Abstract
Parasites often manipulate host immunity for their own benefit, either by exacerbating or suppressing the immune response and this may directly affect the expression of parasite virulence. However, genetic variation in immunodepression, which is a prerequisite to its evolution, and the relationship between immunodepression and virulence, have rarely been studied. Here, we investigated the variation among sibships of the acanthocephalan parasite, Pomphorhynchus laevis, in infecting and in immunodepressing its amphipod host, Gammarus pulex. We also assessed the covariation between infectivity, parasite-induced immune depression and host mortality (parasite virulence). We found that infectivity, the intensity of immunodepression and virulence were variable among parasite sibships. Infectivity and the level of immunodepression were not correlated across parasite sibships. Whereas infectivity was unrelated to host mortality, we found that gammarids that were exposed to the parasite sibships that immunodepressed their hosts the most survived better. This positive covariation between host survival and immunodepression suggests that gammarids exposed to the less immunodepressive parasites could suffer from damage imposed by a higher activity of the phenoloxidase.
Keywords: acanthocephalan, covariation, immune defences, parasite-induced immunodepression, phenoloxidase, virulence
1. Introduction
There is little doubt that the host immune response represents the major defence against parasitic attacks (Wakelin 1996). Upon entering the host, parasites are exposed to the effectors of the immune system, which may result in the clearance of the infection. In response to the threat imposed by the immune system, parasites have evolved a variety of strategies aimed at manipulating host immunity (Damian 1997; Schmid-Hempel 2008). These strategies of immune evasion are supposed to favour the establishment, the growth and the reproduction of the parasite within the host. Immunodepression is probably one of the most widespread strategies of immune evasion. Many parasites interfere with the host immune response by down-regulating immune effectors. Helminth parasites are masters in their ability to depress host immune function (Maizels et al. 2004). Although the mechanisms of evasion and suppression of the host innate immunity have been better investigated and characterized in vertebrate hosts (Maizels & Yazdanbakhsh 2003), helminths seem to adopt the same strategies in their mollusc and arthropod intermediate hosts (Loker 1994; Yoshino & Vasta 1996).
In spite of the adaptive nature of immune evasion for parasite fitness, the study of immunomodulatory strategies has been largely overlooked by evolutionary biologists (Schmid-Hempel 2008). Two questions in particular are still open to debate. In addition to selection, adaptive evolutionary change requires genetic variation in the trait of interest. We currently ignore whether immune evasion is genetically variable. Second, the relationship between strategies of immune evasion and parasite virulence are still unclear. Virulence is defined as the infection-induced damage to the host, often resulting in reduced host fecundity and/or increased host mortality (Read et al. 2004). Virulence is a pivotal trait of parasite life histories and is thought to be involved in trade-offs with other fitness components. Parasites have to balance the benefits of host exploitation (higher within-host multiplication and transmission) and the costs that this might induce in terms of infection-induced host mortality (Frank 1996). These two forces (multiplication rate of the parasite and host mortality) have opposing effects on the evolution of virulence. Accordingly, parasite fitness has been shown to be maximized at intermediate levels of virulence (de Roode et al. 2008).
This theoretical framework has, however, not taken into account the potential effect of immunomodulatory strategies on virulence.
At the moment, two scenarios are conceivable. First, parasites can benefit from a lower level of immune defence, as this should favour parasite replication and transmission. A higher rate of host exploitation will accentuate the deleterious effects of infection and increase virulence. By means of immune evasion mechanisms, parasites can also reduce clearance by the immune system, ensuring a longer survival time within the host. Recently, Frank & Schmid-Hempel (2008) suggested that immune evasion should be selected for in spite of the potential reduction in host survival at any later time (see also Schmid-Hempel 2008). This scenario therefore predicts that immunodepression and virulence should be positively correlated traits. An alternative scenario can, however, produce a different prediction. Although it is often assumed that host mortality is directly related to parasite exploitation (uptake of host resources for parasite growth and replication), infection-induced mortality can also result from the over-activity of the host immune response (Day et al. 2007). Virulence can, therefore, be broken down into two traits: the direct effect of host exploitation and the indirect effect of immunopathology (Graham et al. 2005). Parasite-induced immunodepression, by decreasing the level of activity of the immune effectors, can lower the cost of auto-reactivity. In this case, immunodepression and virulence might be negatively correlated.
The interaction between the freshwater amphipod Gammarus pulex and its acanthocephalan parasite Pomphorhynchus laevis provides an interesting natural system to investigate the evolutionary ecology of immune evasion. Acanthocephalans are macroparasites with a complex life cycle involving a crustacean and a fish as intermediate and definitive hosts, respectively (Kennedy 2006). While the larvae develop within the body cavity of the crustacean intermediate host, they are exposed to the host immune response. We have recently shown that infection by the acanthocephalan P. laevis is associated with weakened immune defences of its intermediate host, G. pulex (Cornet et al. 2009b). The activity of the prophenoloxidase (proPO) system and the number of circulating haemocytes, the major effectors of the crustacean immune system (Cerenius & Söderhäll 2004), were found to be dramatically impaired in infected hosts. In the present article we aimed to (i) assess the variation in infectivity, virulence and immunodepression among parasite sibships, using experimental infections of P. laevis under laboratory conditions, and (ii) estimate the covariation between virulence and immunodepression among parasite families. As previously mentioned, with respect to the covariation between virulence and immunodepression, two predictions can be put forward: positive or negative covariation between immunodepression and virulence. The present study should provide the opportunity to tease apart these two alternative hypotheses.
2. Material and methods
(a). Sampling of hosts and parasites
Gammarus pulex males were collected using a kick sampling method in May and October 2007 in a small tributary of the Suzon River at Val Suzon (in the north of Dijon, eastern France). Animals were maintained in the laboratory under standard conditions (15 ± 1°C; light:dark cycle, 12:12 h) in well aerated tanks filled with dechlorinated UV-treated tapwater and fed with elm leaves. They were acclimatized for four weeks before infection experiments.
Pomphorhynchus laevis parasites used in the experiment came from naturally parasitized chubs (L. cephalus) sampled by electrofishing in the River Ouche at Dijon. Adult parasites were collected from the intestines; eggs were obtained by dissecting female worms and then stored in water (see details in Cornet et al. 2009b).
(b). Infection procedure
Because acanthocephalans reproduce sexually, we could not obtain parasite clones. However, a common feature of acanthocephalan reproduction is that the male seals the female gonopore with secretions produced by the cement apparatus (Dezfuli et al. 1999). The post-copulatory cap prevents multiple matings and ensures egg fertilization by the male (Poulin & Morand 2000). Therefore, a clutch of eggs produced by a female represents a full-sib family, and was considered here as a parasite sibship. In addition, to minimize the risk that the same male had fertilized several females, clutches were sampled from different host fish.
Two sets of experimental infections were carried out in May (spring) and October 2007 (winter), following the protocol described below. Parasite eggs from each female were examined under the microscope to evaluate their number and maturity. Ten clutches of P. laevis of equal maturity were selected, the number of eggs estimated and the solution set at 50 eggs µl−1. Nine and ten different parasite sibships were used for the spring and winter experiments, respectively. Prior to parasite exposure, gammarids were food-deprived for 24 h. Gammarids were placed in pairs in crystallizing dishes and an egg suspension (100 eggs per individual) was deposited on 1 cm2 of elm leaf, on which gammarids were allowed to feed for 48 h. Uninfected leaves were provided to the non-exposed, control group. Approximately 100 gammarids were exposed to the infection by each parasite sibship and randomly maintained in groups of 18 in 0.5 l aquaria.
Beginning at the sixth week after infection, gammarids were inspected under a binocular microscope to ascertain parasite infection. Infection by the acanthocephalan cannot be detected before the parasite reaches the cystacanth stage (end of larval development), which appears as a yellow-orange dot within the body cavity of the host. Therefore, the prevalence of infection was expressed as the number of infected hosts divided by the total number of surviving gammarids at the end of the experiment. This underestimates the proportion of infected gammarids, because infected hosts that died before the parasite reached the cystacanth stage could not be detected. Multi-infections (gammarids harbouring more than one cystacanth) were also recorded. Parasite development time refers to the moment when the cystacanth was first detected.
(c). Haemolymph collection and activities of the proPO system
Haemolymph was collected at the end of the experiment. Haemolymph extracts were sampled as described by Cornet et al. (2009b) by wounding gammarids near the seventh dorsal segment with very fine forceps. Haemolymph (1 µl) was collected into a sterile, pre-chilled glass capillary and flushed with 10 µl of cold phosphate buffer saline (PBS). Samples were immediately frozen in liquid nitrogen and stored at −80°C for use in immune assays to be carried out later.
For each individual haemolymph extract, the activity of naturally activated phenoloxidase (PO) enzymes only (hereafter, PO activity) and the activity of the proenzymes in addition to that of PO (proPO activity) were measured using a spectrophotometric assay (Jacot et al. 2005; Cornet et al. 2009b). PO activity was quantified without further activation, whereas proPO activity required the activation of the proPO into PO with chymotrypsin. Approximately 5 µl of haemolymph extract was added to a microplate well containing 20 µl of PBS and either 140 µl of distilled water to measure PO activity only or 140 µl of chymotrypsin solution (Sigma C-7762, 0.07 mg ml−1 of distilled water) to measure proPO activity. After an incubation period of 4 min, 20 µl of l-Dopa solution (Sigma D-9628, 4 mg ml−1 of distilled water) was added to each well. The reaction was allowed to proceed at 30°C in a microplate reader (Versamax, Molecular Devices) for 40 min. Readings were taken every 15 s at 490 nm and analysed using the software Soft-Max Pro 4.0 (Molecular Devices). Enzyme activity was measured as the slope (Vmax value) of the reaction curve during the linear phase of the reaction and reported relative to the activity of 1 µl of pure haemolymph.
(d). Statistical analyses
One of the nine parasite sibships that were used in the spring experiment provided only two infected gammarids out of the 100 exposed to the infection. The data on this particular parasite sibship were, therefore, excluded from the statistical analyses.
Survival analyses were run using Cox regressions (‘proportional hazards model’). The risk ratio (or hazard ratio) is the probability of the event occurring in time t + 1, given survival to time t. It gives the ratio of hazard functions for the different infected groups (different parasite sibships) relative to the hazard function of the reference group (non-exposed gammarids). A risk ratio greater than 1 implies that the hazard function increases as one moves from the reference group to the treated group. Estimates of the risk ratio given by the model were used afterwards in the statistical analyses as an estimate of parasite virulence (parasite-induced host mortality). Gammarids that were still alive at the end of the experimental period (77 and 63 days for the spring and winter experiments, respectively) were censored in the survival analyses. Because gammarids were maintained in groups (18 animals per aquarium), aquarium identity was included in the statistical model to take into account this shared environment. However, this term was never significant and was removed from the models.
Differences in prevalence of infection were compared using logistic regressions. Development time was analysed using non-parametric tests because the data did not meet the assumption of normality.
All models included ‘season’ as a fixed effect and the ‘parasite sibship’ nested with ‘season’ as a random effect. Variables were transformed as necessary to meet the model assumption of normality and homogeneity of variance. Levels of activity of the phenoloxidase enzyme (PO and proPO activity) were natural-log transformed.
Correlations between traits were assessed using Spearman rank correlations (using mean values per sibship) because of the relatively small number of parasite sibships available (n = 8) (see also Hammerschmidt & Kurtz 2005a).
All tests were performed using JMP v. 5.0 for Windows (SAS Institute, Cary, USA) and referred to two-tailed tests with significant differences considered at the level of p ≤ 0.05.
3. Results
(a). Host survival
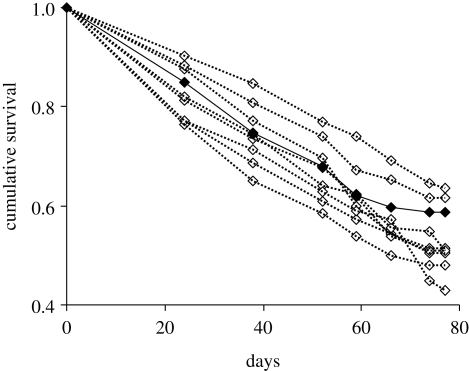
When analysing the entire dataset (spring + winter experiments), we found that survival differed among parasite sibships (global model likelihood ratio χ27 = 36.87, p = 0.0035; ‘season’ χ21 = 3.12, p = 0.0771; ‘parasite sibship(season)’, χ216 = 343.62, p = 0.0061). However, a closer look at the results provided by the two experiments showed that, in winter, host survival did not differ among parasite sibships (χ29 = 13.40, p = 0.1452), whereas parasite sibship explained a significant fraction of variation in host survival in the spring experiment (χ27 = 17.85, p = 0.0127; figure 1). When comparing the survival of infected gammarids (whatever the parasite sibship) with the survival of non-exposed animals we did not find any statistically significant difference (χ21 = 0.88, p = 0.3479). However, given that parasite sibships varied in the amount of mortality induced during the spring experiment, we also compared the survival of non-exposed gammarids to the survival of hosts infected by each parasite sibship. Only two parasite families inflicted a mortality cost on the hosts that significantly differed from the non-exposed controls (χ21 = 4.51, p = 0.0336 and χ21 = 4.36, p = 0.0366, respectively). The other comparisons between parasite-induced mortality per sibship and mortality of non-exposed control provided non-significant results (all p > 0.25).
Figure 1.
Cumulative survival of the gammarids exposed to sibships of Pomphorhynchus laevis (open diamonds, dashed lines) and of non-exposed control individuals (filled diamonds, solid line) in the spring experiment.
(b). Infection success
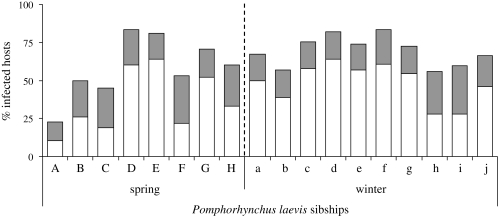
Prevalence was affected by both the ‘season’ (χ21 = 10.28, p < 0.0013) and ‘parasite sibship(season)’ (χ216 = 76.26, p < 0.0001). Infection rates were more variable in spring (from 22.9 to 83.3%) than in winter (from 55.7 to 83.6%) with differences in infection success among parasite families in both seasons (figure 2; spring, LR χ27 = 65.30, p < 0.0001; winter LR χ29 = 23.47, p = 0.0052).
Figure 2.
Gammarus pulex prevalence of infection by sibships of the acanthocephalan Pomphorhynchus laevis in the spring and winter experiments. The proportion of single- and multi-infections are represented by open and shaded bars, respectively.
Parasite sibships also differed in the proportion of multi-infections (figure 2; global model χ217 = 40.94, p = 0.0007; ‘parasite sibship(season)’ χ216 = 37.54, p = 0.0018). The proportion of multi-infections was much more pronounced in spring than in winter (‘season’ effect χ21 = 5.15, p = 0.0232).
(c). Developmental time
We found a strong influence of season when the experiment was carried out on development time to the cystacanth stage (Wilcoxon test Z = 20.44, p < 0.0001), with an average developmental time of 86 (inter-quartile range 81–88) and 61 days (inter-quartile range 61–67) in spring and winter, respectively. A slightly significant difference among parasite families in the developmental time was found in spring (Kruskal–Wallis test χ27 = 14.06, p = 0.0501; O'Brien variance test F7,249 = 5.97, p < 0.0001) whereas the parasites developed in a more synchronized way in winter; O'Brien F9,267 = 1.27, p = 0.2551) therefore erasing the variation among parasite families (Kruskal–Wallis test χ29 = 9.14, p = 0.4245).
(d). Infection-induced immune depression
The influence of multi-infections on the level of immunodepression was tested using a model including the ‘parasite sibship’ and the effect ‘multi-infection’ nested within the ‘parasite sibship’ factor (accounting for the influence of each parasite family). Neither PO nor proPO activity were influenced by multi-infections and this pattern was observed for both spring and winter experiments (for all ‘multi-infection(parasite sibship)’ effects, p ≥ 0.3847). All infected individuals were therefore analysed together.
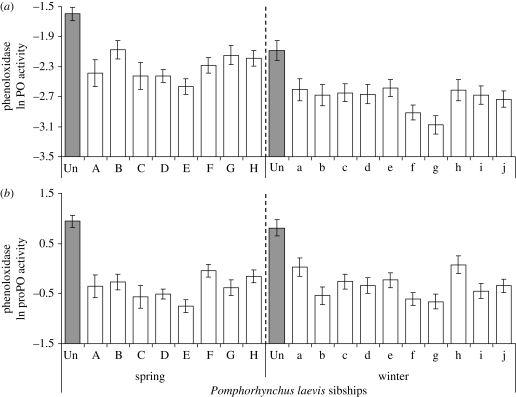
Infection was associated with a reduced level of immune defence by comparison with non-exposed, control individuals (figure 3a,b). PO and proPO activities were lower in gammarids infected by any parasite family compared to control individuals (Dunnett's pairwise comparison tests with control referring to the ‘control’ group of gammarids, α = 0.05) in spring (ANOVA, PO activity F8,305 = 7.34, p < 0.0001; proPO activity F8,305 = 18.17, p < 0.0001), as well as in winter (PO activity F10,302 = 4.39, p < 0.0001; proPO activity F10,302 = 5.92, p < 0.0001).
Figure 3.
Levels of activity of the phenoloxidase enzyme, (a) PO activity and (b) proPO activity measured on gammarids (Gammarus pulex) experimentally infected by sibships of the acanthocephalan Pomphorhynchus laevis in the spring and winter experiments, means ± s.e. Shaded bars represent the values of immune activity of non-exposed control amphipods (Un). Immune data were natural-log transformed.
Although parasitized gammarids had a lower immunocompetence, the extent to which P. laevis parasites depressed the phenoloxidase activities of their hosts was variable among sibships (figure 3a,b). Overall, there was a large sibship variation in immune functions. PO activity was mainly influenced by the ‘season’ and the ‘parasite sibship’ effects (table 1) whereas the ‘parasite sibship’ mostly affected the variation in proPO activity (table 1). When the results were analysed separately by season, the results remained statistically significant for both PO and proPO activities. For the spring experiment, the variation among parasite families was greater for proPO than for PO activity (F7,250 = 3.26, p = 0.0025 and F7,250 = 2.12, p = 0.0416, respectively). In winter, the variability of immunodepression among parasite families was lower both for PO (F9,269 = 1.94, p = 0.0469) and proPO activity (F9,269 = 2.00, p = 0.0400).
Table 1.
Variation in the activities of the phenoloxidase enzyme (PO and proPO activity) measured in Gammarus pulex experimentally infected by Pomphorhynchus laevis as a function of ‘season’ of exposure and ‘parasite sibship’ nested within the ‘season’.
| dependent variable | source of variation | Fd.f. | p |
|---|---|---|---|
| PO activity | global model | 5.30 (17,519) | < 0.0001 |
| season | 25.51 (1,519) | < 0.0001 | |
| parasite sibship(season) | 2.01 (16,519) | 0.0110 | |
| ProPO activity | global model | 2.35 (17,519) | 0.0017 |
| season | 0.15 (1,519) | 0.7045 | |
| parasite sibship(season) | 2.50 (16,519) | 0.0011 |
The level of PO activity was higher in spring than in winter (mean ± s.e. on transformed data: spring, −2.32 ± 0.04; winter, −2.73 ± 0.04; t1,535 = 7.50, p < 0.0001; figure 3a) whereas the proPO activity remained similar in both experiments (spring, −0.37 ± 0.05; winter, −0.35 ± 0.05; t1,535 = −0.235, p = 0.8146; figure 3b). This does not necessarily mean that parasites depressed the immune defences of their hosts more in the winter experiment, but might rather reflect a natural seasonal variation of the basal level of immune defences. Indeed, PO activity measured in non-exposed gammarids was also higher in spring than in winter (spring, −1.60 ± 0.11; winter, −2.09 ± 0.15; t1,70 = 2.61, p = 0.0109).
(e). Correlations between host survival, immunodepression and developmental time
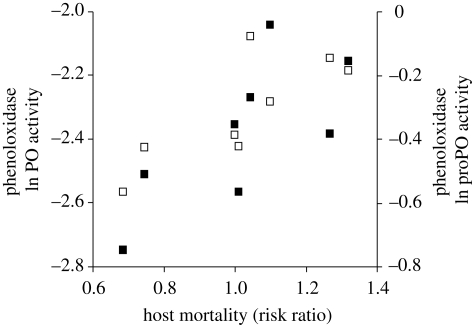
The study of the covariation between traits was restricted to the spring experiment, where most of the variation in traits occurred. Interestingly, when looking at gammarids that had been exposed to the different parasite sibships, we found that mortality was positively related to the PO activity (rs = 0.81, n = 8, p = 0.0149; figure 4) and proPO activity (rs = 0.71, n = 8, p = 0.0465; figure 4). In other words, gammarids that were exposed to the parasite sibships that induced a higher immunodepression had a higher survival rate during the course of the experiment in comparison to the hosts that were exposed to sibships that immunodepressed to a lesser extent. This result therefore suggests that parasite virulence and host immune depression are negatively genetically correlated, at least under the laboratory conditions experienced by the gammarids in the present study. Infectivity was not correlated with virulence or immunodepression among parasite sibships (p > 0.1).
Figure 4.
Covariation between host mortality during the course of the infection (risk ratio) and PO activity (open squares) and proPO activity (filled squares) measured in the spring experiment. Each symbol represents the mean values per parasite sibship. Immune data were natural-log transformed.
4. Discussion
Sibships of the acanthocephalan P. laevis varied considerably in the infection parameters of its intermediate host, the amphipod G. pulex. To our knowledge, this is the first report of variation in parasite infectivity, immunodepression and virulence in an amphipod–acanthocephalan system attributable to parasite ‘genotype’. Although parasite virulence, infectivity and immunodepression are clearly traits whose expression depends on the interaction between the host and the parasite (e.g. Salvaudon et al. 2007), we used different parasite sibships to infect hosts that were sampled in the same population and randomly distributed among the parasite families. This should have ensured that the variation observed for the traits measured is mostly attributable to the parasites.
One of the main purposes of this study was to test whether parasite sibships altered the immune system of their host differently. The level of immune defence of infected gammarids was lowered, as already found in this host–parasite system (Cornet et al. 2009b). In addition, the down-regulation of the level of activity of phenoloxidase (PO and proPO activities) was variable among the parasite sibships. This result suggests genetic variation among P. laevis strains in their ability to immunodepress their host. Nevertheless, we cannot exclude the possibility that environmental sources of variation (such as maternal effects) might also have helped to inflate the observed within-family resemblance in the ability to immunodepress the hosts.
The proPO cascade is one of the major immune effectors of invertebrates; its activation leads to the production of cytotoxic molecules (Nappi & Ottaviani 2000), quinones and melanin (Cerenius & Söderhäll 2004) that participate in pathogen clearance. Up-regulation of proPO genes following infections has been reported in Drosophila (Wertheim et al. 2005) and in Daphnia (Labbé & Little 2009), supporting its role in the resistance to pathogens (Cerenius et al. 2008). Therefore, it is not surprising that this immune pathway is impaired by Acanthocephala. Indeed, acanthocephalan acanthors trigger an accumulation of haemocytes and a high PO activity when penetrating the midgut and the larvae are rapidly surrounded by a capsule of PO-active haemocytes (Volkmann 1991; Taraschewski 2000). However, if a few days after the infection the parasite has not yet been melanized, the haemocyte capsule is lysed, PO activity is strongly reduced and the parasite migrates within the body cavity where it develops. Acanthocephalan parasites, therefore, clearly rely on down-regulation of the host immune response to survive. The variation among parasite strains also suggests that immunodepression in G. pulex is not an all-or-nothing mechanism. The mechanism by which P. laevis inhibits the proPO cascade is still unknown (Cornet et al. 2009b); it may involve excretory–secretory products released by the parasite (for examples in other systems see Shelby et al. 2000; Beck & Strand 2007) or the expression of specific molecules at the parasite surface (Weston et al. 1999). In the tapeworm Schistocephalus solidus, the variation in sugar composition of the parasite surface has been shown to be correlated with variability in infectivity and virulence (Hammerschmidt & Kurtz 2005b). Because of its resemblance with cestodes, differences in infectivity and immunodepression induced by P. laevis sibships might be related to similar mechanisms.
Within-population variation in infection success has been reported for several host–parasite associations (Carius et al. 2001; Schulenburg & Ewbank 2004; Salvaudon et al. 2007) and more recently for a parasite with a complex life cycle, S. solidus, in its intermediate copepod host (Hammerschmidt & Kurtz 2005a). In the present study, parasites from different sibships (a surrogate of different genotypes) differed in their infection success, ranging from 23 to 84 per cent. Because the infection status could only be detected with the observation of the cystacanth (the final larval stage), the estimates of infectivity per parasite family should be taken as an underestimation. Indeed, infected gammarids may have died before their infection status was assessed.
Infected, immunodepressed gammarids can pay an increased cost due to parasite growth as well as the cost of contracting opportunist diseases (Cornet et al. 2009b). As such, one might have expected higher mortality in hosts infected with highly immunodepressive parasite families. Interestingly, contrary to this prediction, we found that gammarids infected by parasite sibships with high immunodepressive effects were those that survived the best, within the sample of exposed gammarids. We suggest that this result might stem from the reduction in the immunopathology cost paid by immunodepressed hosts. Virulence (infection-induced mortality) is the consequence of parasite exploitation (resource uptake) and the cost of an over-reacting immune response (immunopathology) triggered by the infection (Graham et al. 2005; Day et al. 2007). The general activity of the proPO system leads to the production and release of cytotoxic molecules (quinones, reactive superoxide and hydroxyl radicals; Nappi & Ottaviani 2000) in the haemolymph that can harm host tissues (Sadd & Siva-Jothy 2006). PO production has already been shown to be positively correlated with risk of mortality in healthy, non-infected beetles Popillia japonica (Tucker & Stevens 2003), supporting the idea that PO activation triggers a cost in terms of auto-reactivity. Parasite-induced immunodepression might, therefore, protect the host from the negative effect of immune activation (van Riet et al. 2007) and paradoxically result in the observed positive correlation between host survival and the amount of immune depression among exposed individuals. We would like to emphasize that the positive correlation between survival and immunodepression holds for exposed gammarids. Because non-exposed, non-infected animals have a stronger immune response than infected hosts, one might argue that non-infected animals should pay the highest immunopathology cost and thus have the poorest survival prospects. This view, however, confounds two sources of mortality. As mentioned previously, virulence is the result of the additive/interactive effect of the parasite diverting resources from the host, interfering with host homeostasis, and altering the immune response. This means that it is not straightforward to compare the survival prospect of infected/immunodepressed animals with the survival of non-infected/immunocompetent gammarids, because this confounds two factors: the effect of the infection and the effect of immunodepression.
We should also keep in mind that the experiment was run under optimal laboratory conditions, with clean, renewed, aerated water, food ad libitum and low or no risk of suffering from opportunistic infections. The relative costs (better parasite growth, opportunistic infections) and benefits (protection from immunopathology) of acanthocephalan-induced immunodepression are likely to differ across a range of environmental conditions and need to be assessed in a more natural context. One can speculate that in a microorganism-rich environment, the cost of contracting opportunistic infections for infected gammarids might outweigh any benefit due to a reduced immunopathological risk.
In this study we used host survival as a proxy of fitness and, symmetrically, host mortality as a measure of parasite virulence. However, the debilitating effect of parasitism can obviously shape other host fitness-linked traits, such as reproductive output. Moreover, acanthocephalans can manipulate host reproduction (castrating parasites; Bollache et al. 2002) to save energy for host survival (and therefore for their own survival). Similarly, parasite-induced immunodepression might benefit host survival by resource reallocation. This adds a supplementary layer of complexity to the relationship between immunodepression and virulence, because castrating parasites that immunomodulate their hosts might improve host survival, not because of the reduction of the immunopathology cost, but simply because of the reduction of the cost of reproduction and/or the energetic cost of immunity. To our knowledge this issue has not yet been explored.
We did not find any correlation between the prevalence of infection, parasite development and the level of immunodepression among parasite sibships. Parasite traits in the intermediate host seem to be independent at the family level, in the sense that there is no relationship between infection success and host exploitation. Other factors such as potential constraints among parasite traits need to be taken into account to understand the evolution of virulence in complex life cycles (Gandon 2004).
Two sets of experiments were carried out using the same protocol in spring and winter. The results obtained for parasite traits between the sessions differed slightly. In winter, the prevalence of infection was higher (from 56 to 84%), parasite development was on average shortened by 20 days, and cystacanths appeared in a more synchronous way. We also noticed a difference in the activity of the proPO system, in particular a seasonal variation in PO activity in uninfected gammarids that in turn affected the level of immunodepression among parasite families between the spring and winter experiments. Investment in the proPO system (estimated by proPO activity) was similar across the seasons. It has already been shown that in gammarids PO activity is more plastic and is submitted to regulation according to environmental variables more than proPO activity, which appears more physiologically constrained (Cornet et al. 2009a). Environmental variables such as food or temperature can affect the outcome of host–parasite interactions (Blanford et al. 2003; Vale et al. 2008). The hosts and parasites were sampled on two occasions and we were unable to control for this external variation. We attempted to reduce the environmental variation by maintaining the hosts in controlled conditions in the laboratory. However, we cannot exclude a seasonality effect in the physiology of hosts and the level of immune defence of hosts (Sandland & Minchella 2003) nor parasite ‘qualities’ that could explain the higher infectivity and faster development found in winter. Nevertheless, higher prevalence in autumn and winter has been reported in the field for P. laevis infection in Gammarus balcanicus (Dudinak & Spakulova 2003).
Working with complex natural host–parasite systems allows relevant questions on the evolution and ecology of interactions to be addressed. Nevertheless, it also sets a series of limitations, mostly due to the experimental difficulties in completing the parasitic cycle (and the two host species) under controlled laboratory conditions. For instance, one important issue is whether the observed reduction in the immune response of infected animals is the cause or the consequence of infection. Gammarids with initially low levels of constitutive immune defences might be more prone to the infection (and to parasite-induced mortality). In this case, the observed difference in the immune response between infected and non-exposed gammarids would merely reflect a differential susceptibility instead of a parasite strategy to escape host defences. However, the results of a previous experiment strongly suggest that any difference in immune response between infected and non-infected hosts is due to parasite-induced immunodepression. Cornet et al. (2009b) compared the level of PO activity in non-exposed controls, exposed but non-infected and infected hosts. Contrary to the prediction of differential susceptibility, they found that only the infected group had a lower immune activity and that non-exposed and exposed but non-infected hosts had a similar level of PO activity. Overall, these results strongly suggest that the observed variation of PO activity of infected animals among the different parasite sibships was not due to initial differences in susceptibility. These findings are also relevant with respect to the potential bias induced by non-random mortality, in particular due to pre-existing individual differences in PO activity.
In conclusion, we have documented among-sibship variation in some of the key variables affecting the outcome of the infection of gammarids by the acanthocephalan P. laevis. Infection success, the amount of immunodepression and virulence were variable among parasite sibships. To our knowledge this study is also the first to explore the correlation between virulence and the intensity of parasite-induced immunodepression. The finding of a positive correlation between host survival and intensity of immunodepression emphasizes the need to combine in a common framework the consequences of parasite exploitation and an over-expression of host immune defences on the evolution of parasite virulence (Day et al. 2007; Schmid-Hempel 2008).
Acknowledgements
This study was supported by a grant from the Conseil Régional de Bourgogne (06512AA07579-FABER 2006-178) to G.S. and an ANR grant (BLAN07-3_183300) to T.R. Doctoral grants for S.C. and N.F. were from the Conseil Régional de Bourgogne and the MENESR, respectively. We are grateful to D. Promislow and two anonymous referees for valuable comments on an earlier version of the manuscript and to B. Wilson for helpful corrections.
References
- Beck M. H., Strand M. R.2007A novel polydnavirus protein inhibits the insect prophenoloxidase activation pathway. Proc. Natl Acad. Sci. USA 104, 19 267–19 272 (doi:10.1073/pnas.0708056104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanford M., Thomas B., Pugh C., Pell J. K.2003Temperature checks the Red Queen? Resistance and virulence in a fluctuating environment. Ecol. Lett. 6, 2–5 (doi:10.1046/j.1461-0248.2003.00387.x) [Google Scholar]
- Bollache L., Rigaud T., Cézilly F.2002Effects of two acanthocephalan parasites on the fecundity and pairing status of female Gammarus pulex (Crustacea: Amphipoda). J. Invertebr. Pathol. 79, 102–110 (doi:10.1016/S0022-2011(02)00027-7) [DOI] [PubMed] [Google Scholar]
- Carius H. J., Little T. J., Ebert D.2001Genetic variation in a host–parasite association: potential for coevolution and frequency-dependent selection. Evolution 55, 1136–1145 [DOI] [PubMed] [Google Scholar]
- Cerenius L., Söderhäll K.2004The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 198, 116–126 (doi:10.1111/j.0105-2896.2004.00116.x) [DOI] [PubMed] [Google Scholar]
- Cerenius L., Lee B. L., Söderhäll K.2008The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 29, 263–271 (doi:10.1016/j.it.2008.02.009) [DOI] [PubMed] [Google Scholar]
- Cornet S., Biard C., Moret Y.2009aVariation in immune defence among populations of Gammarus pulex (Crustacea: Amphipoda). Oecologia 159, 257–269 (doi:10.1007/s00442-008-1211-y) [DOI] [PubMed] [Google Scholar]
- Cornet S., Franceschi N., Bauer A., Rigaud T., Moret Y.2009bImmune depression induced by acanthocephalan parasites in their intermediate crustacean host: consequences for the risk of super-infection and links with host behavioural manipulation. Int. J. Parasitol. 39, 221–229 (doi:10.1016/j.ijpara.2008.06.007) [DOI] [PubMed] [Google Scholar]
- Damian R. T.1997Parasite immune evasion and exploitation: reflections and projections. Parasitology 115, S169–S175 [DOI] [PubMed] [Google Scholar]
- Day T., Graham A. L., Read A. F.2007Evolution of parasite virulence when host responses cause disease. Proc. R. Soc. B 274, 2685–2692 (doi:10.1098/rspb.2007.0809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roode J. C., Yates A. J., Altizer S.2008Virulence-transmission trade-offs and population divergence in virulence in a naturally occuring butterfly parasite. Proc. Natl Acad. Sci. USA 105, 7489–7494 (doi:10.1073/pnas.0710909105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfuli B. S., Capuano S., Pironi F., Mischiati C.1999The origin and function of cement gland secretion in Pomphorhynchus laevis (Acanthocephala). Parasitology 119, 649–653 (doi:10.1017/S0031182099005193) [DOI] [PubMed] [Google Scholar]
- Dudinak V., Spakulova M.2003The life cycle and seasonal changes in the occurrence of Pomphorhynchus laevis (Palaeacanthocephala, Pomphorhynchidae) in a small isolated lake. Parasite 10, 257–262 [DOI] [PubMed] [Google Scholar]
- Frank S. A.1996Models of parasite virulence. Q. Rev. Biol. 71, 37–78(doi:10.1086/419267) [DOI] [PubMed] [Google Scholar]
- Frank S. A., Schmid-Hempel P.2008Mechanisms of pathogenesis and the evolution of parasite virulence. J. Evol. Biol. 21, 396–404 (doi:10.1111/j.1420-9101.2007.01480.x) [DOI] [PubMed] [Google Scholar]
- Gandon S.2004Evolution of multihost parasites. Evolution 58, 455–469 [PubMed] [Google Scholar]
- Graham A. L., Allen J. E., Read A. F.2005Evolutionary causes and consequences of immunopathology. Annu. Rev. Ecol. Evol. Syst. 36, 373–397 (doi:10.1146/annurev.ecolsys.36.102003.152622) [Google Scholar]
- Hammerschmidt K., Kurtz J.2005aEvolutionary implications of the adaptation to different immune systems in a parasite with a complex life cycle. Proc. R. Soc. B 272, 2511–2518 (doi:10.1098/rspb.2005.3241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K., Kurtz J.2005bSurface carbohydrate composition of a tapeworm in its consecutive intermediate hosts: individual variation and fitness consequences. Int. J. Parasitol. 35, 1499–1507 (doi:10.1016/j.ijpara.2005.08.011) [DOI] [PubMed] [Google Scholar]
- Jacot A., Scheuber H., Kurtz J., Brinkhof M. W. G.2005Juvenile immune system activation induces a costly upregulation of adult immunity in field crickets Gryllus campestris. Proc. R. Soc. B 272, 63–69 (doi:10.1098/rspb.2004.2919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C. R.2006Ecology of the Acanthocephala Cambridge, UK: Cambridge University Press [Google Scholar]
- Labbé P., Little T. J.2009ProPhenolOxidase in Daphnia magna: cDNA sequencing and expression in relation to resistance to pathogens. Dev. Comp. Immunol. 33, 674–680 (doi:10.1016/j.dci.2008.11.012) [DOI] [PubMed] [Google Scholar]
- Loker E. S.1994On being a parasite in an invertebrate host: a short survival course. J. Parasitol. 80, 728–747 (doi:10.2307/3283252) [PubMed] [Google Scholar]
- Maizels R. M., Yazdanbakhsh M.2003Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3, 733–744 (doi:10.1038/nri1183) [DOI] [PubMed] [Google Scholar]
- Maizels R. M., Balic A., Gomez-Escobar N., Nair M., Taylor M. D., Allen J. E.2004Helminth parasites — masters of regulation. Immunol. Rev. 201, 89–116 (doi:10.1111/j.0105-2896.2004.00191.x) [DOI] [PubMed] [Google Scholar]
- Nappi A. J., Ottaviani E.2000Cytotoxicity and cytotoxic molecules in invertebrates. BioEssays 22, 469–480 (doi:10.1002/(SICI)1521-1878(200005)22:5<469::AID-BIES9>3.0.CO;2-4) [DOI] [PubMed] [Google Scholar]
- Poulin R., Morand S.2000Testes size, body size and male–male competition in acanthocephalan parasites. J. Zool. Lond. 250, 551–558 [Google Scholar]
- Read A. F., Gandon S., Nee S., MacKinnon M. J.2004The evolution of pathogen virulence in response to animal and public health interventions. In Infectious disease and host–pathogen evolution (ed. Dronamraju K. R.). pp. 265–292 Cambridge, UK: Cambridge University Press [Google Scholar]
- Sadd B. M., Siva-Jothy M. T.2006Self-harm caused by an insect's innate immunity. Proc. R. Soc. B 273, 2571–2574 (doi:10.1098/rspb.2006.3574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvaudon L., Heraudet V., Shykoff J. A.2007Genotype-specific interactions and the trade-off between host and parasite fitness. BMC Evol. Biol. 7, 189 (doi:10.1186/1471-2148-7-189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandland G., Minchella D. J.2003Costs of immune defence: an enigma wrapped in an environmental cloak? Trends Parasitol. 19, 571–574 (doi:10.1016/j.pt.2003.10.006) [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P.2008Parasite immune evasion: a momentous molecular war. Trends Ecol. Evol. 23, 318–326 [DOI] [PubMed] [Google Scholar]
- Schulenburg H., Ewbank J. J.2004Diversity and specificity in the interaction between Caenorhabditis elegans and the pathogen Serratia marcescens. BMC Evol. Biol. 4, 49 (doi:10.1186/1471-2148-4-49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby K. S., Adeyeye O. A., Okot-Kotber B. M., Webb B. A.2000Parasitism-linked block of host plasma melanization. J. Invertebr. Pathol. 75, 218–225 (doi:10.1006/jipa.2000.4925) [DOI] [PubMed] [Google Scholar]
- Taraschewski H.2000Host–parasite interactions in Acanthocephala: a morphological approach. Adv. Parasitol. 46, 1–179 (doi:10.1016/S0065-308X(00)46008-2) [DOI] [PubMed] [Google Scholar]
- Tucker T. M., Stevens L.2003Geographical variation and sexual dimorphism of phenoloxidase levels in Japanese beetles (Popillia japonica). Proc. R. Soc. Lond. B 270, S245–S247 (doi:10.1098/rsbl.2003.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale P. F., Stjernman M., Little T. J.2008Temperature-dependent costs of parasitism and maintenance of polymorphism under genotype-by-environment interactions. J. Evol. Biol. 21, 1418–1427 [DOI] [PubMed] [Google Scholar]
- van Riet E., Hartgers F. C., Yazdanbakhsh M.2007Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 212, 475–490 [DOI] [PubMed] [Google Scholar]
- Volkmann A.1991Localization of phenoloxidase in the midgut of Periplaneta americana parasitized by larvae of Moniliformis moniliformis (Acanthocephala). Parasitol. Res. 77, 616–621 (doi:10.1007/BF00931025) [DOI] [PubMed] [Google Scholar]
- Wakelin D.1996Immunity to parasites: how parasitic infections are controlled, 2nd edn.Cambridge, UK: Cambridge University Press [Google Scholar]
- Wertheim B., Kraai Jeveld B., Schuster E., Blanc E., Hopkins M., Pletcher S., Strand M., Patridge L., Godfray H. C.2005Genome-wide gene expression in response to parasitoid attack in Drosophila. Genome Biol. 6, R94 (doi:10.1186/gb-2005-6-11-r94) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston D., Patel B., Van Voorhis W. C.1999Virulence in Trypanosoma cruzi infection correlates with the expression of a distinct family of sialidase superfamily genes. Mol. Biochem. Parasitol. 98, 105–116 (doi:10.1016/S0166-6851(98)00152-2) [DOI] [PubMed] [Google Scholar]
- Yoshino T. P., Vasta G. R.1996Parasite–invertebrate host immune interactions. In Advances in comparative and environmental physiology, vol. 24 (ed. Cooper E. L.), pp. 125–167 Berlin, Germany: Springer [Google Scholar]