Abstract
Recent fossil discoveries have demonstrated that Africa and Asia were epicentres for the origin and/or early diversification of the major living primate lineages, including both anthropoids (monkeys, apes and humans) and crown strepsirhine primates (lemurs, lorises and galagos). Competing hypotheses favouring either an African or Asian origin for anthropoids rank among the most hotly contested issues in paleoprimatology. The Afrocentric model for anthropoid origins rests heavily on the >45 Myr old fossil Algeripithecus minutus from Algeria, which is widely acknowledged to be one of the oldest known anthropoids. However, the phylogenetic position of Algeripithecus with respect to other primates has been tenuous because of the highly fragmentary fossils that have documented this primate until now. Recently recovered and more nearly complete fossils of Algeripithecus and contemporaneous relatives reveal that they are not anthropoids. New data support the idea that Algeripithecus and its sister genus Azibius are the earliest offshoots of an Afro–Arabian strepsirhine clade that embraces extant toothcombed primates and their fossil relatives. Azibius exhibits anatomical evidence for nocturnality. Algeripithecus has a long, thin and forwardly inclined lower canine alveolus, a feature that is entirely compatible with the long and procumbent lower canine included in the toothcomb of crown strepsirhines. These results strengthen an ancient African origin for crown strepsirhines and, in turn, strongly challenge the role of Africa as the ancestral homeland for anthropoids.
Keywords: Algeria, primate evolution, toothcombed primates, activity pattern
1. Introduction
Among the few early Paleogene continental sites from Afro–Arabia that have yielded a diverse mammalian fauna, the Glib Zegdou and Gour Lazib localities in Hammada du Dra, southwestern Algeria, are famous for their fossil primates dating from the early or early middle Eocene (between approx. 52 and 46 Ma). These primates document an important but poorly known phase of the early Paleogene primate radiation in Africa. Azibius and Dralestes were tentatively thought to be related to the ‘plesiadapiforms’ (=‘archaic’ primates) (Sudre 1975; Tabuce et al. 2004) and Algeripithecus plus Tabelia (Godinot & Mahboubi 1992, 1994; Godinot 1994) were considered as basal anthropoids, closely related to the much later Eocene–Oligocene forms documented from the Fayum in Egypt (Simons 1992, 1995, 1997a; Seiffert et al. 2005a; Seiffert et al. in press); a fossil record that has engendered the hypothesis that Africa is the homeland of the Anthropoidea clade (Simons & Rasmussen 1994b). Simultaneously, discoveries of basal anthropoids in the Eocene of southern and eastern Asia have demonstrated that Asia has also played a critical role in the origin and early radiation of anthropoid primates (Beard et al. 1994, 1996, 2004; Kay et al. 1997; Jaeger & Marivaux 2005; Bajpai et al. 2008). The various geographical scenarios for our basal history thus depend on the putative anthropoid status of Algeripithecus and Tabelia, these critical issues being linked to the fragmentary nature of their fossil remains.
In the framework of our paleontological project in the early Tertiary of North Africa, since 2003, we have focused our yearly field researches on the vast outcrops situated in the Gour Lazib area, including the Glib Zegdou outlier. Intensive survey of the red to yellow siltstones and sandstones of fluvial origin of the continental Glib Zegdou Formation has allowed the discovery of well-preserved craniodental remains of at least 30 eutherian species documenting several primates, hyraxes, rodents, elephant-shrews, insectivoran-grade mammals, chiropterans, creodonts and ‘condylarths’ (Adaci et al. 2007; Tabuce et al. 2007). The mammalian fauna from the Glib Zegdou Formation is currently the most diverse from the entire Afro–Arabian Eocene. The new fossil primates demonstrate that Azibius is a senior synonym of Tabelia and that Dralestes is invalid because its hypodigm includes specimens of both Azibius and Algeripithecus. Our study reveals a close relationship between Azibius and Algeripithecus, which are now the only known valid genera within the Azibiidae. The phylogenetic study of azibiids highlights their strepsirhine affinities and rejects the anthropoid status of Algeripithecus as a result.
2. Systematic paleontology
Order Primates, Linnaeus, 1758
Suborder Strepsirhini, Geoffroy, 1812
Family Azibiidae, Gingerich, 1976
(a). Included genera
Azibius Sudre, 1975 and Algeripithecus Godinot & Mahboubi, 1992.
(b). Emended diagnosis
Azibiids (figures 1 and 2) differ from all other primates in having high, mesio-distally aligned P3–4 cusps, forming a blade-like structure; P3–4 also present a degree of overlapping and a mesial crown elevation associated with an inclination of the lingual cingulid. On M1–2, azibiid traits are the distally offset metaconid, the absence of hypoconulid, and the narrow sub-circular talonid basin, which is open lingually by a deep notch in front of the entoconid. The P3–4 have a peak-shaped lateral profile resulting from the labially inclined protocone and the very high and vertical sharp edge aspect of the ectoloph. The bunodont M1–2 have a large hypocone, a thick lingual cingulum, and lack a distinct metaconule.
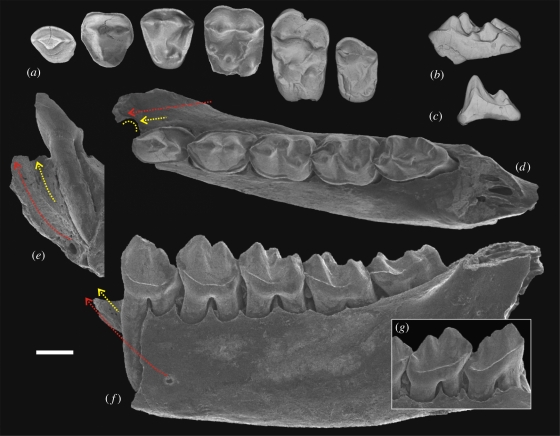
Figure 1.
Algeripithecus minutus. (a) Composite of isolated P2 (HGL50/297, reversed), P3 (HGL50/298, reversed), P4 (HGL50/299, reversed), M1 (GZC7), M2 (GZC1) and M3 (HGL50-321) in occlusal views. (b) M2 (GZC1). (c) P4 (HGL50/299) in mesial and distal views, respectively. (d–f) Mandible with P3–M3 and alveoli for C–P2 (denoted by red and yellow arrows, respectively) (HGL50/397) (d) in occlusal, (e) mesial and (f) labial views. (g) P3–4 (HGL50/397) in lingual view. Scale bar, 1 mm.
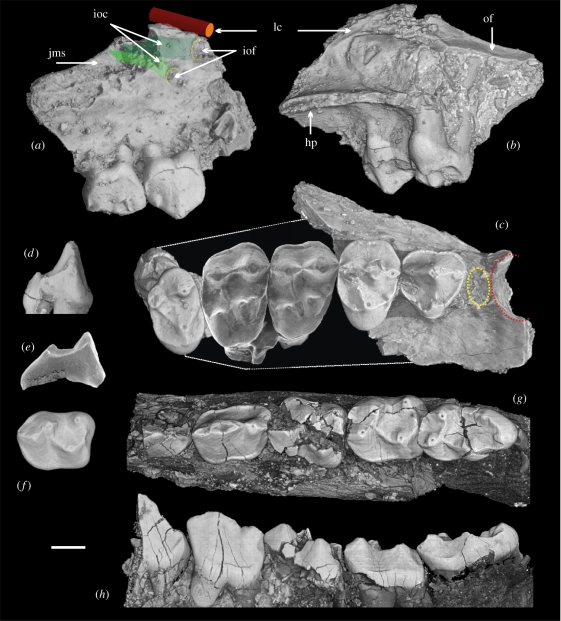
Figure 2.
Azibius trerki. (a,b) Maxilla with P3–4 (HGL51/46) in labial (a) and disto-lingual (b) views. (c) Composite of maxilla with M1–2 (HGL50/396, reversed), P3–4 and M3 with alveoli for C–P2 (denoted by red and yellow circles, respectively) (HGL51/46) in occlusal view. (d) P3 (HGL51/46) and (e) P4 (GZC41) in distal view. (f) M1 (HGL50/248, reversed) in occlusal view. (g,h) Mandible with P3–M3 (HGL50/256) in occlusal (g) and labial (h) views. Abbreviations: hard palate, hp; infra-orbital canals, ioc; infra-orbital foramens, iof; jugo-maxillary suture, jms; lacrimal canal, lc; orbital floor, of. Scale bar, 1 mm.
(c). Remarks
Algeripithecus is smaller than Azibius (table S2 in the electronic supplementary material). Based on the various available equations, deriving from the regressions of M1 area against body mass of living primates (e.g. Conroy 1987; Bajpai et al. 2008), the estimated body mass of Algeripithecus ranges from 65 to 85 g and Azibius from 115 to 160 g. Algeripithecus differs also from Azibius by rather less bunodont teeth, a higher metaconid (or possibly protostylid) on P3–4, a longer trigonid and shorter talonid on M1, a smaller third lobe on M3, the lack of both paraconule and parastyle on P4, and by a more reduced M3. Azibiids are only known from the late early or early middle Eocene (Mahboubi 1995; Mebrouk & Feist 1999; Adaci et al. 2007) of the Glib Zegdou Formation, Gour Lazib area, Algeria. However, another taxon, close to Algeripithecus, could also be present in the contemporaneous site of Chambi, Tunisia (Seiffert et al. in press).
3. Results and discussion
(a). The euprimate affinities of Azibiidae
Sudre (1975) described Azibius as a possible ‘paromomyiform’ (=‘Plesiadapiformes’). Later, Gingerich (1976) suggested Azibius is a euprimate (primates of modern aspect), and erected the Azibiinae, a new subfamily of Adapidae. He thereby rejected the assumption proposed by Szalay (1975) according to which Azibius was a hyopsodontid-like condylarth. More recently, Holroyd & Simons (1991) have reinforced the adapid status of Azibius, while Hartenberger et al. (1997) have suggested a macroscelidid affinity. Finally, after the discovery of Dralestes, which was considered to be the sister taxon of Azibius, Tabuce et al. (2004) suggested that azibiids are related to carpolestid plesiadapiforms, reviving Sudre's initial attribution. This hypothesis was criticized by Godinot (2006) and Silcox (2008), who favoured euprimate affinities for azibiids. The abundant new material reveals that Dralestes is invalid because its hypodigm contains specimens of both Azibius and Algeripithecus (table S1 in the electronic supplementary material). The supposed carpolestid affinities of Azibius relied on the morphology of the P4, which are enlarged in both taxa compared to the molars, exodaenodont (a lobe of enamel overhangs the labial side of the roots), and show apical cusps aligned anteroposteriorly in a blade-like structure. However, from a detailed comparison, Silcox (2008) suggested that this blade-like morphology is non-homologous between Azibius and carpolestids. We agree with her opinion because the P3 of azibiids, previously unknown, are also enlarged, exodaenodont, high-crowned and have a similar shearing morphology as the P4, while the P3 of carpolestids is reduced and lacks the blade-like morphology. Most importantly, the new material reveals that the upper tooth originally thought to be the M2 of ‘Dralestes’ (GZC-41, holotype) is in fact a P4 of Azibius. This new dental allocation clearly precludes any carpolestid affinities for azibiids. The bona fide upper and lower molars of Azibius show, as do those of Algeripithecus, a euprimate morphology.
(b). Testing the anthropoid status of Algeripithecus and Azibius
Algeripithecus was successively considered as a propliopithecid, a proteopithecid or a parapithecoid anthropoid (Godinot & Mahboubi 1992; Godinot 1994; Seiffert et al. 2005a, in press). Its upper molars strongly resemble those of Biretia, a primitive parapithecoid from the late middle Eocene of Algeria and the early late Eocene of Egypt (de Bonis et al. 1988; Seiffert et al. 2005a). Common traits include a similar degree of bunodonty, the same occlusal outline, the robustness and the postero-lingual position of the hypocone, and the important development of the lingual cingulum (figure 1a). Upper molars of Biretia differ in having an uninterrupted lingual cingulum and enlarged conules. Further differences that are evident on premolars and molars clearly distinguish parapithecoids from azibiids: P2 of Biretia is three-rooted, P4 is less molariform due to the absence of the metacone, the roots of P3–4 are oblique in orientation, their crown is low, their metaconid is offset lingually and the entoconid is present on P4; the paraconid occurs on M1, the metaconid is transverse to the protoconid on M2–3, the cristid obliqua reaches the trigonid wall at a more labial point, the hypoconulid is enlarged and central on the postcristid of M1–2, and M3 is reduced. Azibiids also lack some dental features of later parapithecoids (Abuqatrania, Apidium and Parapithecus; Seiffert et al. in press) such as the bulbous conules on upper molars and the absence of lower molar protocristids. Other basal anthropoid families such as proteopithecids and eosimiids are also distinct from azibiids, and all dental resemblances represent only primitive retentions or convergences (see text 2 in the electronic supplementary material). Azibiids also differ from most omomyiforms by their large hypocone and the loss of the paraconid. Some superficial similarities on lower premolars with microchoerines and anaptomorphines are the anterior elevation of the thick basal cingulid and the pronounced overlapping of P3–4 (Godinot 2006). However, the buccolingually enlarged and molariform P4 of omomyiforms are clearly distinct from the mesiolingually elongated and blade-like P4 of azibiids.
In addition to this purely dental evidence, the newly recovered fossils of Algeripithecus and Azibius include dentary and maxilla, which are decisive in reconstructing the affinities of azibiids. The maxilla of Azibius lacks P1 and shows above P2–3 a very anterior position of the infraorbital foramina (IOF) (figure 2a–c). In occlusal view, the lateral maxillary broadening starts at the level of the P2. By comparisons with extant and extinct primates, these characters indicate that Azibius possesses a short rostrum. The pronounced curve and the thickening of the mandible under P4 in Algeripithecus are also indicative of a short jaw (figure 1d). Besides, Azibius presents a reduced suborbital depth of the maxilla above P4. This feature is also observed in the omomyid Necrolemur, in the platyrrhine Aotus, as well as in most small and medium-sized modern strepsirhines (e.g. Loris, Galago, Microcebus). Tarsius and Biretia megalopsis show an extreme compression of the suborbital region in having a complete orbitopalatal fusion and an exposure of the lingual roots of molars in the orbit floor (Seiffert et al. 2005a). The reduced suborbital depth of the maxilla and the orbitopalatal fusion are observed in primates having large orbits and a nocturnal activity pattern. Comparative scans performed using conventional and synchrotron microtomography (see text 3 in the electronic supplementary material) of the maxilla through the lingual P4 root of some of these primates (see figure S1 in the electronic supplementary material) show that Azibius is comparable to Aotus and intermediate in condition between Tarsius and Loris, thereby suggesting very large orbits in Azibius probably associated with a nocturnal activity pattern. The cumulative area (0.74 mm2) of the two IOFs in Azibius is greater than it is in Tarsius, most small anthropoids and living strepsirhines of equivalent weight (e.g. Galago senegalensis, Loris tardigradus; Muchlinsky 2008; Rossie et al. 2006). Several omomyiforms (e.g. Shoshonius, Necrolemur) have a large IOF, like Azibius. In mammals, the IOF transmits the infraorbital nerve and a small artery to the maxillary region; a large IOF is correlated with an increase in vibrissa number, which is characteristic of nocturnal species (Muchlinsky 2008). The large IOF in Azibius thus indicates a fine sensory acuity of the face, and strengthens the hypothesis of a nocturnal activity pattern. Comparative three-dimensional microtomographic reconstructions show that the course of the lacrimal canals in Azibius is oblique rostroventrally (figure 2a–b) as in all living strepsirhines, adapiforms (e.g. Adapis) and omomyiforms (e.g. Microchoerus; figure 3). In contrast, Tarsius and all anthropoids (Rossie et al. 2006), including taxa with long snouts such as Theropithecus, show a lacrimal canal that is vertical and oriented perpendicular to the infraorbital canal (figure 3). The maxilla of Azibius, although partially preserved, thus reveals distinctive cranial traits that allow us to exclude anthropoid affinities for azibiids.
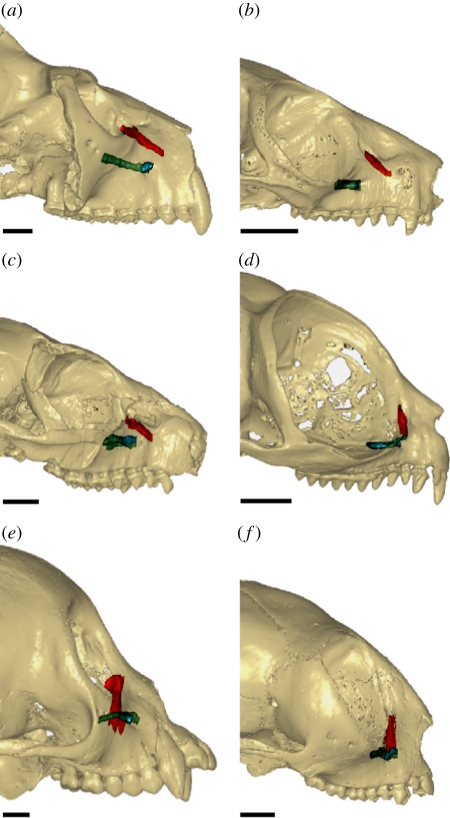
Figure 3.
Variability in the configuration of the lacrimal and infraorbital canal in extant and extinct primates (virtual three-dimensional surfaces derived from µCT image data; see text 3 and table S3 in the electronic supplementary material). (a) Adapis sp., (b) Microcebus murinus, (c) Microchoerus erinaceus, (d) Tarsius syrichta, (e) Cebus apella and (f) Aotus trivirgatus. Note how these canals tend to be perpendicular in anthropoids and Tarsius, but form a narrower angle in Azibius trerki (figure 2), crown and stem strepsirrhines and Microchoerus, an extinct omomyiform primate. Scale bar, 5 mm.
Consequently, the first unquestionable occurrence of anthropoids in Afro–Arabia is the parapithecoid Biretia from the late middle to early late Eocene of Algeria (Bir El Ater) and Egypt (Birket Qarun Locality 2) (de Bonis et al. 1988; Seiffert et al. 2005a). This result, along with the recent discovery of a putative early Eocene eosimiid in India (Bajpai et al. 2008; see also Rose et al. 2009) and the well-supported basal position of this primate family within the anthropoids (Kay et al. 1997; Jaeger & Marivaux 2005; Bajpai et al. 2008), seems to support a South Asian origin for anthropoids (Beard 2004, 2006) and a subsequent dispersal into Africa during the middle Eocene. Such a mammalian dispersal event between Africa and South Asia has also been proposed for several groups including anthracotheriid artiodactyls, and anomaluroid and hystricognathous rodents (Tabuce & Marivaux 2005; Gheerbrant & Rage 2006).
However, this paleobiogeographical issue for the early evolution of anthropoids is complicated by (i) the morphological gap between African parapithecoids and Asian eosimiids and (ii) the poorly documented omomyiform Altiatlasius from the late Paleocene of Morocco (Sigé et al. 1990), which was recently reconsidered either as a stem primate (Tabuce et al. 2004; Marivaux 2006; Silcox 2008) or a possible eosimiid-like anthropoid (Beard 2004, 2006; Seiffert et al. 2005a; Marivaux 2006; Bajpai et al. 2008; our phylogenetic results, see figure S2 in the electronic supplementary material). As such, Altiatlasius could be the earliest anthropoid, a record that equivocally supports an African origin of anthropoids during the late Paleocene, or their early dispersal into Africa from Asia, at least during the Paleocene (Beard 2006). This would extend the root of the Anthropoidea clade back to the Paleocene. In the absence of any undisputed Altiatlasius descendant in Africa during the early to middle Eocene (with the exception of two half-teeth, putatively related to Altiatlasius, from the Lutetian of Morocco (Tabuce et al. 2005), a Paleocene African origin of anthropoids would imply a big gap of about 20 Myr in the anthropoid fossil record in Africa, pre-dating the first appearance of the earliest parapithecoids. The existence of such a long ghost lineage seems speculative given the debate surrounding the phylogenetic affinities of Altiatlasius. Indeed, the extremely fragmentary nature of Altiatlasius cautions against over-interpreting its affinities, especially in light of the example of Algeripithecus.
(c). Higher-level affinities of the Azibiidae
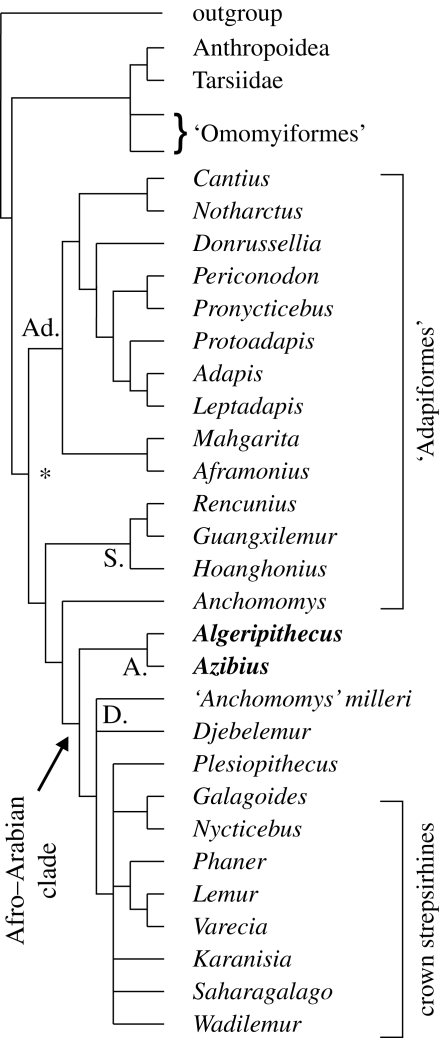
Phylogenetic analyses of primates based on craniodental and postcranial characters show that azibiids are nested within the Strepsirhini clade, setting Algeripithecus clearly apart from the Anthropoidea clade. The analysis reveals two major groups within Strepsirhini (figure 4): (i) the paraphyletic adapiforms mainly known from the Eocene of Europe, North America and Asia (Godinot 1998; Marivaux et al. 2006); and (ii) an Eocene–Oligocene Afro–Arabian clade that gave rise to living crown strepsirhines (lemurs, lorises, galagos) (Seiffert et al. 2003, 2005b; Godinot 2006). Within this Afro–Arabian clade, azibiids are successive sister taxa of djebelemurines (Djebelemur, Hartenberger & Marandat 1992; ‘Anchomomys’ milleri, Simons 1997b) and a group that embraces Plesiopithecus (Simons 1992; Simons & Rasmussen 1994a) and crown strepsirhines, including their late Eocene–Oligocene fossil representatives from the Fayum, that is, Wadilemur, Saharagalago and Karanisia (Seiffert et al. 2003, 2005b; figure 4), and possibly Omanodon and Shizarodon from the Oligocene of Oman (Gheerbrant et al. 1993; Godinot 2006). Also, noteworthy is the putative lemuriform Bugtilemur from the Asian Oligocene, which is now regarded as a peculiar adapiform without direct affinities with crown strepsirhines (Marivaux et al. 2006). Within the paraphyletic adapiforms, the European cercamoniine Anchomomys appears to be the sister taxa of the Afro–Arabian strepsirhine clade. Anchomomys does not present, however, the derived characters of the Paleogene Afro–Arabian taxa, notably the overlapping and the anterior coronal elevation of lower premolars. The tree topology could be explained by some convergences on molars between Anchomomys species and ‘Anchomomys’ milleri, hence the initial name given to this Fayum primate (see also Seiffert et al. 2005b). Considering the middle Eocene age of the oldest Anchomomys and the much earlier age of both azibiids and Djebelemur, it is difficult to conceive that a European ‘anchomomyine’ lineage, having developed a hypocone, gave rise to the Afro–Arabian strepsirhine clade.
Figure 4.
Phylogenetic position of the azibiids Algeripithecus and Azibius (in bold) within the Strepsirhini clade (denoted by the asterisk). This topology results from an analysis, including 83 primate taxa and 341 dental, cranial and postcranial characters, having generated 369 equally most parsimonious trees of 2851 steps each (consistency index, CI = 0.1887; retention index, RI = 0.5335) (see text 2 and figure S2 in the electronic supplementary material). Abbreviations: Azibiidae, A.; Adapidae, Ad.; Djebelemurinae, D.; Sivaladapidae, S.
Within this group, the djebelemurines and crown strepsirhines have more derived lower molars than the azibiids by the development of a mesial fovea built by the connection of the long curved paracristid with the premetacristid. Except on the M3 of Azibius, where this mesial fovea occurs, azibiids show instead a short premetacristid on M2–3 (generally absent on M1), which is usually isolated from the paracristid. Lower molars of Omanodon and Plesiopithecus also display this incomplete mesial fovea. Furthermore, azibiids along with Djebelemur, Plesiopithecus and Wadilemur differ from Karanisia, Saharagalago and ‘Anchomomys’ milleri by the absence of the posterolingually protruding entoconid lobe on lower molars; in Azibius the entoconid is moderately expanded posteriorly on the M1. On upper molars, azibiids are more primitive than crown strepsirhines by the association of a larger paraconule, a symmetrical crown without waisting of the distal border, and by the absence of the connection between the mesiolingually directed hypometaconulecrista and the postprotocrista (except on some M1 of Algeripithecus). Azibius and Plesiopithecus differ in numerous premolar and molar traits but they share bunodont molars and, more importantly, a cranium with a short rostrum and very large orbits indicative of a nocturnal activity pattern. As many adapiforms are diurnal according to their rather small orbit sizes, they appear much more distant from crown strepsirhines than azibiids. This is critical regarding the ancestry of crown strepsirhines as this group was indeed considered as emerging from a nocturnal lineage (e.g. Godinot 2006). However, recent results based on opsin genes have shown that ancestral strepsirhines were diurnal or cathemeral, and that nocturnality has evolved several times within crown strepsirhines and haplorhines as well (Tan et al. 2005; Ankel-Simons & Rasmussen 2008). Despite the apparent lability of nocturnality, it is worth pointing out that some stem strepsirhines, such as Azibius, have already achieved this trait early in the Tertiary.
(d). The early diversity of ‘pre-toothcombed’ primates in Africa
The lower toothrow of crown strepsirhines is characterized by an unusual incisor–canine toothcomb, in which the incisiform canine is reduced and strongly procumbent (Rosenberger & Strasser 1985). Wadilemur and Karanisia provide the earliest fossil evidence for such a lower dental structure (Seiffert et al. 2003, 2005b). Djebelemurines are considered to be stem strepsirhines because they do not develop the toothcomb, their canine being only moderately reduced. The anterior dentition of azibiids is unknown, but the alveoli in front of P3 in the mandible of Algeripithecus show that the canine alveolus is long, thin and forwardly inclined, suggesting that this tooth would be incisiform and procumbent (figure 1d–f). Such a canine may indicate that azibiids were toothcombed primates. The broadening of the maxilla at the level of the large canine in Azibius is also characteristic of the dental arcade morphology of some extant strepsirhines. However, as azibiids seem to diverge cladistically before djebelemurines (figure 4), a group without the toothcomb, this implies either the absence of a ‘true’ toothcomb in azibiids or its secondary loss in djebelemurines, a hypothesis that appears unlikely. Even if further evidence is required to ascertain the presence of a ‘true’ toothcomb in azibiids, it appears that Algeripithecus is unique among early Paleogene African primates documented to date in having a long and procumbent lower canine. This morphology seems compatible with the incisor–canine functional unit that pre-dates the toothcomb of crown strepsirhines. The toothcomb of crown strepsirhines is coupled with a P2, which is often caniniform and commonly higher and/or larger than the other premolars. These derived traits, observed in Miocene and Recent strepsirhines (Fleagle 1999), are achieved as early as the late Eocene in Wadilemur (Seiffert et al. 2005b). The tall and sub-caniniform P2, along with the loss of P1 and the reduced and procumbent canine, were proposed as synapomorphies of crown strepsirhines (Rasmussen & Nekaris 1998). Therefore, even if Algeripithecus possesses the two latter traits, its reduced P2 seems to preclude direct relationships between azibiids and crown strepsirhines. However, this character is not as definite as Karanisia (a crown strepsirhine or even a stem lorisiform based on molar characters) and seems also to present a reduced P2, thereby suggesting that the toothcomb could pre-date the specialization of the P2 within Paleogene Afro–Arabian strepsirhines.
Thus, the question of whether azibiids are stem or crown strepsirhines is unresolved based on the known morphology of their front dentition. However, the phylogenetic analysis assessed on the global morphological evidence suggests that azibiids are the earliest offshoot of stem strepsirhines to the exclusion of adapiforms. Azibiids are furthermore characterized by numerous autapomorphic features such as a pronounced bunodonty of molars, a large hypocone, a loss of paraconid and high-crowned posterior premolars forming a blade-like structure. As Plesiopithecus does by its very large procumbent lower canine, azibiids exemplify, therefore, an aberrant group of stem strepsirhines, reinforcing the diversity, unsuspected for a long time, of Afro–Arabian Paleogene strepsirhines. The apparent high degree of specialization of the azibiid family, associated with its late early or early middle Eocene age reveals the antiquity of this Afro–Arabian clade. As a result, we cannot exclude the possibility that this clade is rooted in a primitive, yet unknown, African lineage older than the earliest Eocene. This working hypothesis is tantalizing, especially in view of the age of Altiatlasius, testifying to the presence of primates as early as the late Paleocene in Africa. The divergence time of crown strepsirhines, estimated by recent molecular analysis as between 67 and 84 Ma (Horvath et al. 2008), and the possibility that Algeripithecus displays a true toothcomb are two interesting arguments in favour of the great antiquity of ‘pre-toothcombed’ primates in Africa.
Aknowlegements
The vice-chancellors of Tlemcen and Oran Universities, the authorities from Bechar and Tindouf districts, assisted fieldwork in the Gour Lazib area. We thank also the staff of beamlines ID19 and ID17 from the ESRF, P. Wyss (EMPA, Dübendorf) and C. Zollikofer (AIM, Zurich) for access to µCT facilities. We thank A.-L. Charruault and A. Ramdarshan for technical assistance, M. Vianey-Liaud and N. Mestres-Francès (UM2, Montpellier), M. Ponce de León (AIM), and C. Denys and J. Cuisin (MNHN, Paris) for access to comparative material. We are grateful to E. R. Seiffert and K. C. Beard for suggestions and helpful comments on the manuscript. This research was supported by the French ANR-PALASIAFRICA Program (ANR-08-JCJC-0017), CNRS-Eclipse and CS-UM2 grants. This is ISE-M publication 2009-091.
References
- Adaci M., et al. 2007Nouveaux sites à vertébrés paléogènes dans la région des Gour Lazib (Sahara Nord-occidental, Algérie). C. R. Palevol. 6, 535–544 (doi:10.1016/j.crpv.2007.09.001) [Google Scholar]
- Ankel-Simons F., Rasmussen D. T.2008Diurnality, nocturnality and the evolution of primate visual systems. Yearbook Phys. Anthro. 137, 100–117 (doi:10.1002/ajpa.20957) [DOI] [PubMed] [Google Scholar]
- Bajpai S., Kay R. F., Williams B. A., Das D. P., Kapur V. V., Tiwari B. N.2008The oldest Asian record of Anthropoidea. Proc. Natl Acad. Sci. USA 105, 11093–11098 (doi:10.1073/pnas.0804159105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard K. C.2004The hunt for the dawn monkey: unearthing the origins of monkeys, apes and humans Berkeley, CA: University of California Press [Google Scholar]
- Beard K. C.2006Mammalian biogeography and anthropoid origin. In Primate biogeography (eds Lehman S. M., Fleagle J. G.), pp. 439–467 New York, NY: Springer [Google Scholar]
- Beard K. C., Qi T., Dawson M. R., Wang B., Li C.1994A diverse new primate fauna from middle Eocene fissure-fillings in southeastern China. Nature 368, 604–609 (doi:10.1038/368604a0) [DOI] [PubMed] [Google Scholar]
- Beard K. C., Tong Y., Dawson M. R., Wang J., Huang X.1996Earliest complete dentition of an anthropoid primate from the Late–Middle Eocene of Shanxi province, China. Science 272, 82–85 (doi:10.1126/science.272.5258.82) [Google Scholar]
- Bonis L. de, Jaeger J.-J., Coiffait B., Coiffait P.-E.1988Découverte du plus ancien primate catarrhinien connu dans l'Eocène supérieur d'Afrique du Nord. C. R. Acad. Sci. Paris 306, 929–934 [Google Scholar]
- Conroy G. C.1987Problems of body-weight estimation in fossil primates. Int. J. Primato. 8, 115–137 (doi:10.1007/BF02735160) [Google Scholar]
- Fleagle J. G.1999Primate adaptation and evolution, 2nd edition San Diego, CA: Academic Press [Google Scholar]
- Gheerbrant E., Rage J.-C.2006Paleobiogeography of Africa: How distinct from Gondwana and Laurasia? Palaeogeogr. Palaeoclimateol. Palaeoecol. 241, 224–246 [Google Scholar]
- Gheerbrant E., Thomas H., Roger J., Sen S., Al-Sulaimani Z.1993Deux nouveaux primates dans l'Oligocène inférieur de Taqah (Sultanat d'Oman): premiers Adapiformes (?Anchomomyini) de la péninsule arabique? Palaeovertebrata 22, 141–201 [Google Scholar]
- Gingerich P. D.1976Cranial anatomy and evolution of early Tertiary Plesiadapidae (Mammalia, Primates). Pap. Paleont. 15, 1–141 [Google Scholar]
- Godinot M.1994Early North African primates and their significance for the origin of simiiformes (=Anthropoidea). In Anthropoid origins (eds Fleagle J. G., Kay R. F.), pp. 235–295 New York, NY: Plenum Press [Google Scholar]
- Godinot M.1998A summary of Adapiform systematics and phylogeny. Folia Primatol. 69, 218–249 (doi:10.1159/000052715) [Google Scholar]
- Godinot M.2006Lemuriform origins as viewed from the fossil record. Folia Primatol. 77, 446–464 (doi:10.1159/000095391) [DOI] [PubMed] [Google Scholar]
- Godinot M., Mahboubi M.1992Earliest known simian primate found in Algeria. Nature 357, 324–326 (doi:10.1038/357324a0) [DOI] [PubMed] [Google Scholar]
- Godinot M., Mahboubi M.1994Les petits primates simiiformes de Glib Zegdou (Eocène inférieur à moyen d'Algérie). C. R. Acad. Sci. Paris 319, 357–364 [Google Scholar]
- Hartenberger J.-L., Marandat B.1992A new genus and species of an early Eocene primate from North Africa. J. Hum. Evol. 7, 9–16 [Google Scholar]
- Hartenberger J.-L., Crochet J.-Y., Martinez C., Feist M., Godinot M., Mannai Tayech B., Marandat B., Sigé B.1997Le gisement de mammifères de Chambi (Eocène, Tunisie centrale) dans son contexe géologique. Apport à la connaissance de l′évolution des mammifères en Afrique. In Actes du congrès BiochroM'97 (eds Aguilar J.-P., Legendre S., Michaux J.), pp. 263–274 Montpellier, France: Mém. Trav. EPHE, Inst. Montpellier [Google Scholar]
- Holroyd P. A., Simons E. L.1991The phyletic relationships of Azibius. Am. J. Phy. Anthro. 12, 94 [Google Scholar]
- Horvath J. E., Weisrock D. W., Embry S. L., Fiorentino I., Balhoff J. P., Kappeler P., Wray G. A., Willard H. F., Yoder A. D.2008Development and application of a phylogenomic toolkit: resolving the evolutionary history of Madagascar's lemurs. Genome Res. 18, 489–499 (doi:10.1101/gr.7265208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J.-J., Marivaux L.2005Shaking the earliest branches of anthropoid primate evolution. Science 310, 244–245 (doi:10.1126/science.1118124) [DOI] [PubMed] [Google Scholar]
- Kay R. F., Ross C. F., Williams B. A.1997Anthropoids origins. Science 275, 797–804 (doi:10.1126/science.275.5301.797) [DOI] [PubMed] [Google Scholar]
- Mahboubi M.1995Etude géologique et paléontologique des formations continentales paléocènes et éocènes d'Algérie. Oran: Université d'Oran, Institut des Sciences de la Terre [Google Scholar]
- Marivaux L.2006The eosimiid and amphipithecid primates (Anthropoidea) from the Oligocene of the Bugti Hills (Balochistan, Pakistan): new insight into early higher primate evolution in South Asia. Paleovertebrata 34, 29–109 [Google Scholar]
- Marivaux L., Chaimanee Y., Tafforeau P., Jaeger J.-J.2006A new strepsirrhine primate from the late Eocene of peninsular Thailand (Krabi). Am. J. Phy. Anthro. 130, 425–434 (doi:10.1002/ajpa.20376) [DOI] [PubMed] [Google Scholar]
- Mebrouk F., Feist M.1999Nouvelles charophytes de l'Eocène continental de l'Algérie. Géol. Méditerranéenne 26, 29–45 [Google Scholar]
- Muchlinsky M. N.2008The relationship between the infraorbital foramen, infraorbital nerve and maxillary mechanoreception: implications for interpreting the paleoecology of fossil mammals based on infraorbital foramen size. Anat. Record 291, 1221–1226 [DOI] [PubMed] [Google Scholar]
- Rasmussen D. T., Nekaris K. A.1998Evolutionary history of lorisiform primates. Folia Primatol. 69, 250–285 [DOI] [PubMed] [Google Scholar]
- Rose K. D., Rana R. S., Sahni A., Kumar K., Missiaen P., Singh L., Smith T.2009Early Eocene primates from Gujarat, India. J. Hum. Evol. 56, 366–404 (doi:10.1016/j.jhevol.2009.01.008) [DOI] [PubMed] [Google Scholar]
- Rosenberger A. L., Strasser E.1985Toothcomb origins: support for the grooming hypothesis. Primates 26, 73–84 (doi:10.1007/BF02389048) [Google Scholar]
- Rossie J. B., Ni X., Beard K. C.2006Cranial remains of an Eocene tarsier. Proc. Natl Acad. Sci. USA 103, 4381–4385 (doi:10.1073/pnas.0509424103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffert E. R., Simons E. L., Attia Y.2003Fossil evidence for an ancient divergence of lorises and galagos. Nature 422, 421–424 (doi:10.1038/nature01489) [DOI] [PubMed] [Google Scholar]
- Seiffert E. R., Simons E. L., Clyde W. C., Rossie J. B., Attia Y., Brown T. M., Chatrath P. S., Mathison M. E.2005aBasal anthropoids from Egypt and the antiquity of Africa's higher primate radiation. Science 310, 300–304 (doi:10.1126/science.1116569) [DOI] [PubMed] [Google Scholar]
- Seiffert E. R., Simons E. L., Ryan A. S., Attia Y.2005bAdditional remains of Wadilemur elegans, a primitive stem galagid from the late Eocene of Egypt. Proc. Natl Acad. Sci. USA 102, 11396–11401 (doi:10.1073/pnas.0505310102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffert E. R., Simons E. L., Fleagle J. G., Godinot M.In press Paleogene anthropoids. In Cenozoic mammals of Africa (eds Sanders W. J., Werdelin L.). Berkeley, CA: University of California Press [Google Scholar]
- Sigé B., Jaeger J.-J., Sudre J., Vianey-Liaud M.1990Altiatlasius koulchii n. gen. et sp., primate omomyidé du Paléocène supérieur du Maroc, et les origines des euprimates. Palaeontographica 214, 31–56 [Google Scholar]
- Silcox M. T.2008The biogeographic origins of primates and euprimates: east, west, north or south of Eden? In Mammalian evolutionary morphology: a tribute to Fredrick S. Szalay (eds Sargis E. J., Dagosto M.), pp. 199–231 Dordrecht, The Netherlands: Springer [Google Scholar]
- Simons E. L.1992Diversity in the early Tertiary anthropoidean radiation in Africa. Proc. Natl Acad. Sci. USA 89, 10743–10747 (doi:10.1073/pnas.89.22.10743) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons E. L.1995Skulls and anterior teeth of Catopithecus (Primates, Anthropoidea) from the Eocene and Anthropoid origins. Science 268, 1885–1888 (doi:10.1126/science.7604261) [DOI] [PubMed] [Google Scholar]
- Simons E. L.1997aPreliminary description of the cranium of Proteopithecus sylviae, an Egyptian Late Eocene anthropoidean primate. Proc. Natl Acad. Sci. USA 94, 14970–14975 (doi:10.1073/pnas.94.26.14970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons E. L.1997bDiscovery of the smallest Fayum Egyptian primates (Anchomomyini, Adapidae). Proc. Natl Acad. Sci. USA 94, 180–184 (doi:10.1073/pnas.94.1.180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons E. L., Rasmussen D. T.1994aA remarkable cranium of Plesiopithecus teras (Primates, Prosimii) from late Eocene of Egypt. Proc. Natl Acad. Sci. USA 91, 9946–9950 (doi:10.1073/pnas.91.21.9946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons E. L., Rasmussen D. T.1994bA whole new world of ancestors: Eocene anthropoideans from Africa. Evol. Anthro. 3, 128–139 (doi:10.1002/evan.1360030407) [Google Scholar]
- Sudre J.1975Un prosimien du Paléogène ancien du Sahara nord-occidental: Azibius trerki n. g. n. sp. C. R. Acad. Sci. Paris 280, 1539–1542 [Google Scholar]
- Szalay F. S.1975Phylogeny, adaptations and dispersal of the tarsiiform primates. In A phylogeny of the primates. A multidisciplinary approach (eds Luckett W. P., Szalay F. S.), pp. 357–404 New York, NY: Plenum Press [Google Scholar]
- Tabuce R., Adnet S., Cappetta H., Noubhani A., Quillevéré F.2005Aznag (bassin d'Ouarzazate, Maroc), nouvelle localité à sélaciens et mammifères de l'Eocène d'Afrique. Bull. Soc. Géol. France 176, 381–400 (doi:10.2113/176.4.381) [Google Scholar]
- Tabuce R., Mahboubi M., Tafforeau P., Sudre J.2004Discovery of a highly specialized Plesiadapiformes (Mammalia, Primates) in the Eocene of Africa. J. Hum. Evol. 47, 305–321 (doi:10.1016/j.jhevol.2004.08.005) [DOI] [PubMed] [Google Scholar]
- Tabuce R., Marivaux L.2005Mammalian interchanges between Africa and Eurasia: an analysis of temporal constraints for the plausible Paleogene anthropoid dispersions. Anthropol. Sci. 112, 27–32 [Google Scholar]
- Tabuce R., Marivaux L., Adaci M., Bensalah M., Hartenberger J.-L., Mahboubi M., Mebrouk F., Tafforeau P., Jaeger J.-J.2007Early Tertiary mammals from North Africa reinforce the molecular Afrotheria clade. Proc. R. Soc. B 274, 1159–1166 (doi:10.1098/rspb.2006.0229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y., Yoder A. D., Yamashita N., Li W.-H.2005Evidence from opsin genes rejects nocturnality in ancestral primates. Proc. Natl Acad. Sci. USA 102, 14712–14716 (doi:10.1073/pnas.0507042102) [DOI] [PMC free article] [PubMed] [Google Scholar]