Abstract
Cerebral lateralization refers to the division of information processing in either hemisphere of the brain and is a ubiquitous trait among vertebrates and invertebrates. Given its widespread occurrence, it is likely that cerebral lateralization confers a fitness advantage. It has been hypothesized that this advantage takes the form of enhanced cognitive function, potentially via a dual processing mechanism whereby each hemisphere can be used to process specific types of information without contralateral interference. Here, we examined the influence of lateralization on problem solving by Australian parrots. The first task, a pebble-seed discrimination test, was designed for small parrot species that feed predominately on small seeds, which do not require any significant manipulation with the foot prior to ingestion. The second task, a string-pull problem, was designed for larger bodied species that regularly use their feet to manipulate food objects. In both cases, strongly lateralized individuals (those showing significant foot and eye biases) outperformed less strongly lateralized individuals, and this relationship was substantially stronger in the more demanding task. These results suggest that cerebral lateralization is a ubiquitous trait among Australian parrots and conveys a significant foraging advantage. Our results provide strong support for the enhanced cognitive function hypothesis.
Keywords: parrots, behaviour, discrimination, lateralization, problem solving, cognition
1. Introduction
For over a century, cerebral lateralization (the division of information processing in either hemisphere of the brain) was considered to be a uniquely human trait because of its close relationship with the control of speech and other ‘higher order’ cognitive functions (Corballis 2002). Recent studies, however, have shown that it is a ubiquitous characteristic found in a wide range of animals and it is likely to have ancient evolutionary origins (Vallortigara 2000; Byrne et al. 2002; Rogers & Vallortigara 2008).
Cerebral lateralization can be readily quantified by examining traits such as hand preferences while manipulating objects or eye preferences when viewing certain scenes. This is because the stimuli emitted from an object or scene are preferentially processed in one hemisphere of the brain or the other, and there is often a high concordance between lateralized motor control and information processing (Rogers 2009). In a foraging context, for example, the hemisphere used to discriminate between food and non-food items may cause the preferential use of an associated limb to manipulate potential food items (Rogers & Anson 1979; Andrew 1988; Rogers et al. 2004). Thus, measuring hand and eye preferences provide an excellent, non-invasive method of quantifying cerebral lateralization.
Historically, most studies have focused on neurological mechanisms underlying lateralization, but recently there has been a surge of interest in identifying its function and potential fitness benefits (Ghirlanda & Vallortigara 2004; Brown 2005; Vallortigara & Rogers 2005; Brown et al. 2007; Ghirlanda et al. 2009). The presence of lateralized information processing can have varying influences on the everyday behaviour of animals, such as their social interactions, foraging and anti-predator behaviour. For example, baboons are more aggressive to individuals on their left-hand side (Casperd & Dunbar 1996); toads are more likely to strike at moving prey items in the right hemifield (Vallortigara et al. 1998) and fish from high predation areas prefer to inspect predators using their right eye (Brown et al. 2004).
Rogers (2000) suggested that enhanced cognitive ability is one of the potential benefits of cerebral lateralization because animals with strongly lateralized brains may have the ability to act directly on many sources of information at the same time. To date, only a few studies have experimentally examined this hypothesis. Lateralized individuals are better able to distinguish food grains from pebbles compared with non-lateralized individuals (Gunturkun et al. 2000), and this disparity is enhanced in the presence of predators (Rogers et al. 2004). Similarly, chimpanzees that fish for termites using one hand are more efficient than ambidextrous individuals (Marchant & McGrew 1996). So far, however, studies to determine whether lateralization is related to higher order problem-solving abilities have been largely restricted to humans and primates (Horster & Ettlinger 1985; Bradshaw 1988; Gazzaniga 2000).
Lateralization has been extensively studied in the chicken as a model for understanding the underlying neurodevelopment in the avian brain (Rogers & Andrew 2002). Among birds, corvids and parrots are renowned for their cognitive capacity (Griffin & Speck 2004; Pepperberg 2004; Kacelnik et al. 2006), but surprisingly there have been limited observations of lateralized behaviours in these species. New Caledonian crows have a lateralized approach to tool making (Hunt et al. 2001), and many species of parrot have strong preferences for using the left or right foot while manipulating food items both at the species and individual level (Friedmann & Davis 1938; Rogers 1980; Harris 1989; C. Brown 2009, unpublished data). Thus, these taxa present an ideal opportunity for addressing questions pertaining to the possible link between lateralization and enhanced cognitive function.
Here, we used 40 individuals from eight species of Australian parrots to investigate the effect of lateralization on the ability to solve a discrimination task and a novel problem. The two tasks differed in the degree of difficulty. The first task was a relatively simple discrimination task that required the parrots to pick out seeds from a background of similar sized pebbles. The second task was far more cognitively demanding: obtaining a food item suspended on the end of a string. This latter task requires multiple, coordinated beak–foot actions to gain access to the food reward and the generation of an understanding of the functional relationship between the reward, the string and themselves. Success in this task has been used previously to assess problem-solving skills in a range of avian species (Pepperberg 2004; Heinrich & Bugnyar 2005). We explicitly tested the enhanced cognition hypothesis and expected that strongly lateralized individuals would outperform non-lateralized individuals in both tasks. Further, we expected the relationship between lateralization and cognitive performance would be substantially stronger in the more demanding task.
2. Material and methods
Five individual captive-reared, adult parrots from each of eight species were examined. Multiple species were employed to illustrate the ubiquitous nature of the trait in Australian parrots and maximize the range of lateralized individuals in the dataset. Each species also differs in its primary diet, which can be broadly split into small or large seed eating groups (Lindsey 1998; McNaughton 2004) (table 1). Small seeds, such as grass seeds, rarely require manipulation with the foot prior to ingestion, whereas large seed pods, such as those from banksias or eucalypts, are frequently held by the foot and manipulated in tandem with the beak. With these broad foraging categories in mind, two tasks were designed to examine the relationship between foraging success and lateralization. The first task was a relatively simple discrimination test and the second task was a more difficult task requiring repeated beak–foot coordinated actions.
Table 1.
Study species information.
| species | common name | primary diet | feeding mode |
|---|---|---|---|
| Nymphicus hollandicus | cockatiel | small seeds from native or cultivated plants | beak only |
| Melopsittacus undulatus | budgerigar | small seeds of grass tussocks | beak only |
| Eolophus roseicapilla | galah | small seeds of native or cultivated grasses | beak and foot |
| Callocephalon fimbriatum | gang-gang cockatoo | large seeds of native trees | beak and foot |
| Calyptorhynchus banksii | red-tailed black cockatoo | large seeds of native trees | beak and foot |
| Cacatua galerita | sulphur-crested cockatoo | large seeds, nuts and bulbs | beak and foot |
| Alisterus scapularis | Australian king parrot | large seeds, berries, fruits and nuts | beak and foot |
| Polytelis swainsonii | superb parrot | large seeds, eucalyptus blossoms and fruits | beak and foot |
(a). Quantification of laterality
Our prior observations indicated that four of the test species were primarily left footed, two species were primarily right footed and the remaining two were non-lateralized (C. Brown 2009, unpublished data), and thus including these species in the analysis potentially provided a broad range of lateralized individuals. Individual variation also exists within this broad species level pattern; therefore, it was necessary to quantify the foot and eye preferences of each individual prior to testing. The pattern and strength of cerebral lateralization of each individual was established by examining their foot and eye preferences while manipulating food objects. Foot preferences were first established by observing which foot each parrot used to manipulate pieces of fruit offered on the end of a platform over a 10 min interval. If individuals did not adequately complete this task, a container of seed was offered in association with a small perch. In order to access the seed, subjects had to hold on to the perch with one foot and the seed container with the other foot. The primary foot used to manipulate the fruit or hold the seed container over each feeding trial was recorded. The primary eye used to fixate on the food was also noted during these observations. The primary foot and eye used during each trial was defined as that used to manipulate or fixate on the food for more than 50 per cent of the foraging trial. An individual was given a score of 1 for being primarily left biased or 0 for being primarily right biased during each trial. Each individual was tested 10 times, foot and eye preferences were summed to give a laterality score ranging from 0 to 10 (i.e. 100% right–100% left preference). This score was then transposed to proportion data ranging from 0 to 100 per cent left preference to aid interpretation. In addition, an absolute laterality score was calculated to examine the strength of lateralization irrespective of the preference direction (the absolute value of |laterality score −5|), which ranged from 0 (i.e. not lateralized) to 5 (i.e. very strongly lateralized).
(b). Experiment 1: pebble-seed discrimination task
All 40 subjects were tested in the pebble-seed discrimination task (sensu Rogers et al. 2004) to analyse the relationship between the extent of eye lateralization and visual foraging performance. This task required the birds to peck at 35 seeds scattered on a background on to which 50 small pebbles had been adhered. The pebbles and seeds were similar in colour and shape. This test is of particular interest because it presented the parrot with a situation similar to that frequently encountered in their home cages. The birds were not fed prior to the task and were thus highly motivated to forage. The number of seeds eaten and the number of pecks in 2.5 min were tabulated. Discrimination performance was calculated as the number of consumed seeds divided by the total number of pecks. Each bird was tested once. A polynomial regression was used to examine the relationship between the pattern of eye lateralization and discrimination performance at the level of the individual. The association between the strength of lateralization and seed-pebble discrimination performance was examined using a linear regression. It was expected that strongly lateralized individuals would be more successful in discriminating seeds from pebbles than weakly lateralized individuals.
(c). Experiment 2: string-pull problem
In the second task, subjects were tested on their ability to obtain an item suspended from a string, which involved multiple, coordinated beak–foot actions to succeed (Heinrich 1995). Here, a favourite food item was suspended at the end of a 50 cm string hung from the end of a perch. The birds were not fed prior to this task. If test subjects did not succeed, make an attempt or demonstrate interest within 5 min, the trial was terminated. Each bird was exposed to the apparatus 10 times and the proportion of successful food retrievals was recorded. A polynomial regression was used to determine whether the pattern of foot lateralization (as determined above) was related to individual success in obtaining the food item. The association between strength of lateralization and level of string-pulling success was also examined using a linear regression.
3. Results
(a). Foot and eye preferences in the test subjects
Examination of the foot and eye preferences revealed a large degree of variation between individuals. Some interesting discrepancies between eye and foot preferences were noted at the species level (table 2). Cockatiels, for example, showed strong left foot preferences while manipulating food items, but preferred to fixate on the food using their right eye. Similarly, all of the gang-gangs were very strongly lateralized in terms of foot preferences (100% left footed for all individuals) but were non lateralized in terms of eye preferences while viewing food items. For the remaining species, however, there was very strong concordance between foot and eye preferences (R2 > 0.95).
Table 2.
Mean eye and foot preferences for each of the eight study species while manipulating food objects and the associated success rates in the two tasks.
| species | left eye preference (mean% ± s.e.) | left foot preference (mean% ± s.e.) | discrimination success (mean% ± s.e.) | string-pull success (mean% ± s.e.) |
|---|---|---|---|---|
| N. hollandicus | 16 ± 5.1 | 90 ± 4.5 | 79.2 ± 4.41 | 0 ± 0 |
| M. undulatus | 46 ± 10.3 | 50 ± 15.8 | 68 ± 6.1 | 0 ± 0 |
| E. roseicapilla | 52 ± 12 | 58 ± 14.6 | 81.6 ± 2.7 | 66 ± 5.1 |
| C. fimbriatum | 58 ± 11.6 | 100 ± 0 | 79.4 ± 2.5 | 88 ± 5.8 |
| C. banksii | 86 ± 5.1 | 86 ± 6 | 85.4 ± 2.0 | 86 ± 5.1 |
| C. galerita | 88 ± 3.7 | 94 ± 4 | 84.2 ± 3.1 | 88 ± 4.9 |
| A. scapularis | 22 ± 5.8 | 12 ± 3.7 | 72.4 ± 3.7 | 82 ± 5.8 |
| P. swainsonii | 26 ± 4 | 38 ± 8.6 | 71 ± 4.3 | 62 ± 3.7 |
(b). Experiment 1: pebble-seed discrimination task
The pattern of eye lateralization exhibited by the individual test subjects was significantly related to pebble-seed discrimination performance explaining 35 per cent of the variance (F1,38 = 8.907, p = 0.004, R2 = 0.35; figure 1). Individuals with stronger eye asymmetries were more successful in discriminating seeds from pebbles (F1,38 = 12.622, p = 0.001, R2 = 0.25; figure 2). There were clear differences between each of the species tested (F7,32 = 2.844, p = 0.020), with three species, the budgerigar, king and superb parrots scoring particularly poorly (table 2; see table S3 in the electronic supplementary material). Success in this task was not related to foraging mode (F1,38 = 2.342, p = 0.134, figure 3).
Figure 1.
The relationship between the pattern of eye lateralization and pebble-seed discrimination performance. The different symbols represent individuals from eight different species (black diamond, C. galerita; black square, E. roseicapilla; black triangle, C. fimbriatum; black circle, C. banksii; grey diamond, N. hollandicus; grey square, A. scapularis; grey triangle, P. swainsonii; grey circle, M. undulatus).
Figure 2.
The relationship between the strength of laterality and pebble-seed discrimination performance. The different symbols represent individuals from eight different species (black diamond, C. galerita; black square, E. roseicapilla; black triangle, C. fimbriatum; black circle, C. banksii; grey diamond, N. hollandicus; grey square, A. scapularis; grey triangle, P. swainsonii; grey circle, M. undulatus).
Figure 3.
Mean ± s.e. pebble-seed discrimination success for each of the eight species of Australian parrots tested. Species depicted in hatched bars forage using their beak alone, while those in grey bars forage using both their beaks and feet in coordinated movements.
(c). Experiment 2: string-pull problem
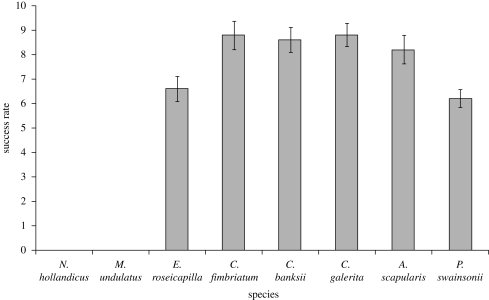
The success rate of the string-pull problem varied significantly between species (F7,32 = 72.053, p < 0.001, figure 4, table 2). All six species that successfully mastered the vertical string-pulling task use their feet to manipulate food items extensively during foraging. One sulphur-crested cockatoo, two gang-gangs, one red-tailed black cockatoo and one Australian king parrot solved the string-pull problem on their first exposure. Despite the fact that the galah (E. roseicapilla) reportedly feeds on small seeds, individuals from this species regularly used their feet to manipulate potential food items during our observations. The remaining two species failed the task entirely; cockatiel (N. hollandicus) and budgerigar (M. undulatus), both of which feed on small seeds and do not use their feet when feeding. Individuals from the species that failed the task were excluded from the regression analysis examining the relationship between foot laterality and success in the string-pull task.
Figure 4.
Mean ± s.e. success in solving the string-pull task for each of the eight species of Australian parrots tested. Each individual was tested 10 times. Species depicted in hatched bars forage using their beak alone, while those in grey bars forage using both their beaks and feet in coordinated movements. Only the latter species solved the task, hence no hatched bars are shown.
Regression analysis revealed that the pattern of individual foot laterality exhibited by the birds was strongly related to success in obtaining an item suspended on the end of a string, explaining over 60 per cent of the variation (F1,27 = 11.342, p = 0.002, R2 = 0.61; figure 5). The association between individual strength of lateralization and success in the string-pulling problem was also highly significant (F1,28 = 48.534, p < 0.001, R2 = 0.63; figure 6).
Figure 5.
The relationship between the pattern of foot lateralization and success at the string-pull task. Species that failed the task are not shown. The different coloured points represent individuals from six different species (black diamond, C. galerita; black square, E. roseicapilla; black triangle, C. fimbriatum; black circle, C. banksii; grey square, A. scapularis; grey triangle, P. swainsonii).
Figure 6.
The relationship between individual strength of lateralization and success at the string-pull task. Species that failed the task are not shown. The different coloured points represent individuals from six different species (black diamond, C. galerita; black square, E. roseicapilla; black triangle, C. fimbriatum; black circle, C. banksii; grey square, A. scapularis; grey triangle, P. swainsonii).
4. Discussion
It has been proposed that the ubiquitous nature of cerebral lateralization is indicative of its role in key fitness traits such as foraging or predator avoidance (Brown et al. 2004; Vallortigara & Rogers 2005). Here we have shown that strongly lateralized parrots were more adept at discriminating between food versus non-food items and solving a complex problem than non-lateralized parrots. It was revealed that the pattern of lateralization (left or right bias) played little role in the probability of success in these tasks, rather the strength of lateralization was the primary predictor of performance. Thus, parrots with very strong foot or eye biases were more adept at solving both tasks than individuals with limited laterality. Moreover, individual laterality scores explained a substantially greater amount of the variance in performance in the more cognitively demanding task. Our results support the notion that cerebral lateralization serves to increase cognitive capacity generally, perhaps through enhanced simultaneous processing of multiple sources of information (Gunturkun et al. 2000; Rogers et al. 2004).
The results of the pebble-seed discrimination task showed a large variance in individual success rate, ranging from 55 per cent for non-lateralized individuals to 95 per cent for strongly lateralized individuals, representing an almost twofold difference in discrimination ability. It is unlikely that this variance in performance stems from differential visual perception processes because all subjects had experienced seeds numerous times prior to testing. Nor was there any indication that the performance of any of the birds was limited by the number of seeds on offer (see table S3 in the electronic supplementary material). The potential for discriminating between food and non-food items has obvious fitness benefits, and it is clear that an increase in visual asymmetry enhances success in visually guided foraging tasks. Interestingly, these results only partially support findings from previous studies. Strongly lateralized pigeons tested on a similar task also showed enhanced foraging success, supporting the notion that strong lateralization leads to a general enhancement of cognitive ability (Gunturkun et al. 2000). Lateralized chicks, however, performed the pebble-seed discrimination task better than non-lateralized chicks but only when they were simultaneously exposed to a predator (Rogers et al. 2004). Rogers and colleagues argue that strongly lateralized chicks employ their left hemisphere for solving the foraging task, while using their right hemisphere for predator vigilance at the same time. The subtle differences in findings between the studies may be a reflection of the study species and methodologies employed. A predator was not deployed by Gunturkun et al. (2000) or in the present study; however, it is likely that the presence of an unfamiliar observer may have been perceived as a potential threat, thus the subjects may have been suffering from divided attention at the time of testing in both studies. These conditions closely mimic those experienced by animals in their natural environment where the need to pay attention to multiple stimuli and process them simultaneously is paramount.
In contrast to the pebble-seed discrimination task, the string-pull problem represents a far more complex problem probably requiring higher order cognitive processes for successful completion. In the present study, five parrots from four species solved the problem on their first exposure. Previous studies have assessed ‘insight’ in avian species using the same apparatus as that used here and suggested that birds which successfully complete the task on first exposure demonstrate an understanding of intentional means-end behaviour (Heinrich 1995; Pepperberg 2004; Heinrich & Bugnyar 2005). That is, the subjects have an appreciation of a cause–effect relationship between the string, the food reward and themselves and immediately understand how to solve the problem. In such a task, it is evident that multiple sources of information must be acted on simultaneously while also performing coordinated motor skills. As expected from the enhanced cognition theory, strongly lateralized parrots solved this problem far more successfully than non-lateralized parrots. Indeed, 63 per cent of the variation in problem-solving ability was explained by the strength of an individual's laterality. Thus, it was evident that the strength of lateralization was the primary factor driving success in the string-pull problem and was exemplified by the fact that one fully left and one fully right footed individual solved the task on every exposure (figure 5).
Only those species of parrots that used their feet while foraging solved the string-pull problem. Studies on ravens exposed to this task suggest that an understanding of means-end relationships or causal understanding is not the only factor involved in solving the problem. It is likely that prior experience with similar tasks (both during ontogeny and over evolutionary time) and learning capacity also play a role (Heinrich 1995). Analysis of function has been shown in New Caledonian crows, which regularly make and use tools to forage on otherwise inaccessible prey (Hunt 1996; Kacelnik et al. 2006). Captive-reared ravens regularly pull up a string to lift up a food reward but failed a counterintuitive task where they were required to pull down on a string to lift up a food reward (Heinrich & Bugnyar 2005), suggesting that the task has to bear some similarity to the types of problems individuals frequently face in the wild. Similarly, chimpanzees know how to stack boxes, reach for food and pick up sticks and can combine all these elements in order to solve a novel problem. Rumbaugh et al. (1996) use the term ‘emergents’ to set this type of insight-based behaviour apart from trial and error learning.
The use of the feet to manipulate objects when foraging provides a significant advantage in the string-pull problem because solving the task requires beak–foot coordination. Prior experience of similar tasks during ontogeny is likely to aid the development of the appropriate motor skills and the repeated use of the same foot may facilitate this process (Marchant & McGrew 1996). When taken together, our results suggest that success at this task requires a blend of fine motor coordination, cognitive processing power and prior experience with similar problems. Those species that failed to solve this task rarely use their feet during foraging, probably never encounter similar problems in their natural environment and, therefore, lack the capacity to deal with them (Healy 1992; Heinrich & Bugnyar 2005).
In summary, individual parrots with strongly lateralized brains solved both a simple discrimination task and a more complex problem more efficiently than parrots with less strongly lateralized brains. These data support the notion that cerebral lateralization is strongly associated with important fitness traits such as foraging efficiency and anti-predator behaviour (Brown et al. 2004), and probably explains its widespread occurrence in the animal kingdom.
Acknowledgements
This project was conducted with permission from the Macquarie University Ethics Board, permit number 2007/034.
We thank the large number of staff from various zoos, animal parks and pet stores, as well as parrot breeders for permitting us to conduct research on their birds. Also thanks to M. D. Magat for participating in data collection.
References
- Andrew R. J.1988The development of visual lateralization in the domestic chick. Behav. Brain Res. 29, 201–209 (doi:10.1016/0166-4328(88)90025-3) [DOI] [PubMed] [Google Scholar]
- Bradshaw J. L.1988The evolution of human lateral asymmetries: new evidence and second thoughts. J. Hum. Evol. 17, 615–637 (doi:10.1016/0047-2484(88)90088-7) [Google Scholar]
- Brown C.2005Cerebral lateralisation, ‘social constraints,’ and coordinated anti-predator responses. Behav. Brain Sci. 28, 591–592 (doi:10.1017/S0140525X05240104) [Google Scholar]
- Brown C., Gardner C., Braithwaite V. A.2004Population variation in lateralized eye use in the poeciliid Brachyraphis episcopi. Proc. R. Soc. Lond. B 271, S455–S457 (doi:10.1098/rsbl.2004.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C., Western J., Braithwaite V. A.2007The influence of early experience on, and inheritance of, cerebral lateralization. Anim. Behav. 74, 231–238 (doi:10.1016/j.anbehav.2006.08.014) [Google Scholar]
- Byrne R. A., Kuba M., Griebel U.2002Lateral asymmetry of eye use in Octopus vulgaris. Anim. Behav. 64, 461–468 (doi:10.1006/anbe.2002.3089) [Google Scholar]
- Casperd J. M., Dunbar R. I. M.1996Asymmetries in the visual processing of emotional cues during agonistic interactions by gelada baboons. Behav. Process. 37, 57–65 (doi:10.1016/0376-6357(95)00075-5) [DOI] [PubMed] [Google Scholar]
- Corballis M. C.2002From hand to mouth: the origins of language Princeton, NJ: Princeton University Press [Google Scholar]
- Friedmann H., Davis M.1938‘Left-handedness’ in parrots. Auk 55, 478–480 [Google Scholar]
- Gazzaniga M. S.2000Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain 123, 1293–1326 (doi:10.1093/brain/123.7.1293) [DOI] [PubMed] [Google Scholar]
- Ghirlanda S., Vallortigara G.2004The evolution of brain lateralization: a game theoretical analysis of population structure. Proc. R. Soc. Lond. B 271, 853–857 (doi:10.1098/rspb.2003.2669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirlanda S., Frasnelli E., Vallortigara G.2009Intraspecific competition and coordination in the evolution of lateralization. Phil. Trans. R. Soc. B 364, 861–866 (doi:10.1098/rstb.2008.0227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. R., Speck G. B.2004New evidence of animal consciousness. Anim. Cogn. 7, 5–18 (doi:10.1007/s10071-003-0203-x) [DOI] [PubMed] [Google Scholar]
- Gunturkun O., Diekamp B., Manns M., Nottelmann F., Prior H., Schwarz A., Skiba M.2000Asymmetry pays: visual lateralization improves discrimination success in pigeons. Curr. Biol. 10, 1079–1081 (doi:10.1016/S0960-9822(00)00671-0) [DOI] [PubMed] [Google Scholar]
- Harris L. J.1989Footedness in parrots: three centuries of research, theory, and mere surmise. Can. J. Psychol. 43, 369–396 [DOI] [PubMed] [Google Scholar]
- Healy S.1992Optimal memory: towards an evolutionary ecology of animal cognition. Trends Ecol. Evol. 7, 399–400 (doi:10.1016/0169-5347(92)90019-8) [DOI] [PubMed] [Google Scholar]
- Heinrich B.1995An experimental investigation of insight in common ravens (Corvus corax). Auk 112, 994–1003 [Google Scholar]
- Heinrich B., Bugnyar T.2005Testing problem solving in ravens: string-pulling to reach food. Ethology 111, 962–976 (doi:10.1111/j.1439-0310.2005.01133.x) [Google Scholar]
- Horster W., Ettlinger G.1985An association between hand preference and tactile discrimination performance in the Rhesus monkey. Neuropsychologia 23, 411–413 (doi:10.1016/0028-3932(85)90027-2) [DOI] [PubMed] [Google Scholar]
- Hunt G. R.1996Manufacture and use of hook-tools by New Caledonian crows. Nature 379, 249–251 (doi:10.1038/379249a0) [Google Scholar]
- Hunt G. R., Corballis M. C., Gray R. D.2001Laterality in tool manufacture by crows. Nature 414, 707 (doi:10.1038/414707a) [DOI] [PubMed] [Google Scholar]
- Kacelnik A., Chappell J., Weir A. A. S., Kenward B.2006Cognitive adaptations for tool-related behaviour in New Caledonian crows. In Comparative cognition: experimental explorations of animal intelligence (eds Wasserman E. A., Zentall T. R.), pp. 515–528 Oxford, UK: Oxford University Press [Google Scholar]
- Lindsey T.1998Parrots of Australia Sydney, NSW: New Holland Publishers [Google Scholar]
- Marchant L. F., McGrew W. C.1996Laterality of limb function in wild chimpanzees of Gombe National Park: comprehensive study of spontaneous activities. J. Hum. Evol. 30, 427–443 (doi:10.1006/jhev.1996.0036) [Google Scholar]
- McNaughton M.2004Australian parrots and finches Melbourne, VIC: Bluestone Press [Google Scholar]
- Pepperberg I. M.2004‘Insightful’ string-pulling in grey parrots (Psittacus erithacus) is affected by vocal competence. Anim. Cogn. 7, 263–266 (doi:10.1007/s10071-004-0218-y) [DOI] [PubMed] [Google Scholar]
- Rogers L. J.1980Lateralisation in the avian brain. Bird Behav. 2, 1–12 (doi:10.1016/j.anbehav.2003.12.014) [Google Scholar]
- Rogers L. J.2000Evolution of hemispheric specialization: advantages and disadvantages. Brain Lang. 73, 236–253 (doi:10.1006/brln.2000.2305) [DOI] [PubMed] [Google Scholar]
- Rogers L. J.2009Hand and paw preferences in relation to the lateralized brain. Phil. Trans. R. Soc. B 364, 943–954 (doi:10.1098/rstb.2008.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L. J., Andrew R. J.2002Comparative vertebrate lateralization New York, NY: Cambridge University Press [Google Scholar]
- Rogers L. J., Anson J. M.1979Lateralisation of function in the chicken fore-brain. Pharmacol. Biochem. Behav. 10, 679–686 (doi:10.1016/0091-3057(79)90320-4) [DOI] [PubMed] [Google Scholar]
- Rogers L. J., Zucca P., Vallortigara G.2004Advantages of having a lateralized brain. Proc. R. Soc. Lond. B 271, S420–S422 (doi:10.1098/rsbl.2004.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L. J., Vallortigara G.2008From antenna to antenna: lateral shift of olfactory memory in honeybees. PLoS One 3, e2340 (doi:10.1371/journal.pone.0002340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh D. M., Washburn D. A., Hillix W. A.1996Respondents, operants, and emergents: toward an integrated perspective on behavior. In Learning as a self-organizing process (eds Pribram K., King J.), pp. 57–73 Hillsdale, NJ: Erlbaum [Google Scholar]
- Vallortigara G.2000Comparative neuropsychology of the dual brain: a stroll through animals’ left and right perceptual worlds. Brain Lang. 73, 189–219 (doi:10.1006/brln.2000.2303) [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Rogers L. J.2005Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575–589 (doi:10.1017/S0140525X05000105) [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Rogers L. J., Bisazza A., Lippolis G., Robins A.1998Complementary right and left hemifield use for predatory and agonistic behaviour in toads. NeuroReport 9, 3341–3344 (doi:10.1097/00001756-199810050-00035) [DOI] [PubMed] [Google Scholar]