses of log-transformed mean vigilance (Sokal & Rohlf 1995). All tests were two-tailed and carried out in SPSS 16.0 for Macintosh (SPSS Inc., Chicago, IL, USA).
3. Results
(a). Morphology of primary feathers
The eighth primary was much narrower than adjacent primary feathers and was equally attenuated in males and females (sex difference: t-test, t18 = 0.40, p = 0.70; figure 1). Considering primary eight and its two adjacent feathers, there was no difference between the sexes in mean width (sex: F1,18 = 0.09, p = 0.76) nor in wing ‘shape’ (sex by primary interaction, F2,36 = 1.44, p = 0.25). Despite the similarities in feather width, males had longer wings than females (mean ± s.d.: 168.0 ± 2.0 versus 158.9 ± 1.1 mm; t18 = 4.0, p = 0.001).
(b). Overall whistle structure
The crested pigeon wing whistle was composed of three element types, two tonal elements and an atonal ‘clap’, which were repeated cyclically in rapid succession as expected from sounds caused by flapping flight (figure 2). Tone 1 had a mean fundamental peak frequency (±s.d.) of 1303 ± 100.5 Hz, while Tone 2 had a higher fundamental peak frequency of 2937 ± 208.9 Hz (paired t-test: t29 = 48.1, p < 0.001; n = 30). The clap element was a short, broad-frequency sound that usually appeared immediately after Tone 1, but was not always present and could also appear after Tone 2. The video sample of 10 different whistles contained the same number of wingbeats as sound element cycles in the video frame as expected from sounds caused by flapping flight (n = 10; number of wingbeats ranged from 2.5 to 7.5 and sound element cycles were defined by the alternation of Tones 1 and 2).
Figure 2.
Wing whistle produced by a crested pigeon during a routine take-off. (a) Spectrogram produced in Raven Pro 1.3 using the settings given in the text for acoustic analysis; (b) waveform, with a linear sound pressure scale. The inset shows one ‘element cycle’ with a single Tone 1 (T1), clap and Tone 2 (T2); claps are most easily visualized as vertical lines in the main spectrogram (a).
(c). Acoustic differences between alarmed and non-alarmed whistles
Alarmed whistles were louder and had a more rapid tempo than non-alarmed whistles (see sound file in the electronic supplementary material). The mean (±s.e.) amplitude of the first 0.5 s was 67.6 ± 0.6 dB in alarmed and 62.2 ± 0.6 dB in non-alarmed whistles (independent t-test: t28 = 6.7, p < 0.001), and the sound element cycle rate, corresponding to the wingbeat rate, was 13.74 ± 0.10 Hz in alarmed and 12.31 ± 0.13 Hz in non-alarmed whistles (t28 = 8.8, p < 0.001). Consistent with a greater tempo, there were more sound elements in alarmed than in non-alarmed whistles (23.0 ± 0.70 versus 19.3 ± 0.64, t28 = 4.0, p < 0.001). In contrast to differences in amplitude and timing, there was no difference in Tone 2 fundamental frequency between alarmed and non-alarmed whistles (2947 ± 56.8 and 2928 ± 52.8 Hz, respectively, t28 = 0.24, p = 0.8), and alarmed whistles had only a slightly higher Tone 1 fundamental frequency than non-alarmed whistles (1341 ± 27.8 versus 1264 ± 20.1 Hz, t28 = 2.3, p = 0.03).
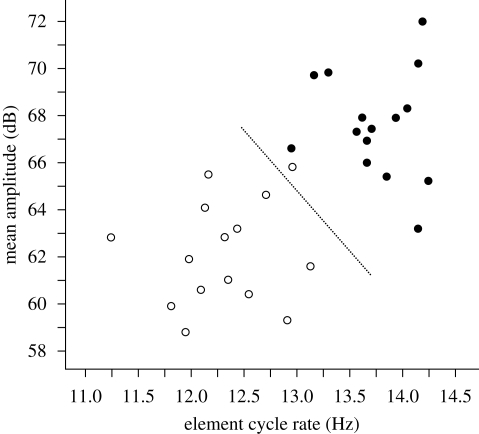
Both sound amplitude and element cycle rate weighted strongly in a discriminant function analysis and together classified 29 out of 30 whistles correctly (figure 3; Wilks' Lambda = 0.191; χ2= 44.7, d.f. = 2, p < 0.001; standardized discriminant function coefficients: amplitude 0.59, cycle rate 0.79). One non-alarmed whistle was classified as an alarmed whistle, and this was also the only one misclassified in a cross-validation analysis. Neither fundamental frequencies nor the total number of elements explained additional variation in the discriminant function.
Figure 3.
Acoustic comparison of wing whistles produced in alarmed and non-alarmed take-offs. Amplitude is the mean over the first 0.5 s; element cycle rate corresponds to the flap rate (see text). A discriminant function analysis classified all but one non-alarmed whistle correctly using these two measures; dashed line shows classification threshold. Filled circles, alarmed; open circles, non-alarmed.
(d). Playback experiment
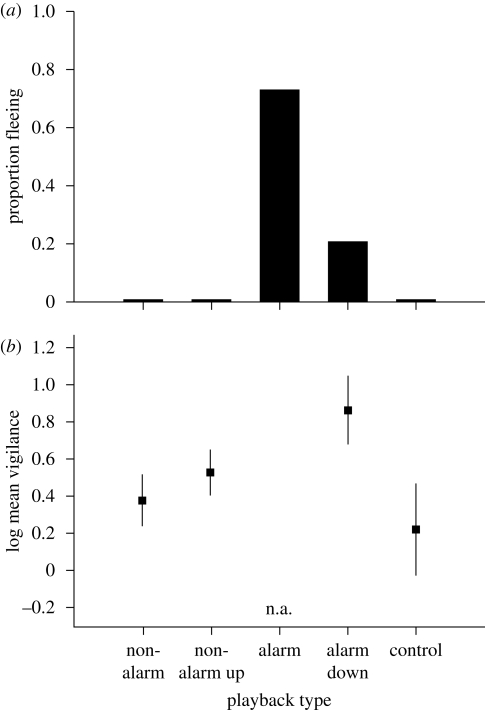
Flocks flew immediately in 11 out of 15 cases in response to playback of alarmed whistles at natural amplitude but never to non-alarmed whistles at natural amplitude (figure 4a; Cochran Q = 36.3, d.f. = 4, p < 0.001, across all treatments). This difference was not simply owing to the higher amplitude of alarmed whistles, as flocks never flew to non-alarmed whistles that were amplified up to match alarmed whistles, nor to the control sounds that were played back at alarmed-whistle amplitude. However, the high alarmed-whistle amplitude did increase the probability of flight, as alarmed whistles amplified down to match natural non-alarmed whistles provoked flight in only three of 15 cases.
Figure 4.
Response of crested pigeon flocks to playback of alarmed and non-alarmed wing whistles: (a) proportion of flocks fleeing; (b) mean time individuals spent vigilant among those that did not flee (with 95% confidence interval). Playbacks included alarmed and non-alarmed whistles at their natural amplitude, alarmed whistles at reduced amplitude to match non-alarmed whistles (alarm down), non-alarmed whistles at increased amplitude to match alarmed whistles (non-alarm up) and a control sound (parrot contact call, at alarmed amplitude). n = 15 matched playback sets for fleeing and 12 matched sets for vigilance because three flocks fled to the alarmed down treatment and so vigilance could not be measured. Vigilance data are unavailable for the alarmed playback because most flocks fled. n.a., not applicable.
Although most birds did not flee to alarmed whistles amplified down to match non-alarmed whistles, individuals in those 12 flocks remaining were nonetheless more vigilant than those after non-alarmed or control playbacks (figure 4b; repeated-measures ANOVA, F3,33 = 11.5, p < 0.001 comparing all but alarm at natural amplitude; paired t-test, t11 = 6.6, p < 0.001, matching alarmed at reduced amplitude with non-alarmed at the same, natural amplitude). Within natural non-alarmed whistles, vigilance increased with increasing element cycle rate, consistent with graded information on the type of flight (log vigilance = −2.17 + 0.21 × cycle rate; F1,13 = 4.64, p = 0.05, n = 15, r2 = 0.26). There was no similar pattern among the 12 flocks that remained after reduced-amplitude alarmed whistles, perhaps because they were already at heightened vigilance (F1,10 = 0.049, p = 0.5). Overall, flocks showed the greatest response to alarmed whistles, an intermediate response to alarmed whistles at reduced amplitude and little response to non-alarmed whistles or the control sound.
4. Discussion
Crested pigeons produced louder flight whistles with a faster tempo when taking off in alarm than when taking off in normal flight. Playback experiments showed that other individuals used these differences adaptively, as they took off in alarm only after alarmed whistles. Furthermore, playback of amplitude-manipulated whistles showed that the flight response required structural differences between alarmed and non-alarmed flight whistles, not simply the difference in amplitude. The flight whistle is a distinctive sound that is probably produced by vibration of the highly modified eighth primary feather, suggesting that it is a signal rather than a by-product of flight. To our knowledge, this is the first description of differences between alarmed and non-alarmed non-vocal sounds, and a demonstration that a mechanical flight noise can act like an alarm signal and alert flock members of danger.
The flight whistle of the crested pigeon is a complex sound consisting of three different components, repeated cyclically with one cycle of elements per wingbeat. Two components are tonal sounds with multiple harmonics, while the third is a short, broadband clap. High-speed video is necessary to examine the timing and mechanism of production of each element, but it is likely that the harmonic sounds are produced by the vibration of the eighth and possibly adjacent primaries during upstrokes and downstrokes, while claps are produced through contact of feathers at the top or bottom or both of the wingbeat (Norberg 1991; Bostwick & Prum 2003; Bostwick 2006; Clark & Feo 2008). The eighth primary is equally narrow in both sexes, consistent with all individuals producing whistles. The constant (Tone 2) or nearly constant (Tone 1) acoustic frequency of tonal elements regardless of wingbeat frequency suggests that these sounds are produced by feather vibration rather than being acoustic whistles, which is consistent with previous studies of sound production in birds (Clark & Feo 2008; C. J. Clark 2009, personal communication).
Flight whistles produced in alarm were acoustically distinct from those produced in normal flight, showing that the wing whistle encodes reliable information about alarm despite being produced in all flapping flight. Alarmed whistles were louder and produced at a greater tempo, which is consistent with a faster or steeper take-off and changes in wingbeat kinematics including greater wingbeat frequency (§1). The mean amplitude increase of 5.4 dB is easily detectable by birds, as should be the differences in timing (Dooling 2004).
The playback experiment showed that crested pigeons used the acoustic information in the wing whistle in an adaptive way, fleeing immediately over 70 per cent of the time after hearing a whistle given in alarm but never fleeing after a whistle given in normal flight. This response to playback shows that the birds did not need to see a predator or use other cues from real pigeons. For example, the use of playback ruled out the possibilities that birds were responding to an individual bird flying without first giving intention movements (Davis 1975), or took their cue from the simultaneous departure of multiple individuals (Lima 1995; Cresswell et al. 2000). This result reveals a novel method by which members of a group can benefit from collective vigilance, without having to rely on alarm calls or rules of thumb based on the timing of departure of other individuals (Lima 1995; Cresswell et al. 2000).
The playbacks using manipulated amplitudes showed that birds used the information in both structure and amplitude when responding to flight whistles. Non-alarmed whistles amplified up to match natural alarmed whistles never prompted birds to flee, showing that the structure of alarmed whistles was essential in identifying alarmed flight. Alarmed whistles amplified down to match non-alarmed whistles prompted flight in only 20 per cent of cases, but nonetheless caused greater vigilance than the non-alarmed whistles, again showing that whistle structure affected meaning. Overall, the whistle's structure appeared to determine whether it was interpreted as an alarmed or a non-alarmed flight, while the amplitude affected whether the response would be immediate flight or increased vigilance.
Continuous variation in whistle structure and amplitude, and variability in pigeon response according to those features, suggests that whistles convey information about the urgency of the situation, comparable to graded alarm calls (Blumstein & Armitage 1997; Manser et al. 2001; Leavesley & Magrath 2005; Templeton et al. 2005). Vigilance increased with tempo within natural non-alarmed whistle playbacks, suggesting that tempo encodes graded information. Similarly, a reduction in the amplitude of alarmed whistles led to a reduced probability of take-off, suggesting that a quieter whistle indicates reduced threat, either because the bird is interpreted as taking off less rapidly or is further away.
The crested pigeon's whistle could be an alarm signal or cue. It would be an alarm ‘signal’ if it benefited the signaller and evolved to convey information about danger to others, whereas it would be a ‘cue’ if it provided information about danger but did not evolve for that purpose (Maynard Smith & Harper 2003). An individual could benefit from signalling alarm by prompting simultaneous flight and so reducing the risk of being singled out by a predator (Sherman 1985), or warning relatives or mates of danger (Sherman 1977; Greisser & Ekman 2004; Krams et al. 2006). The distinctive wing whistle is likely to be produced at least partly by the vibration of the highly modified eighth primary feather, so it is likely to be a signal and not simply a side effect of flight, but it might have evolved to signal something other than alarm. For example, the flight whistle might have evolved as a courtship signal used in display flight (Frith 1982; Baptista et al. 1997). We suggest that signalling alarm might also be one facet of a flight-contact signal, which enables acoustic tracking of others by encoding flight information such as current direction of movement and relative movement, as well as changes in direction or speed that result from changes in wing kinematics. In sustained flight, it could enable flock cohesion, and on the ground convey information about arrival, departure and speed of take-off.
Regardless of whether it is a signal or cue of alarm, the wing whistle is likely to provide reliable information on the degree of danger because it is intrinsically related to the type of take-off, probably cannot be omitted and is likely to be energetically costly to fake. An individual detecting extreme danger is likely to take off more quickly or more steeply (§1), creating whistles with a greater amplitude and a faster tempo. Furthermore, a physiological cost of taking off rapidly is paid even if the signal is used deceptively when no predator is present, which reinforces reliability. Birds might conceivably suppress the whistle by removing feathers or ruffling vanes (C. J. Clark 2009, personal communication), but we found no evidence for such modification in the eighth or any other primary in the skins studied at the Australian Wildlife Collection. If the whistle is an alarm signal, it is therefore likely to be an honest index signal of danger (Maynard Smith & Harper 2003). By contrast, vocal alarm signals are likely to be costly only if a predator is present, and so costs are unlikely to enforce honesty (Searcy & Nowicki 2005), and deceptive alarm calls can be common (Munn 1986; Møller 1988, 1990; Ridley et al. 2007). If the whistle is a cue, it raises the possibility that wing sounds could be a very general mechanism whereby individuals in flocks gather acoustic information on danger, contributing to the evolution of grouping even in species with no significant feather modification, but with audible whooshing flight sounds. Small passerine species appear unable to distinguish easily alarmed from routine take-off (Lima 1995), but the wing sounds of larger species are likely to be louder and therefore act as a cue or be modified into signals that more efficiently provide information on wingbeat kinematics. Similarly, individuals can gain information from the alarmed behaviour in other animals, including insects (Treherne & Foster 1981), fishes (Krause 1993; Mathis et al. 1996) and mammals (Randall 2001), suggesting further studies of cue reliability and the evolution of index signals.
Acknowledgements
The research was carried out under an ethics permit from the Australian National University.
We thank Bob Phillips and Jim Forge for help with electronics, Stephen Bartnik and Ric Hingee for assistance in the field, Leo Joseph for access to the Australian National Wildlife Collection and Christopher J. Clark, Ana Dalziell, Pam Fallow, Tonya Haff, Ben Hatchwell, Megan Higgie, Michael Jennions and anonymous reviewers for comments on the manuscript. The research was carried out with support from an Australian Research Council Discovery Grant to R.D.M.
References
- Baptista L. F., Trail P. W., Horblit H. M.1997Family Columbidae (pigeons and doves). In Handbook of the birds of the world, vol. 4: sandgrouse to cuckoos (eds del Hoyo J., Elliott A., Sargatal J.), pp. 60–243 Barcelona, Spain: Lynx Edicions [Google Scholar]
- Blumstein D. T., Armitage K. B.1997Alarm calling in yellow-bellied marmots. 1. The meaning of situationally variable alarm calls. Anim. Behav. 53, 143–171 (doi:10.1006/anbe.1996.0285) [Google Scholar]
- Bostwick K. S.2006Mechanisms of feather sonation in Aves: unanticipated levels of diversity. Acta Zool. Sinica 52, 68–71 [Google Scholar]
- Bostwick K. S., Prum R. O.2003High-speed video analysis of wing-snapping in two manakin clades (Pipridae: Aves). J. Exp. Biol. 206, 3693–3706 (doi:10.1242/jeb.00598) [DOI] [PubMed] [Google Scholar]
- Bostwick K. S., Zyskowski K.2001Mechanical sounds and sexual dimorphism in the crested doradito. Condor 103, 861–865 (doi:10.1650/0010-5422(2001)103[0861:MSASDI]2.0.CO;2) [Google Scholar]
- Charif R. A., Waack A. M., Strickman L. M.2008Raven Pro 1.3 user's manual Ithaca, NY: Cornell Laboratory of Ornithology [Google Scholar]
- Clark C. J.2008Fluttering wing feathers produce flight sounds of male streamertail hummingbirds. Biol. Lett. 4, 341–344 (doi:10.1098/rsbl.2008.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. J., Feo T. J.2008The Anna's hummingbird chirps with its tail: a new mechanism of sonation in birds. Proc. R. Soc. B 275, 955–962 (doi:10.1098/rspb.2007.1619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman S. W.2008Mourning dove (Zenaida macroura) wing-whistles may contain threat-related information for con- and hetero-specifics. Naturwissenschaften 95, 981–986 (doi:10.1007/s00114-008-0404-x) [DOI] [PubMed] [Google Scholar]
- Cresswell W., Hilton G. M., Ruxton G. D.2000Evidence for a rule governing the avoidance of superfluous escape flights. Proc. R. Soc. Lond. B 267, 733–737 (doi:10.1098/rspb.2000.1064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.1975Socially induced flight reactions in pigeons. Anim. Behav. 23, 597–601 (doi:10.1016/0003-3472(75)90136-0) [Google Scholar]
- Dooling R.2004Audition: can birds hear everything they sing? In Nature's music: the science of birdsong (eds Marler P., Slabbekoorn H.), pp. 206–225 San Diego, CA: Elsevier [Google Scholar]
- Elgar M. A.1989Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biol. Rev. 64, 13–33 (doi:10.1111/j.1469-185X.1989.tb00636.x) [DOI] [PubMed] [Google Scholar]
- Frith H. J.1982Pigeons and doves of Australia Sydney, Australia: Rigby [Google Scholar]
- Greisser M., Ekman J.2004Nepostic alarm calling in the Siberian jay, Perisoreus infaustus. Anim. Behav. 67, 933–939 [Google Scholar]
- Higgins P. J., Davies S. J. J. F.1996Handbook of Australian, New Zealand and Antarctic birds. Volume 3: snipe to pigeons Melbourne, Australia: Oxford University Press [Google Scholar]
- Hunter T. A.2008On the role of wing sounds in hummingbird communication. Auk 125, 532–541 (doi:10.1525/auk.2008.06222) [Google Scholar]
- Johnston R. F.1960Behavior of the Inca dove. Condor 62, 7–24 (doi:10.2307/1365655) [Google Scholar]
- Krams I., Krama T., Igaune K.2006Alarm calls of wintering great tits Parus major: warning a mate, reciprocal altruism or a message to the predator? J. Avian Biol. 37, 131–136 (doi:10.1111/j.0908-8857.2006.03632.x) [Google Scholar]
- Krause J.1993Transmission of fright reaction between different species of fish. Behaviour 127, 37–48 (doi:10.1163/156853993X00416) [Google Scholar]
- Krause J., Ruxton G. D.2002Living in groups Oxford, UK: Oxford University Press [Google Scholar]
- Kullberg C., Jakobsson S., Fransson T.1998Predator-induced take-off strategy in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1659–1664 [Google Scholar]
- Leavesley A., Magrath R. D.2005Communicating about danger: urgency alarm calling in a bird. Anim. Behav. 70, 365–373 (doi:10.1016/j.anbehav.2004.10.017) [Google Scholar]
- Lima S. L.1994Collective detection of predatory attack by birds in the absence of alarm signals. J. Avian Biol. 25, 319–326 (doi:10.2307/3677279) [Google Scholar]
- Lima S. L.1995Collective detection of predatory attack by social foragers: fraught with ambiguity? Anim. Behav. 50, 1097–1108 (doi:10.1016/0003-3472(95)80109-X) [Google Scholar]
- Magrath R. D., Pitcher B. J., Gardner J. L.2007A mutual understanding? Interspecific responses by birds to each other's aerial alarm calls. Behav. Ecol. 18, 944–951 (doi:10.1093/beheco/arm063) [Google Scholar]
- Manser M. B., Bell M. B., Fletcher L. B.2001The information that receivers extract from alarm calls in suricates. Proc. R. Soc. Lond. B 268, 2485–2491 (doi:10.1098/rspb.2001.1772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant S., Higgins P. J.1993Handbook of Australian, New Zealand and Antarctic birds: raptors to lapwings Melbourne, Australia: Oxford University Press [Google Scholar]
- Mathis A., Chivers D. P., Smith R. J. F.1996Cultural transmission of predator recognition in fishes: intraspecific and interspecific learning. Anim. Behav. 51, 185–201 (doi:10.1006/anbe.1996.0016) [Google Scholar]
- Maynard Smith J., Harper D.2003Animal signals Oxford, UK: Oxford University Press [Google Scholar]
- Møller A. P.1988False alarm calls as a means of resource usurpation in the great tit, Parus major. Ethology 79, 25–30 [Google Scholar]
- Møller A. P.1990Deceptive use of alarm calls by male swallows, Hirundo rustica: a new paternity guard. Behav. Ecol. 1, 1–6 (doi:10.1093/beheco/1.1.1) [Google Scholar]
- Munn C. A.1986Birds that ‘cry wolf’. Nature 319, 143–145 (doi:10.1038/319143a0) [Google Scholar]
- Norberg R. Å.1991The flappet lark Mirafra ufocinnamomea doubles its wingbeat rate to 24 Hz in wing-clap display flight: a sexually selected feat. J. Exp. Biol. 159, 515–523 [Google Scholar]
- Randall J. A.2001Evolution and function of drumming as communication in mammals. Am. Zool. 41, 1143–1156 (doi:10.1668/0003-1569(2001)041[1143:EAFODA]2.0.CO;2) [Google Scholar]
- Ridley A. R., Child M. F., Bell M. B. V.2007Interspecific audience effects on the alarm-calling behaviour of a kleptoparasitic bird. Biol. Lett. 3, 589–591 (doi:10.1098/rsbl.2007.0325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy W. A., Nowicki S.2005The evolution of animal communication: reliability and deception in signaling systems Princeton, NJ: Princeton University Press [Google Scholar]
- Sherman P. W.1977Nepotism and the evolution of alarm calls. Science 197, 1246–1253 (doi:10.1126/science.197.4310.1246) [DOI] [PubMed] [Google Scholar]
- Sherman P. W.1985Alarm calls of Belding's ground squirrels to aerial predators: nepotism or self preservation? Behav. Ecol. Sociobiol. 17, 313–323 (doi:10.1007/BF00293209) [Google Scholar]
- Snow D. W.2004Family Cotingidae (Cotingas). In Handbook of the birds of the world, vol. 9: cotingas to pipits and wagtails (eds del Hoyo J., Elliott A., Christie D. A.), pp. 32–108 Barcelona, Spain: Lynx Edicions [Google Scholar]
- Sokal R. R., Rohlf F. J.1995Biometry New York, NY: W. H. Freeman [Google Scholar]
- Tabachnick B. G., Fidell L. S.2007Using multivariate statistics Boston, CA: Pearson [Google Scholar]
- Templeton C. N., Greene E., Davis K.2005Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308, 1934–1937 (doi:10.1126/science.1108841) [DOI] [PubMed] [Google Scholar]
- Tobalske B. W., Altshuler D. L., Powers D. R.2004Take-off mechanics in hummingbirds (Trochilidae). J. Exp. Biol. 207, 1345–1352 (doi:10.1242/jeb.00889) [DOI] [PubMed] [Google Scholar]
- Treherne J. E., Foster W. A.1981Group transmission of predator avoidance behaviour in a marine insect: the Trafalgar effect. Anim. Behav. 29, 911–917 (doi:10.1016/S0003-3472(81)80028-0) [Google Scholar]
- Van der Veen I., Lindström K. M.2000Escape flights of yellowhammers and greenfinches: more than just physics. Anim. Behav. 59, 593–601 (doi:10.1006/anbe.1999.1331) [DOI] [PubMed] [Google Scholar]
- Veasey J. S., Metcalfe N. B., Houston D. C.1998A reassessment of the effect of body mass upon flight speed and predation risk in birds. Anim. Behav. 56, 883–889 (doi:10.1006/anbe.1998.0880) [DOI] [PubMed] [Google Scholar]