Abstract
Background
In many parts of the world, the exponential growth rate of infections during the initial epidemic phase has been used to make statistical inferences on the reproduction number, R, a summary measure of the transmission potential for the novel influenza A (H1N1) 2009. The growth rate at the initial stage of the epidemic in Japan led to estimates for R in the range 2.0 to 2.6, capturing the intensity of the initial outbreak among school-age children in May 2009.
Methods
An updated estimate of R that takes into account the epidemic data from 29 May to 14 July is provided. An age-structured renewal process is employed to capture the age-dependent transmission dynamics, jointly estimating the reproduction number, the age-dependent susceptibility and the relative contribution of imported cases to secondary transmission. Pitfalls in estimating epidemic growth rates are identified and used for scrutinizing and re-assessing the results of our earlier estimate of R.
Results
Maximum likelihood estimates of R using the data from 29 May to 14 July ranged from 1.21 to 1.35. The next-generation matrix, based on our age-structured model, predicts that only 17.5% of the population will experience infection by the end of the first pandemic wave. Our earlier estimate of R did not fully capture the population-wide epidemic in quantifying the next-generation matrix from the estimated growth rate during the initial stage of the pandemic in Japan.
Conclusions
In order to quantify R from the growth rate of cases, it is essential that the selected model captures the underlying transmission dynamics embedded in the data. Exploring additional epidemiological information will be useful for assessing the temporal dynamics. Although the simple concept of R is more easily grasped by the general public than that of the next-generation matrix, the matrix incorporating detailed information (e.g., age-specificity) is essential for reducing the levels of uncertainty in predictions and for assisting public health policymaking. Model-based prediction and policymaking are best described by sharing fundamental notions of heterogeneous risks of infection and death with non-experts to avoid potential confusion and/or possible misuse of modelling results.
Background
The reproduction number, R, the average number of secondary cases generated by a typical (or "average") single primary case, of influenza A (H1N1) 2009 is a summary measure of the transmission potential in the population of interest. It has been estimated using the early epidemic growth data in different locations across the world [1-12]. The estimations have been based primarily on models that include one or a limited number of aspects of heterogeneous transmission. The scientific community has been attracted to R because it provides a first aggregated measure of the overall transmissibility of an emerging infection [13]. Further, the estimate of R, based on homogeneously or nearly homogeneously mixing population models that by design ignore most individual differences, is not only used to assess the initial growth of an epidemic but also the extent to which the population will experience infection by the end of a first pandemic wave [14,15]. Except for a unique study estimating R using a data set of international spread [7], the exponential growth rate, r, of cases during the initial epidemic phase has been investigated using a simple procedure that involves translating r into R through the use of the estimator R = 1/M(-r) where M(-r) is the moment-generating function of the generation time distribution, given the growth rate r [16]. Naturally, the higher the growth rate r of the number of cases, the larger the estimate of R.
The majority of R estimates for this ongoing pandemic have ranged from 1.1-1.8 [17] while our estimate of R in Japan was in the range of 2.0-2.6 under the assumption of a mean generation time of 1.9 days through May 2009 [5]. The most plausible reason for this estimate, as noted in our earlier study [5], involved the role of initial conditions as the very early growth was driven by the high contact rates that are common to school settings [17,18]. In addition to the phenomenological explanation, it is important to assess whether or not the methodology used to estimate R was adequate. We do this here using data that go beyond those used in our estimation of R for the earlier epidemic period of May 2009 in Japan. Here we provide an updated estimate of R for the novel influenza A (H1N1) 2009 in Japan, summarising the relevant methodological issues in estimating R from the growth rate of cases and initiating a dialogue on how estimates of the transmission potential should be shared with non-experts, including the general public.
Discussion
The epidemic data in Japan
Figure 1 shows the epidemic curve of influenza A (H1N1) 2009 for Japan from May to July 2009. Starting with the illness onset of an index case on 5 May, 4986 confirmed cases, all diagnosed by means of RT-PCR, were reported to the government during this period. On 22 July, the Ministry of Health, Labour and Welfare of Japan decided not to mandate its local health sectors to notify all the confirmed cases, and thereafter the local sectors gradually ceased counting all the cases. The first pandemic wave in Japan continued to grow steadily thereafter hitting the first peak in November [19].
Figure 1.
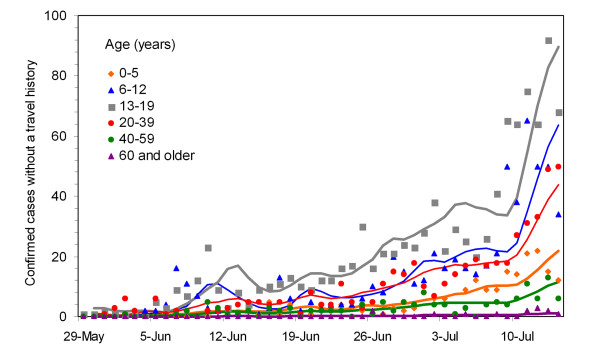
Temporal distribution of confirmed cases of influenza A (H1N1) 2009 virus infection in Japan from May to July 2009 (n = 3,480). All the confirmed cases were diagnosed by RT-PCR. The horizontal axis represents the date of onset. Cases are stratified by (A) age and (B) travel history. Here "cases with travel history" are associated with overseas travel within 10 days preceding onset of illness and those with such a history are referred to as imported cases in our analysis.
Since our original data indicate that the 97.5 percentile point of the reporting delay distribution (i.e., the time from illness onset to notification) is 8 days, we analyse a total of 3480 cases that developed the disease on or before 14 July. Figure 1A shows the temporal distribution stratified by age-group of all identified cases. Of the 3480 cases, 67.0% were among individuals 19 years of age or less. The population of those aged from 20-39 years accounted for 24.2% of the total, and the remaining (older adult) cases accounted for only 8.8%. The contributions from imported cases to the early epidemic growth in this island nation, in addition to the local (indigenous) transmissions, are also critical (Figure 1B). Of the 3840 confirmed cases, 694 (19.9%) had a history of overseas travel within 10 days preceding the onset of illness, and we refer to them as imported cases in the present study.
Growth rates of two different phases
We proceed to compare two different growth rates (Figure 2) in order to explore the patterns that led to our past R estimates for Japan in [5]. The growth rates of cases in the very initial phase (i.e., from 5 to 17 May), which corresponds to the period examined in our earlier study [5], and those that followed the generation of secondary cases caused by school clusters (i.e., from 29 May to 14 July) are compared. Over these periods we observe that the proportion of cases attributed to the 0-19 age grouping decreased from 83.0% to 67.0%.
Figure 2.
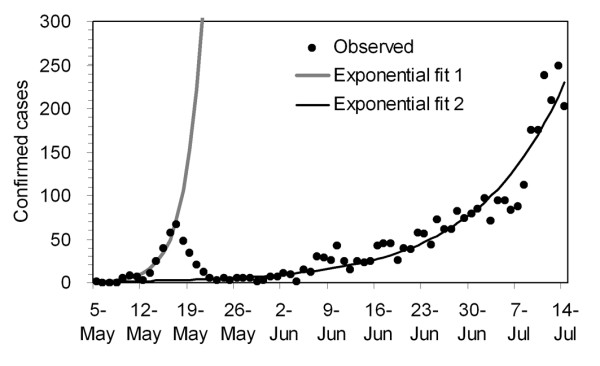
Simple extrapolation of the exponential growth of cases. Two exponential fits are compared with the observed number of confirmed cases. Exponential fit 1 employs the data set from 5 May to 17 May during which clusters of cases in a few high schools fuelled the epidemic. Exponential fit 2 draws the best fit to the data from 29 May to 14 July representing the spread of influenza into the wider population. The growth rates for fits 1 and 2 are estimated at 0.37 and 0.08 per day, respectively.
We model the expected value of the incidence of illness onset at calendar time t as E(c(t)) = kexp(r(t-τ)) where k is a constant, r is the growth rate of the corresponding period, and τ is the starting time point of exponential growth (assumed as 5 May and 29 May, respectively). Minimizing the sum of squares between the observed data and expectation, r is estimated as 0.37 and 0.08 per day, respectively, for the former and latter periods. The estimate for the former period is smaller than that reported in our earlier study in May (i.e., 0.47 per day) [5], because of our use of refined dates of onset and the use of a simpler statistical method in the present study. The estimates of the exponential growth rates differ by almost a factor of five (i.e., 0.37/0.08) in the two windows in time, indicating that the cases in the former period experienced a 1.3 times greater daily growth rate (i.e., exp(0.37)/exp(0.08)) than those in the latter period. A glance at the age-specific data show that the disease spread from an initial cluster that mix primarily in an assortative manner into the "general" Japanese population is the most likely key to this dramatic difference. Since the latter period reflects the early population-wide spread of H1N1 involving the entire Japanese community, R for this period is estimated using the following methodology.
Modelling methods
We employ an age-structured model to derive an estimate for R since the transmission of influenza A (H1N1) 2009 is known to differ greatly among age groups [1,3,5,9]. Spatial heterogeneity, social heterogeneity (e.g. differing patterns of transmission between household-, school- and workplace-settings), or potential changes in behaviour are mostly ignored.
The square matrix with generic entry Rij, the average number of secondary cases in age-group i generated by a single primary case in age-group j, is referred to as the next-generation matrix [20]. The reproduction number R is defined as the dominant eigenvalue of the next-generation matrix [21]. Since the observed data come as daily reports, we consider the incidence of indigenous, ci, t, and imported cases, bi, t, of age-group i developing the disease on day t in discrete time. Using Rij, the multi-type renewal process, yielding the conditional expectation of indigenous cases on day t, is written as
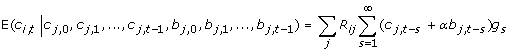 |
(1) |
where α is the relative contribution of imported cases to secondary transmission as compared to indigenous cases (0 ≤ α ≤ 1) and gs is the discretized density function of the generation time of length s days. We introduce the relative reduction α because the physical movements of those with a history of overseas travel were partly restricted during the early epidemic phase in Japan, reducing the number of secondary transmissions. Also, the imported cases most often developed the disease shortly before or after entering Japan. The density of the generation time, gs, is calculated as follows:
| (2) |
where G(s) is the cumulative distribution function of the generation time distribution, which we assume to be known and to follow a gamma distribution. In the early modelling studies, the mean generation time was estimated at 1.9 days [1], 2.6-3.2 days [3] and 2.5 days [4]. From contact tracing data in the Netherlands, the mean and standard deviation (SD) were estimated at 2.7 and 1.1 days, respectively [22]. We adopt 2.7 days as the mean and fix the coefficient of variation to 40.7% as calculated from the Dutch study. We partly address issues of uncertainty by measuring the sensitivity of R to differing mean generation times ranging from 2.1 to 3.3 days.
Rij is modelled as
| (3) |
where R is the reproduction number to be estimated (i.e., scalar quantity), si measures the susceptibility of age-group i given a contact, and mij is the frequency of contact made by an individual in age-group j with that in i (which is assumed known and is extracted from a contact survey in the Netherlands [23]); let S and M be square matrices. S is the diagonal matrix in which the diagonal elements (i, i) are si and the entries outside the main diagonal are all zero. The (i, j) element of M is mij with which we adopt frequency-dependent assumption, and we ignore more detailed contact including the "type" and "duration" [24]. We normalize the product SM (i.e., the dominant eigenvalue of SM is set to 1) so that R scales the next-generation matrix. We aggregate the population into six discrete age groups (0-5, 6-12, 13-19, 20-39, 40-59 years and 60 and older) in order to be able to adhere to the precision of the contact survey [23]; consequently, the next-generation matrix has dimensions 6 × 6 (36 elements).
We estimate eight parameters (i.e., R, α and si for six age-groups) using the renewal equation (1). We assume that variations in secondary transmissions are appropriately captured by a Poisson distribution [25]. The conditional likelihood of observing ci, t on day t given the series of foregoing indigenous cases cj, 0, cj, 1, ..., cj, t-1 and of imported cases bj, 0, bj, 1, ..., bj, t-1, respectively, for all age-groups j, is given by
| (4) |
where E(ci, t) is the conditional expectation (i.e., the right-hand side of (1)) and ci, t is the observed number of cases of age-group i on day t. Maximum likelihood estimates of the parameters are obtained by minimizing the negative logarithm of (4) with the 95% confidence intervals (CI) derived from profile likelihood.
Modelling results
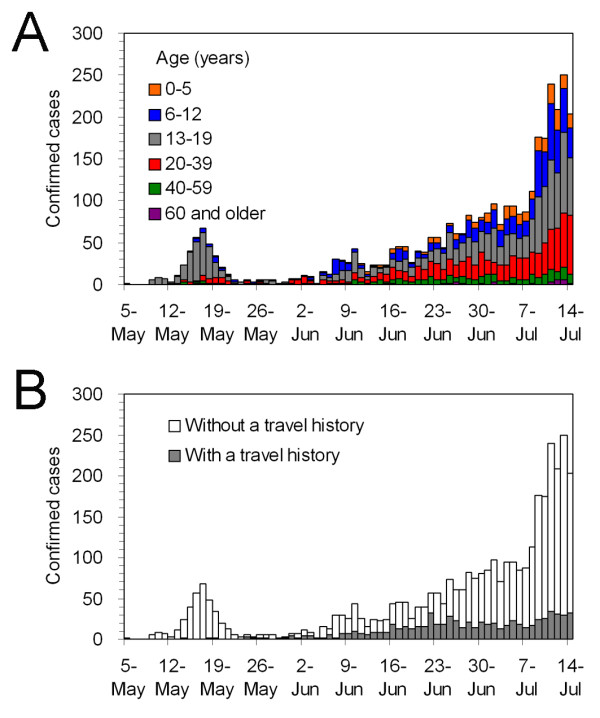
Figure 3 compares the observed and predicted numbers of confirmed cases. The conditional expectation approximately captures the observed age-specific patterns of incidence. The maximum likelihood estimate of the next-generation matrix, Rij, is
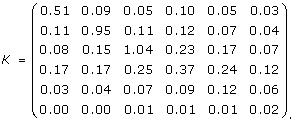 |
(5) |
Figure 3.
Model prediction. Observed (dots) and predicted (lines) age-specific numbers of confirmed cases as a function of onset time are compared. The prediction on day t was conditioned on observations from days 0 to (t-1).
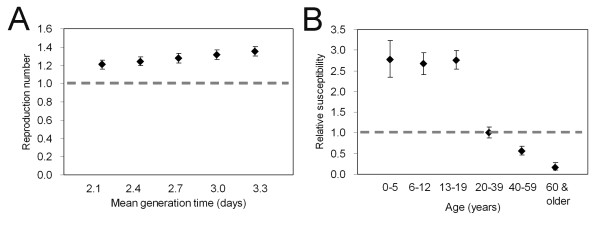
Those aged from 13-19 years appear to be able to maintain the transmission by themselves (i.e., meeting the definition of maintenance host, R33 > 1 [26]). Nevertheless, age groups 1 and 2, children aged from 0-12 years, appear incapable of maintaining transmission (i.e., the dominant eigenvalue of the 2 × 2 matrix involving transmissions among and between those aged from 0-5 and 6-12 years is less than 1). The maximum likelihood estimate of R is 1.28 (95% CI: 1.23, 1.33). The relative contribution of imported cases to secondary transmission, α, is estimated at 0.15 (standard error = 0.14).
Figure 4A examines the sensitivity of R to different mean generation times. If we adopt 2.1 days as the mean, R is estimated at 1.21 (95% CI: 1.16, 1.26). If we adopt 3.3 days, R is 1.35 (95% CI: 1.30, 1.41). Figure 4B captures relative susceptibilities, using those aged from 20-39 years to define the susceptibility baseline. The age-groups 0-5, 6-12 and 13-19 years appear to be 2.77 (95% CI: 2.35, 3.24), 2.67 (95% CI: 2.41, 2.95) and 2.76 (95% CI: 2.55, 2.98) times more susceptible than adults aged 20-39 years. On the other hand, those aged from 40-59 years and 60 years and older are 0.56 (95% CI: 0.45, 0.68) and 0.17 (95% CI: 0.09, 0.28) times as susceptible than those aged 20-39 years. It should be noted that the qualitative pattern of age-dependent susceptibility agrees well with the results of immunological studies [27,28] and a hypothesis about its underlying mechanisms [29].
Figure 4.
Parameter estimates and sensitivity analysis. Panel A examines the sensitivity of the reproduction number to different mean lengths of the generation time ranging from 2.1 to 3.3 days. Panel B shows the estimate of the age-specific relative susceptibility. The expected value of susceptibility for those aged 20-39 years was taken as the reference. In both panels, the whiskers extend to the upper and lower 95% confidence intervals based on the profile likelihood.
Limitation of the growth rate r
As expected from the greatly differing exponential growth rates between early May and from 29 May to 14 July (Figure 2), the reproduction number for the latter period, ranging from 1.21 to 1.35, is much smaller than our previously reported estimate for the former time period when the transmission was mainly confined to school settings [5]. The estimate in the latter period is consistent with the estimates of R in other countries [14,16]. The situation is not straightforward, however, as the estimation was carried out using confirmed cases (which may be biased towards severe cases). Further, it should be noted that various interventions, including reactive school closure and contact tracing, were instituted during the whole period of observation, so the R value for the latter period and especially the entries Rij involving school children might potentially lead to underestimates for R in the present study.
Since the small outbreak in the former time period was restricted to a limited number of schools and the contacts made by the students in Osaka and Kobe (and as Japan was unique in successfully "containing" the local school-based outbreak before actual pandemic overshoot), the depletion of susceptibles in May and undiagnosed cases are unlikely to have played a significant role in our estimates of a smaller R for the epidemic in the latter transmission period, which saw the pandemic takeover. Rather, as we discussed above, the local networks of interactions (i.e., transmission within networks that connect to other networks in time), and consequently the initial conditions (i.e., which network gets infected first), played a key role in our estimates for the initial outbreak growth. The earlier estimates of R captured the initial role in the generation of secondary cases from schools where the frequency of transmission among school children greatly exceeded those of the community (and the "type" of their contact is perhaps more dense (or close) than those in the community [24,30]). Although the sensitivity of R to differing mean generation times was examined within a relatively narrow range, this aspect could not account for the high R estimates obtained in Japan [5].
One important conclusion is drawn from the present study. The lessons learnt from our estimation over the two windows in time has forced us to revisit the role of using summary statistics to characterize transmission potential from the data generated by heterogeneous contact patterns. As a network expands, the structure of networks involved in transmission changes, and consequently the summary statistics of cases, also change in time [13]. The initial summary statistics therefore depend in a rather critical way on the initial conditions (i.e., where and how the disease was introduced) [31], which is not always captured well by homogeneous mixing models. Since the very early stage of this epidemic alone involves primarily a few specific sub-groups of the population, it is difficult to quantify the next-generation matrix fully and estimate a reproduction number that adequately captures the transmission potential for the entire population. Whereas the next-generation matrix includes representative levels of population heterogeneity, the infected individuals during the very initial epidemic stage were clearly not representative of the entire population of interest. The use of the next-generation matrix involves the introduction of a "typical" infectious individual into the population, but such an individual cannot be properly characterized if the matrix involves unavoidable approximations (due to limited availability of structured data) when an outbreak happens to be mostly confined to a single cluster whose average individual is "atypical" of the entire population.
The previously reported expected value, R = 2.3, for the May outbreak might well approximate the intensity of transmissions in schools (and indeed, is consistent with the estimate in school settings in the USA [3]). This is another example of what is often referred as core group effects in the epidemiological literature [32]. Naturally, the use of the empirical data from school clusters does not provide sufficient information to carry out precise estimation of the age-dependent next-generation matrix, so the resulting dominant eigenvalue in the earlier study should not be regarded as R but rather as a measure of transmissibility conditioned on the initial conditions. The need for the collection of additional data may be critical when age-specific transmission is highly assortative and/or when age-specific susceptibility is highly heterogeneous (as is the case for influenza A (H1N1) 2009). Summary statistics based on highly aggregated populations are in general not helpful in identifying the pressure points of a heterogeneous network, which is essential in the identification and assessment of the most effective (e.g., age-specific) intervention policies. In other words, the finer details of epidemic data (i.e., epidemiological information at a local level, e.g., active surveillance of cases) need to be taken into account in the modelling. Not only school outbreaks, but also other social factors and settings (e.g. transportation, hospital settings and mass gatherings) can play enhancing or reducing transmission roles.
In addition to the challenges posed by our need to average over different levels of heterogeneous mixing, quantification of the growth rates involves the additional challenges that come from underreporting, notably ascertainment of cases and reporting bias. Further, imputation of onset dates for missing data is sometimes required, and moreover, the time-varying reporting frequency may even call for naïve adjustment of the growth rate of confirmed cases by the growth rate of hospitalized (or other severe portions of) cases [4]. The data set we examined in Japan involved contact tracing efforts at all local levels, so the growth rate of confirmed symptomatic cases is thought to have captured the actual increase in infection appropriately. Nevertheless, achieving precise estimation of incidence for this mild disease remains an open question, particularly if the proportion of asymptomatic infections among the total of infected individuals is high.
Is the epidemic growth rate useless?
Despite our earlier suggestion of the "biased" estimate of the next-generation matrix, it should be noted that we do not argue that the early growth rate is no longer be used but rather that the context of its use when appropriate should be clarified. The growth rate of cases is, as with most inferences from statistical modelling, context dependent (e.g., presence of initially infected cluster). Given the precise estimate of the generation time distribution, the exponential growth rate is appropriately translated to the reproduction number for a single population [16]. This is also the case, for example, for the multi-type epidemic as outlined below. Discarding imported cases, the continuous-time version of our renewal process (1) is written as
| (6) |
Equation (6) assumes that the generation time is shared among sub-populations. If we further assume that the intrinsic growth rate r is identical among sub-populations then the incidence ci(t) can be written as ([33]):
| (7) |
where k is constant and ωi is the leading eigenvector of the next-generation matrix. Replacing the right-hand side of (7) in (6) leads to
| (8) |
That is,
| (9) |
and thus the estimator of R is given by 1/M(-r) (see Background) [16]. Hence, and not surprisingly, as long as the intrinsic growth rate and generation time are the same among sub-populations, the estimator of R for the multi-type epidemic model can be identical to that of single-host epidemic model [34].
The incorporation of additional levels of detail into the basic model used to generate growth estimates depends on the model's ability to capture the underlying transmission dynamics in the data and the purposes of the research questions or public health policymaking goals. These issues are particularly relevant when a clustering of cases is observed [35,36]; as we saw in Japan, clusters of cases caused a delay in accurately estimating the true population average of transmissibility. The epidemic growth rate remains a useful quantity for estimating the transmission potential at the population level in the absence of obvious clusters of cases and as long as the approximately modelled transmission sufficiently captures the actual heterogeneity. We start from the premise that the use of heterogeneous mixing models is essential in the assessment of critical theoretical claims and policymaking decisions. Hence, it is worth noticing that in this context, technical questions remain regarding the use and applicability of the exponential growth rate. They include (i) the development of methods for estimating the generation time distribution and (ii) the determination of an appropriate length of the exponential growth period [37-39].
How should we communicate the estimate?
Without doubt R is the most widely used measurement of transmissibility and there are many good reasons why this is so. It has a simple formula and it is the simplest and most interpretable quantity to communicate to non-experts. Its limitations become evident when specific decisions must be made including, for example, who should be vaccinated first. Precise estimates of the next-generation matrix capture detailed epidemic dynamics that are key to answering questions like the one posed, but its estimation requires age- and risk-group structured data and a clear identification of the correct exponential growth time window. In the context of the pandemic from 2009, gathering age-specific transmission dynamics information is of the utmost relevance to prediction and policymaking. For instance, given that R = 1.28, one may predict the final size of epidemic, z, the proportion of those who will experience infection by the end of epidemic, by using the final size equation (based on a homogeneous mixing model),
| (10) |
Iteratively solving (10), z is estimated to be 40.3%. Similarly, R = 2.3 for a homogeneously mixing population is translated to z = 86.2%. Nevertheless, if we have the next-generation matrix, Rij, the final size zi of host i is written as ([40])
| (11) |
Using our estimate in (5), the corresponding z1-z6 are estimated at 16.2, 37.1, 47.7, 29.0, 8.7 and 1.0%, respectively. Using the age-specific population size Ni, the final size z for the entire population is calculated as the weighted average,
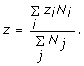 |
(12) |
Extracting the age-specific population estimate in Japan [41], z is estimated at 17.5%, not surprisingly much less than predicted by (10), a value that is indeed close to the actual range of the impact of first pandemic wave in Northern Hemisphere countries [17]. The "real" value of z may be even smaller if we account for additional levels of heterogeneity in transmission. The reproduction number, R, for the entire population may be useful for obtaining a rough estimate of how much vaccine we need (e.g., deciding the total number of vaccines to be manufactured), while Rij is far more essential for structuring the most effective strategy of vaccination and planning the optimal prioritization schemes [42,43]. Given that R can also be calculated from Rij, communicating Rij rather than R to the general public would be the most informative strategy of science communication for modelling results. When one explains the concept of Rij to non-experts, it's ideal to mention the limitation due to its nature of approximation because of limitations in structured data in any empirical observation.
The case fatality ratio (CFR), an epidemiological measurement of virulence, would also benefit from the use of detailed (e.g., age-structured) information. Whereas the confirmed CFR (cCFR) for the entire population conditioned on confirmed cases has been estimated at approximately 0.5% during the very early stage of the pandemic [1,44,45], the symptomatic CFR (sCFR), which is conditioned on symptomatic cases, later appeared to be 0.048% [46]. The CFR estimate for the entire population is regarded as a summary measure of virulence, so the reduced order of virulence (approximately by a factor of 10) provides not only more accurate information but a more useful measure for assessing the impact of this pandemic. Nevertheless, knowledge based on additional information would be even more useful. Ideally, we would like to compare epidemiologically the virulence between different settings (e.g., comparison between age groups or countries) and to use this knowledge in the development of relevant public health policy. Understanding the heterogeneous risks of death by age- and risk-groups is critical in the effective design of intervention strategies. In other words, apart from discussing the changing assessment of the CFR as a whole, CFRi for age-group i is probably more informative than CFR for the entire population.
Conclusions
We analysed the temporal distribution of the influenza A (H1N1) 2009 epidemic in Japan from May-July 2009, estimating the reproduction number for the time period from 29 May to 14 July -- a follow-up to our earlier estimates derived primarily from data on school outbreaks in May. The updated estimate of R, an average over a wider spectrum of the Japanese population, ranged from 1.21 to 1.35. Our higher estimate reported in [5] was based on a data set that was incapable of quantifying the next-generation matrix for the whole country from the growth rate of cases in a restricted school setting alone. The upshot of this analysis is tied to the fact that in regions where disease transmission is highly heterogeneous the initial conditions will have a strong effect on the variation in estimates of the rate of epidemic growth. To quantify R and Rij appropriately from the growth rate of cases, it is essential that the model not only captures the underlying transmission dynamics behind the empirical data but also that we have sufficiently structured data for each sub-population to estimate the role of variability in our estimates. In addition to extending the window in time for developing accurate population level measurements [39], it may be useful to examine additional epidemiological information (e.g., active surveillance of local transmission patterns and the severe portion of cases including hospital admissions).
Communicating R to non-experts is simpler than Rij, but Rij contains the information that is most useful for the development of specific intervention efforts. Developing simple interpretations and effective ways of communicating Rij (i.e., the average number of secondary cases in host i generated by a single primary case in host j) must be pursued. This retrospective study highlights the importance of incorporating the concept of heterogeneous risks of infection and death in the context of the assessment of pandemic potential.
List of abbreviations
R: the reproduction number; RT-PCR: Reverse transcription polymerase chain reaction; CI: confidence intervals; SD: standard deviation; CFR: case fatality ratio.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HN conceived of the study. HN developed methodological ideas, implemented statistical analyses and drafted the manuscript. GC, MS and CCC revised the model and helped improve the manuscript. All authors read and approved the final manuscript.
Contributor Information
Hiroshi Nishiura, Email: h.nishiura@uu.nl.
Gerardo Chowell, Email: gchowell@asu.edu.
Muntaser Safan, Email: muntaser_safan@yahoo.com.
Carlos Castillo-Chavez, Email: ccchavez@asu.edu.
Acknowledgements
The work of H Nishiura was supported by the JST PRESTO program. The authors are grateful to the editor and the three reviewers for their useful comments, advices and expediting publication of this article.
References
- Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, Griffin J, Baggaley RF, Jenkins HE, Lyons EJ, Jombart T, Hinsley WR, Grassly NC, Balloux F, Ghani AC, Ferguson NM, Rambaut A, Pybus OG, Lopez-Gatell H, Alpuche-Aranda CM, Chapela IB, Zavala EP, Guevara DM, Checchi F, Garcia E, Hugonnet S, Roth C. WHO Rapid Pandemic Assessment Collaboration. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324:1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boëlle PY, Bernillon P, Desenclos JC. A preliminary estimation of the reproduction ratio for new influenza A(H1N1) from the outbreak in Mexico, March-April 2009. Euro Surveill. 2009;14(19) doi: 10.2807/ese.14.19.19205-en. pii: 19205. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sugimoto JD, Halloran ME, Basta NE, Chao DL, Matrajt L, Potter G, Kenah E, Longini IM Jr. The transmissibility and control of pandemic influenza A (H1N1) virus. Science. 2009;326:729–733. doi: 10.1126/science.1177373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LF, Wallinga J, Finelli L, Reed C, Riley S, Lipsitch M, Pagano M. Estimation of the reproductive number and the serial interval in early phase of the 2009 influenza A/H1N1 pandemic in the USA. Influenza Other Respi Viruses. 2009;3:267–276. doi: 10.1111/j.1750-2659.2009.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H, Castillo-Chavez C, Safan M, Chowell G. Transmission potential of the new influenza A(H1N1) virus and its age-specificity in Japan. Euro Surveill. 2009;14(19) doi: 10.2807/ese.14.22.19227-en. pii: 19205. [DOI] [PubMed] [Google Scholar]
- Nishiura H, Wilson N, Baker MG. Estimating the reproduction number of the novel influenza A virus (H1N1) in a Southern Hemisphere setting: preliminary estimate in New Zealand. N Z Med J. 2009;122:73–77. [PubMed] [Google Scholar]
- Balcan D, Hu H, Goncalves B, Bajardi P, Poletto C, Ramasco JJ, Paolotti D, Perra N, Tizzoni M, Broeck W Van den, Colizza V, Vespignani A. Seasonal transmission potential and activity peaks of the new influenza A(H1N1): a Monte Carlo likelihood analysis based on human mobility. BMC Med. 2009;7:45. doi: 10.1186/1741-7015-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munayco CV, Gomez J, Laguna-Torres VA, Arrasco J, Kochel TJ, Fiestas V, Garcia J, Perez J, Torres I, Condori F, Nishiura H, Chowell G. Epidemiological and transmissibility analysis of influenza A(H1N1)v in a southern hemisphere setting: Peru. Euro Surveill. 2009;14(32) pii: 19299. [PubMed] [Google Scholar]
- McBryde E, Bergeri I, van Gemert C, Rotty J, Headley E, Simpson K, Lester R, Hellard M, Fielding J. Early transmission characteristics of influenza A(H1N1)v in Australia: Victorian state, 16 May - 3 June 2009. Euro Surveill. 2009;14 doi: 10.2807/ese.14.42.19363-en. pii = 19363. [DOI] [PubMed] [Google Scholar]
- de Silva UC, Warachit J, Waicharoen S, Chittaganpitch M. A preliminary analysis of the epidemiology of influenza A(H1N1)v virus infection in Thailand from early outbreak data, June-July 2009. Euro Surveill. 2009;14 doi: 10.2807/ese.14.31.19292-en. pii = 19292. [DOI] [PubMed] [Google Scholar]
- Pourbohloul B, Ahued A, Davoudi B, Meza R, Meyers LA, Skowronski DM, Villaseñor I, Galván F, Cravioto P, Earn DJ, Dushoff J, Fisman D, Edmunds WJ, Hupert N, Scarpino SV, Trujillo J, Lutzow M, Morales J, Contreras A, Chávez C, Patrick DM, Brunham RC. Initial human transmission dynamics of the pandemic (H1N1) 2009 virus in North America. Influenza Other Respi Viruses. 2009;3:215–222. doi: 10.1111/j.1750-2659.2009.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuite AR, Greer AL, Whelan M, Winter AL, Lee B, Yan P, Wu J, Moghadas S, Buckeridge D, Pourbohloul B, Fisman DN. Estimated epidemiologic parameters and morbidity associated with pandemic H1N1 influenza. CMAJ. 2009. in press . [DOI] [PMC free article] [PubMed]
- Busenberg S, Castillo-Chavez C. A general solution of the problem of mixing of subpopulations and its application to risk- and age-structured epidemic models for the spread of AIDS. IMA J Math Appl Med Biol. 1991;8:1–29. doi: 10.1093/imammb/8.1.1. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Mathematical modelling of the pandemic H1N1 2009. Wkly Epidemiol Rec. 2009;84:341–348. [PubMed] [Google Scholar]
- Brauer F, Feng Z, Castillo-Chavez C. Discrete epidemic models. Math Biosci Eng. 2010;7:1–15. doi: 10.3934/mbe.2010.7.1. [DOI] [PubMed] [Google Scholar]
- Wallinga J, Lipsitch M. How generation intervals shape the relationship between growth rates and reproductive numbers. Proc R Soc Lond Ser B. 2007;274:599–604. doi: 10.1098/rspb.2006.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Transmission dynamics and impact of pandemic influenza A (H1N1) 2009 virus. Wkly Epidemiol Rec. 2009;84:481–484. [PubMed] [Google Scholar]
- Mikolajczyk RT, Akmatov MK, Rastin S, Kretzschmar M. Social contacts of school children and the transmission of respiratory-spread pathogens. Epidemiol Infect. 2008;136:813–822. doi: 10.1017/S0950268807009181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Labor and Welfare, Japan. Influenza A(H1N1) Tokyo: Ministry of Health, Labor and Welfare; http://www.mhlw.go.jp/english/topics/influenza_a/index.html (accessed on 10 December 2009) [Google Scholar]
- Diekmann O, Heesterbeek JAP. Mathematical Epidemiology of Infectious Diseases: Model Building, Analysis and Interpretation. Chichester: John Wiley and Son; 2000. [Google Scholar]
- Diekmann O, Heesterbeek JA, Metz JA. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J Math Biol. 1990;28:365–382. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- Hahné S, Donker T, Meijer A, Timen A, van Steenbergen J, Osterhaus A, van der Sande M, Koopmans M, Wallinga J, Coutinho R. Dutch New Influenza A(H1N1)v Investigation Team. Epidemiology and control of influenza A(H1N1)v in the Netherlands: the first 115 cases. Euro Surveill. 2009;14(27) doi: 10.2807/ese.14.27.19267-en. pii: 19267. [DOI] [PubMed] [Google Scholar]
- Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. Am J Epidemiol. 2006;164:936–944. doi: 10.1093/aje/kwj317. [DOI] [PubMed] [Google Scholar]
- Smieszek T. A mechanistic model of infection: why duration and intensity of contacts should be included in models of disease spread. Theor Biol Med Model. 2009;6:25. doi: 10.1186/1742-4682-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H. Time variations in the transmissibility of pandemic influenza in Prussia, Germany, from 1918-19. Theor Biol Med Model. 2007;4:20. doi: 10.1186/1742-4682-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H, Hoye B, Klaassen M, Bauer S, Heesterbeek H. How to find natural reservoir hosts from endemic prevalence in a multi-host population: A case study of influenza in waterfowl. Epidemics. 2009;1:118–128. doi: 10.1016/j.epidem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. Morb Mortal Wkly Rep. 2009;58:521–524. [PubMed] [Google Scholar]
- Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki-Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Takahashi K, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, Ogasawara K, Kawaoka Y. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert T, Chowell G, Nishiura H, Christensen RA, McCullers JA. Does Glycosylation as a modifier of Original Antigenic Sin explain the case age distribution and unusual toxicity in pandemic novel H1N1 influenza? BMC Infect Dis. 2010;10:5. doi: 10.1186/1471-2334-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton T, Nordvik MK, Liljeros F. Modelling sexually transmitted infections: the effect of partnership activity and number of partners on R0. Theor Popul Biol. 2007;72:389–399. doi: 10.1016/j.tpb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Newman MEJ. The structure and function of complex networks. SIAM Review. 2003;45:167–256. doi: 10.1137/S003614450342480. [DOI] [Google Scholar]
- Hethcote HW, Yorke JA. Gonorrhea Transmission Dynamics and Control (Lecture Notes in Biomathematics, 56) Berlin:Springer-Verlag; 1980. [Google Scholar]
- Goldstein E, Apolloni A, Lewis B, Miller JC, Macauley M, Eubank S, Lipsitch M, Wallinga J. Distribution of vaccine/antivirals and the 'least spread line' in a stratified population. J R Soc Interface. 2009. in press . [DOI] [PMC free article] [PubMed]
- Nishiura H, Chowell G, Heesterbeek H, Wallinga J. The ideal reporting interval for an epidemic to objectively interpret the epidemiological time course. J R Soc Interface. 2010;7:297–307. doi: 10.1098/rsif.2009.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling M. The implications of network structure for epidemic dynamics. Theor Popul Biol. 2005;67:1–8. doi: 10.1016/j.tpb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Smieszek T, Fiebig L, Scholz RW. Models of epidemics: when contact repetition and clustering should be included. Theor Biol Med Model. 2009;6:11. doi: 10.1186/1742-4682-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich KG. Formal kinetics of H1N1 epidemic. Theor Biol Med Model. 2009;6:23. doi: 10.1186/1742-4682-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell G, Castillo-Chavez C. In: Bioterrorism: Mathematical and Modeling Applications in Homeland Security, (Frontiers in Applied Mathematics 28) Banks T, Castillo-Chavez C, editor. Philadelphia, PA: Society for Industrial Mathematics; 2003. Worst-Case scenarios and epidemics. [Google Scholar]
- Bettencourt LMA, Ribeiro RM, Chowell G, Lant T, Castillo-Chavez C. Intelligence and Security Informatics: Biosurveillance (Lecture Notes in Computer Science, volume 4506) Berlin: Springer; 2009. Towards Real Time Epidemiology: Data Assimilation, Modeling and Anomaly Detection of Health Surveillance Data Streams. [Google Scholar]
- Ball F, Clancy D. The final size and severity of a generalised stochastic multitype epidemic model. Adv Appl Prob. 1993;25:721–736. doi: 10.2307/1427788. [DOI] [Google Scholar]
- Statistics Bureau, Ministry of Internal Affairs and Communications. Population estimates. Tokyo: Statistics Bureau (in Japanese) http://www.stat.go.jp/data/nihon/02.htm (accessed on 10 December 2009)
- Longini IM, Ackerman E, Elveback LR. An optimization model for influenza A epidemics. Math Biosci. 1978;38:141–157. doi: 10.1016/0025-5564(78)90023-8. [DOI] [Google Scholar]
- Britton T. On critical vaccination coverage in multitype epidemics. J Appl Prob. 1998;35:1003–1006. doi: 10.1239/jap/1032438395. [DOI] [Google Scholar]
- Garske T, Legrand J, Donnelly CA, Ward H, Cauchemez S, Fraser C, Ferguson NM, Ghani AC. Assessing the severity of the novel influenza A/H1N1 pandemic. BMJ. 2009;339:b2840. doi: 10.1136/bmj.b2840. [DOI] [PubMed] [Google Scholar]
- Nishiura H, Klinkenberg D, Roberts M, Heesterbeek JA. Early epidemiological assessment of the virulence of emerging infectious diseases: a case study of an influenza pandemic. PLoS ONE. 2009;4:e6852. doi: 10.1371/journal.pone.0006852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presanis AM, De Angelis D. New York City Swine Flu Investigation Team. Hagy A, Reed C, Riley S, Cooper BS, Finelli L, Biedrzycki P, Lipsitch M. The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med. 2009;6:e1000207. doi: 10.1371/journal.pmed.1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]