Abstract
Although the impact of environmental changes on the demographic parameters of top predators is well established, the mechanisms by which populations are affected remain poorly understood. Here, we show that a reduction in the thermal stratification of coastal water masses between 2005 and 2006 was associated with reduced foraging and breeding success of little penguins Eudyptula minor, major bio-indicators of the Bass Strait ecosystem in southern Australia. The foraging patterns of the penguins suggest that their prey disperse widely in poorly stratified waters, leading to reduced foraging efficiency and poor breeding success. Mixed water regimes resulting from storms are currently unusual during the breeding period of these birds, but are expected to become more frequent due to climate change.
Keywords: climate change, bio-indicator, thermocline, food webs, top predator
1. Introduction
There is an increasing interest in the use of top predators as bio-indicators of the state of marine ecosystems, to assess indirectly the impact of environmental changes on lower trophic levels (Verity et al. 2002). In the marine environment, top predators that periodically return to land to breed offer the opportunity to investigate changes in the availability of marine resources (Boyd & Murray 2001). Recent studies on the effects of climate change and the role of oceans in world climate regulation have concentrated on the associations of marine predators with large-scale, well-defined oceanic systems, such as oceanographic fronts at ocean basin scales (Cotté et al. 2007) or eddies on circumpolar scales (Biuw et al. 2007). In contrast, research on top predators exploiting coastal ecosystems clearly lacks a comprehensive overview. This is probably because most physical data are collected at scales that would not suit the examination of the biology of species with a reduced foraging range. Nevertheless, local environmental changes are often associated with large-scale phenomena such as changes in sea surface temperature (SST; Georges & Le Maho 2003), which can be compiled over vast areas. Data collected at the mesoscale (1–100 km) are required to understand the mechanisms that link predator–prey interactions to the properties of the habitat, and to predict the evolution of these near-shore systems in the forthcoming years of intensifying climate changes.
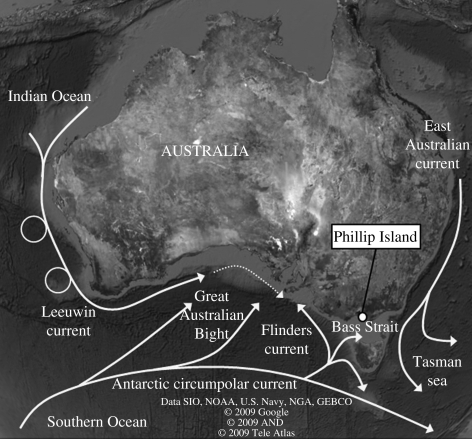
To address this question, we used animal-borne data-recording devices (Ropert-Coudert & Wilson 2005) to monitor the foraging effort and success of a coastal marine predator, the little penguin Eudyptula minor. We determined simultaneously biotic and abiotic characteristics of its environment at the microscale (1 m to 1 km) in terms of prey availability and vertical water temperature regimes. This approach ensures that physical data were collected at the same spatial and temporal scales as modifications in the penguins' behaviour occurred. Little penguins from Phillip Island (32°16′ S, 115°21′ E) that forage in northern Bass Strait, Australia, were a suitable model (figure 1) because the Bass Strait region experiences a great oceanographic variability. The waters of the Bass Strait on its western side are under the influence of two major flows: the Leeuwin current bringing SST anomalies of the Indian Ocean Dipole (IOD; Yamagata et al. 2004), and the Flinders current, which is itself under the influence of the variability of the Antarctic Circumpolar Wave through complex forcings (Middleton et al. 2007). The diving behaviour of the penguins was compared between two years of contrasting environmental conditions: October to December 2005 corresponded to a negative SST anomaly, and October to December 2006 corresponded to a positive SST anomaly (see an illustration of this on the Japanese weather bureau page, which presents SST anomalies provided by the NOAA: http://www.data.jma.go.jp/gmd/cpd/db/elnino/learning/faq/elnino_table.html). Although these anomalies were under the threshold above which they could qualify as La Niña and El Niño events, the SST patterns in 2005 and 2006 resembled those observed during weak La Niña and El Niño events.
Figure 1.
Location of the study site showing the major currents liable to influence the waters of the Bass Strait (excerpt from Google Earth).
2. Material and methods
Twenty penguins (10 in November 2005 and 10 in November 2006, in an equal sample of males and females) guarding 1- to 2-week-old chicks, and conducting one-day foraging trips within 20 km from the colony (Collins et al. 1999), were monitored each year between late October and mid-December. This sample size was defined using power analysis and considering limits regulated by the ethics permit. Breeding success at the level of the colony was measured as the number of chicks fledged per pair (details in Daniel et al. 2007); peak chick mass and initial linear chick growth were also determined (details in Chiaradia & Nisbet 2006).
Twelve-bit, 52 × 15 mm, four-channel data loggers (Little Leonardo, Japan) were used to record depth (resolution 0.05 m) and temperature (0.01°C) every second, and acceleration along the heave and surge axes of birds at 16 Hz. As each flipper beat is measured on the heave axis, we could clearly identify periods of higher frequency than the normal cruising frequency values observed during travel periods (3.16 Hz). Increased flipper beat frequency indicates that a prey has been encountered and chased (Ropert-Coudert et al. 2006a). Although this increase in flipper beat frequency is not a direct measure of prey ingestion, it reflects the rate at which prey are found in the water column, thus providing a good estimate of prey availability. Because our temperature sensors have a slow time response, T0.9 = 15 s, we corrected the temperature associated to depth depper than 5 m following Daunt et al. (2003). This correction allowed us to qualitatively compare changes in the temperature during dives between the two years. Diet samples were collected each year by stomach flushing (Wilson 1984) of ten birds at the same breeding stage, but from a neighbouring colony.
After testing the data for normality and log- or arcsine transformation, we compared diving parameters of birds using Student t-tests for single parameter and generalized linear mixed models, with birds as the random factor, sex and years as fixed factors, for repeated measurements. As dive duration and bottom phase duration depend on the maximum depth of dives, depth was added as a covariate to the models for these parameters. Analyses were conducted using JMP 8 (SAS Institute Inc.). Results are expressed as mean ± s.d. for single parameters, and as least-square mean ± s.e. The significance level was set at p = 0.05.
3. Results
The colony of little penguins at Phillip Island has been closely monitored three times a week since 2000 to determine breeding activity, in particular chick production and growth (Chiaradia & Nisbet 2006). In 2006, the chick production of 0.9 chicks per pair was markedly lower than the 1.4 chicks per pair produced in 2005. By comparison, from 2001 to 2006, chick production averaged 1.1 ± 0.3 chicks per pair (0.6–1.6). Chicks that survived to fledging were 72 g heavier in 2005 than in 2006 (mean 1167 and 1095 g, respectively; t119 = 2.95, p < 0.01), while they grew 5 g heavier per day in 2005 (t117 = 2.59, p < 0.01). Previous differences in these variables resulted mainly from unfavourable conditions at sea, which affect provisioning rates (Chiaradia & Nisbet 2006).
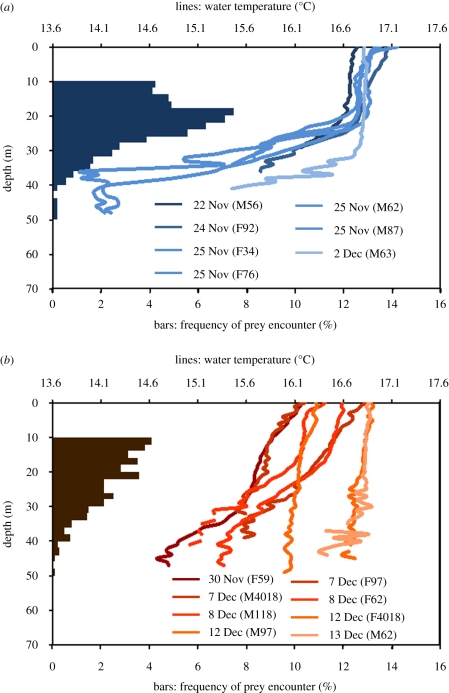
The foraging effort of little penguins did not differ statistically between 2005 and 2006. They performed a similar number of dives per day (t18 = −0.54, p = 0.60; see table 1 for values), which corresponded to a similar amount of time underwater per day (t18 = 0.52, p = 0.61; table 1). In 2005 and 2006, seven and eight penguins, respectively, dived deep enough (>20 m) to investigate the temperature variation in the water column. These birds dived on average to the same depths (F1,13 = 2.60, p = 0.13; table 1), their dive durations were not longer in 2005 than in 2006 (F1,13 = 4.21, p = 0.06; table 1) and the percentage of time spent around the maximum depth of their dives was also similar (F1,13 = 0.22, p = 0.65; table 1). However, the percentage of dives per day when prey was encountered dropped significantly from 19.45 ± 5.57% in 2005 to 12.84 ± 4.72% in 2006 (t13 = −2.49, p = 0.03). Similarly, the time allocated to prey pursuit was significantly lower in 2006 (6.08 ± 4.8% of the dive time) than in 2005 (13.33 ± 4.42%; t13 = −3.00, p = 0.01). The distribution of the depth at which prey were encountered in the water column differed markedly (figure 2). In 2005, the majority of the chases occurred at 20–25 m. Apart from being less numerous, the chases were also less concentrated in 2006, occurring from 10 to 30 m. Also in 2006, the diet of the birds showed an overall decrease in the mass of prey consumed than in 2005, with a particular decrease in abundance of their major prey, barracouta Thyrsites atun (A. Chiaradia, M. G. Forero, M. Cullen & K. A. Hobson 2009, unpublished data). In contrast, penguins consumed a large proportion of Gould's squid Nototodarus gouldi in 2006; a high abundance of squids is usually associated with low breeding success in little penguins (Cullen et al. 1991).
Table 1.
Summary of the dive parameters of male and female little penguins foraging off the coast of Phillip Island in two years of constrasting oceanic conditions (2005 and 2006). Results are presented as mean ± s.d. (number of dives, daily underwater time) or as least-square means ± s.e. (other parameters). The statistics values of tests on single parameters per individual are given in §3 (t-test). The results presented here are generalized linear mixed models.
| 2005 |
2006 |
statistics | |||
|---|---|---|---|---|---|
| males | females | males | females | ||
| number of dives | 608.5 ± 195.3 | 590.7 ± 165.8 | 567.8 ± 273.7 | 611.3 ± 81.8 | see text |
| daily underwater time (h) | 5.9 ± 1.7 | 4.8 ± 1.7 | 4.2 ± 2.3 | 5.1 ± 0.6 | see text |
| dive depth (m) | 8.4 ± 1.1 | 8.5 ± 1.2 | 5.8 ± 1.1 | 8.0 ± 1.1 | year: F1,11 = 2.97, p = 0.11 |
| sex: F1,11 = 1.97, p = 0.19 | |||||
| sex*year: F1,11 = 1.57, p = 0.24 | |||||
| dive duration (s) | 22.2 ± 1.0 | 16.3 ± 1.1 | 17.8 ± 1.0 | 18.1 ± 1.0 | year: F1,11 = 1.41, p = 0.26 |
| sex: F1,11 = 9.00, p = 0.01 | |||||
| sex*year: F1,11 = 11.07, p < 0.01 | |||||
| bottom phase (%) | 0.37 ± 0.02 | 0.26 ± 0.03 | 0.34 ± 0.02 | 0.31 ± 0.02 | year: F1,11 = 0.25, p = 0.62 |
| sex: F1,11 = 11.21, p < 0.01 | |||||
| sex*year: F1,11 = 1.57, p = 0.09 | |||||
Figure 2.
Temperature profiles (lines) of the water masses around Phillip Island, Australia, recorded by miniature bio-loggers attached to foraging little penguins. Note the clear thermal stratification of the water in (a) 2005 (with a thermocline around 20–25 m), compared with the situation in (b) 2006, a weak El Niño year (see text). Bars indicate the distribution of those depths where penguins encountered prey (bars at 0–10 m are not represented as the index of prey encounter at these depths is influenced by biomechanical factors related to the buoyancy of the animal).
This decrease in the foraging efficiency of the birds corresponded to a clear change in the thermal structure of the waters where birds foraged (figure 2). Although the mean temperature from 5 to 25 m depth did not vary between years (2005, 16.6 ± 0.3°C; 2006, 16.7 ± 0.1°C), the stratification was clearly different. In 2005, the water column was thermically stratified: the upper layer of the water column to approximately 22 m was well mixed as it was characterized by a uniform temperature, but this changed under 22 m as a thermocline was observed with an average vertical temperature gradient of 0.12 ± 0.03°C m−1. In contrast, in 2006, such a well-marked thermocline did not appear to exist. During the first days (30 November–8 December) of the observation period in 2006, a weakly mixed upper layer was present with a small temperature gradient (<0.03°C m−1) to at least 40 m. On 11 December, the water column was then thoroughly mixed by a strong breeze with average winds of 55.4 km h−1 (Beaufort scale 7, Australian Bureau of Meteorology). The water column then remained almost perfectly mixed down to 40 m for the duration of the study. The absence of a thermocline in the upper 40 m probably resulted from intense wind-mixing events induced by a series of storms occurring early in the 2006 breeding season (average wind speed in October 2006 at 15.00 h: 36.0 ± 13.1 km h−1, with 4 days >50 km h−1).
4. Discussion
The well-stratified waters observed in 2005 corresponded with a greater rate of prey encounter. Prey were probably less dispersed and this consequently led to a greater foraging success for little penguins. This suggests that the prey of little penguins was mainly concentrated at the thermocline, in a similar manner to Pacific bluefin tuna Thunnus orientalis, whose vertical migration is apparently an immediate response to the depth of a thermocline (Kitagawa et al. 2000). The association between enhanced prey capture and the presence of a thermocline at around 20–25 m depth needs to be regarded in the light of the maximum diving ability of little penguins being 66.7 m (Ropert-Coudert et al. 2006b), which is about 10 m above the seabed of the zone within the foraging range of the birds. The penguins rarely dive to such extreme depths and most of their diving effort takes place in the water mass above the thermocline. Also, breeding success of little penguins is negatively correlated with the bathymetry (depth) of the foraging area; that is, birds having access to shallower waters to forage generally present a higher breeding success than those that forage in deeper waters (Chiaradia et al. 2007). Any physical barriers reducing the escape field of prey, like bathymetry, might optimize the foraging efficiency of penguins. In this regard, the greater prey encounter rate of little penguins diving in stratified waters indicates that the thermocline may act as a physical barrier, either by reducing the fish escape field or by concentrating prey around the thermocline. The same behaviour—that is, predators diving preferentially at a thermocline—has been suggested at a greater oceanic scale for southern elephant seals Mirounga leonina (Boyd & Arnbom 1991). In contrast, the waters in 2006 were well mixed and little penguins encountered prey less frequently. The depth distribution of foraging dives in 2006 indicates that the distribution of prey in the water column was probably more diffuse. In summary, prey availability to top predators may be enhanced in stratified coastal waters but reduced in highly mixed waters.
Given the apparent negative effect that a reduced stratification has on the foraging ability of little penguins, we predict that such unfavourable conditions will occur more frequently in the future. It is now commonly accepted that climate change is accompanied by an increased likelihood of abrupt changes in ecosystems (IPCC 2007), similar to the unusual weather event that took place in the latter part of 2006 in Southern Victoria (see §3). It has been noted that during previous El Niño events (e.g. 1982) the westerly wind around Tasmania strengthened (Harris et al. 1987). This tendency for a strengthening of the westerlies in future climate change has been recently confirmed by model results (e.g. Cai et al. 2005). Stronger westerlies could contribute to an increased degree of vertical mixing in the upper layer of the water column in specific local zones (Kraus & Turner 1967). Note that, at the timescale of our study, one or two strong storms are enough to change the stratification characteristics without these isolated events reflecting the monthly or bi-monthly average conditions at which oceanographic data are collected.
Here, we developed a case study with little penguins, but our results may not be representative of all predators foraging on fish in coastal zones. However, our observations that a thermocline plays an important role in the distribution of marine resources, and may influence the foraging efficiency of predators, are similar to the observations reported for pelagic predators (fish, seals and an offshore penguin species; Boyd & Arnbom 1991; Kitagawa et al. 2000). Further studies are required in other areas, and the role of thermoclines in structuring marine ecosystems should be considered at different spatial and temporal scales. For instance, the recent suggestion to mix ocean water artificially in an attempt to help the planet ‘to heal’ itself (Lovelock & Rapley 2007) could have direct detrimental consequences at local scales on the presence of vertical gradients, and thus potentially on the foraging efficiency of top predators and the availability of their prey.
Acknowledgements
The experiments in this study were approved by Phillip Island Nature Park Animal Experimentation Ethics Committee under research permit numbers 10002669 and 10003419 from the Department of Sustainability and Environment, Victoria.
The authors thank BHP-Billiton, the JSPS and the Australian Academy of Science for financial support, P. Fallow and M. Salton for help in the field, P. Gaspar, Y. du Penhoat, G. Alory and especially G. Meyers, J. Risbey, and M. Pook for positive discussions on the atmospheric and oceanographic anomalies of Bass Strait. We also thank two anonymous referees for their comments and help in improving our manuscript tremendously.
References
- Biuw M., et al. 2007Variations in behavior and condition of a Southern Ocean top predator in relation to in situ oceanographic conditions. Proc. Natl Acad. Sci. USA 104, 13 705–13 710 (doi:10.1073/pnas.0701121104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd I. L., Arnbom T.1991Diving behaviour in relation to water temperature in the southern elephant seal: foraging implications. Polar Biol. 11, 259–266 (doi:10.1007/BF00238460) [Google Scholar]
- Boyd I. L., Murray A.2001Monitoring a marine ecosystem using responses of upper trophic level predators. J. Anim. Ecol. 70, 747–760 (doi:10.1046/j.0021-8790.2001.00534.x) [Google Scholar]
- Cai W., Shi G., Cowan T., Bi D., Ribbe J.2005The response of the southern annular mode, the east Australian current, and the southern mid-latitude ocean circulation to global warming. Geophys. Res. Lett. 32, L23706 (doi:10.1029/2005GL024701) [Google Scholar]
- Chiaradia A., Nisbet I. C.2006Plasticity in parental provisioning and chick growth in Little Penguins Eudyptula minor in years of high and low breeding success. Ardea 94, 257–270 [Google Scholar]
- Chiaradia A., Ropert-Coudert Y., Kato A., Mattern T., Yorke J.2007Diving behaviour of Little Penguins from four colonies across their whole distribution range: bathymetry affecting diving effort and fledging success. Mar. Biol. 151, 1535–1542 (doi:10.1007/s00227-006-0593-9) [Google Scholar]
- Collins M., Cullen J. M., Dann P.1999Seasonal and annual foraging movements of little penguins from Phillip Island, Victoria. Wildl. Res. 26, 705–721 (doi:10.1071/MU02020) [Google Scholar]
- Cotté C., Park Y.-H., Guinet C., Bost C.-A.2007Movements of foraging king penguins through marine mesoscale eddies. Proc. R. Soc. B 274, 2385–2391 (doi:10.1098/rspb.2007.0775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen J. M., Montague T. L., Hull C.1991Food of Little Penguins Eudyptula minor in Victoria: comparison of three localities between 1985 and 1988. Emu 91, 318–341 (doi:10.1071/MU9910318) [Google Scholar]
- Daniel T., Chiaradia A., Logan M., Quinn G. P., Reina R. D.2007Synchronized group association in little penguins Eudyptula minor. Anim. Behav. 74, 1241–1248 (doi:10.1016/j.anbehav.2007.01.029) [Google Scholar]
- Daunt F., Peters G., Scott B., Grémillet D., Wanless S.2003Rapid-response recorders reveal interplay between marine physics and seabird behaviour. Mar. Ecol. Prog. Ser. 255, 282–288 (doi:10.3354/meps255283) [Google Scholar]
- Georges J.-Y., Le Maho Y.2003Responses of marine and insular ecosystems to climate change. C. R. Geosci. 335, 551–560 (doi:10.1016/S1631-0713(03)00101-9) [Google Scholar]
- Harris G. P., Davies P., Nunez M., Meyers G.1987Interannual variability in climate and fisheries in Tasmania. Nature 333, 754–757 (doi:10.1038/333754a0) [Google Scholar]
- IPCC 2007Climate change 2007: the physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.). Cambridge, UK: Cambridge University Press [Google Scholar]
- Kitagawa T., Nakata H., Kimura S., Itoh T., Tsuji S., Nitta A.2000Effect of ambient temperature on the vertical distribution and movement of Pacific bluefin tuna Thunnus thynnus orientalis. Mar. Ecol. Prog. Ser. 206, 251–260 (doi:10.3354/meps206251) [Google Scholar]
- Kraus E. B., Turner J. S.1967A one-dimensional model of the seasonal thermocline. II. The general theory and its consequences. Tellus 19, 98–105 [Google Scholar]
- Lovelock J. E., Rapley C. G.2007Ocean pipes could help the Earth to cure itself. Nature 449, 403 (doi:10.1038/449403a) [DOI] [PubMed] [Google Scholar]
- Middleton J. F., et al. 2007El Niño effects and upwelling off South Australia. J. Phys. Oceanogr. 37, 2458 (doi:10.1175/JPO3119.1) [Google Scholar]
- Ropert-Coudert Y., Wilson R. P.2005Trends and perspectives in animal-attached remote sensing. Front. Ecol. Environ. 3, 437–444 (doi:10.1890/1540-9295(2005)003[0437:TAPIAR]2.0.CO;2) [Google Scholar]
- Ropert-Coudert Y., Kato A., Wilson R. P., Cannell B.2006aForaging strategies and prey encounter rate of free-ranging Little Penguins. Mar. Biol. 149, 139–148 (doi:10.1007/s00227-005-0188-x) [Google Scholar]
- Ropert-Coudert Y., Chiaradia A., Kato A.2006bAn exceptionally deep dive by a little penguin, Eudyptula minor. Mar. Ornithol. 34, 71–74 [Google Scholar]
- Verity P. G., Smetacek V., Smayda T. J.2002Status, trends and the future of the marine pelagic ecosystem. Environ. Conserv. 29, 207–237 (doi:10.1017/S0376892902000139) [Google Scholar]
- Wilson R. P.1984An improved stomach pump for penguins and other seabirds. J. Field Ornithol. 55, 109–112 [Google Scholar]
- Yamagata T., et al. 2004Coupled ocean–atmosphere variability in the tropical Indian Ocean. Geophys. Monogr. 147, 189–212 [Google Scholar]