Abstract
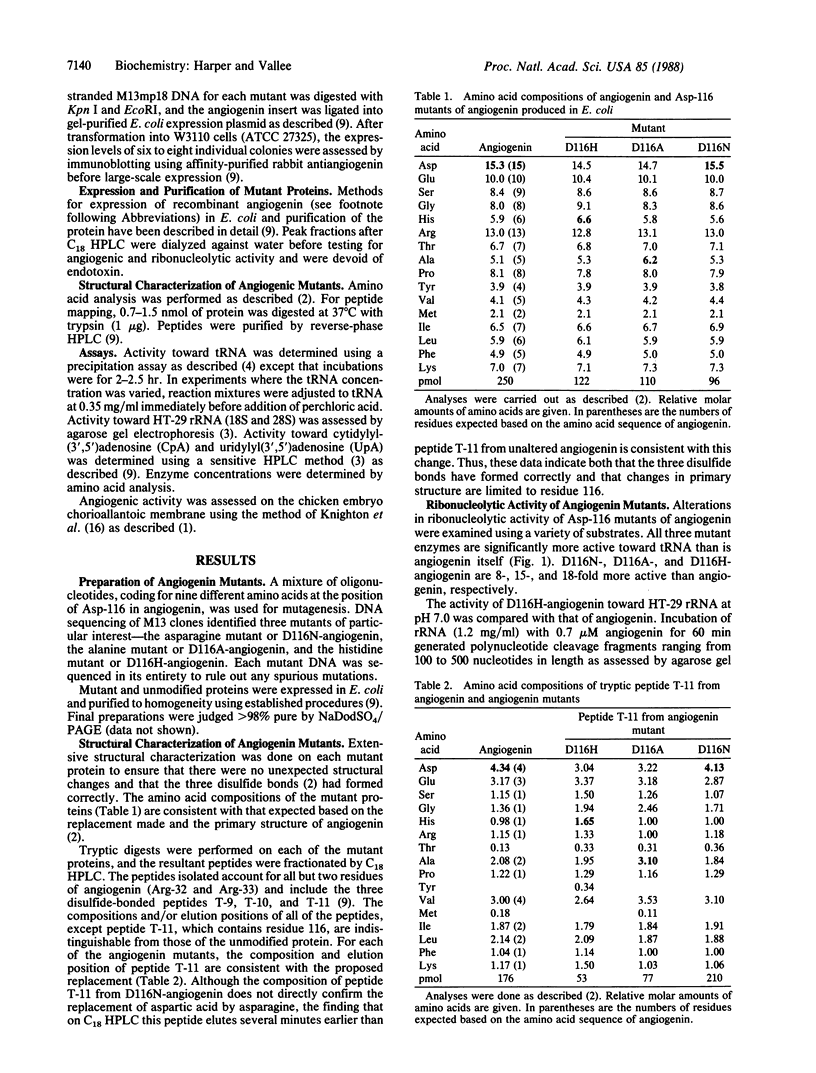
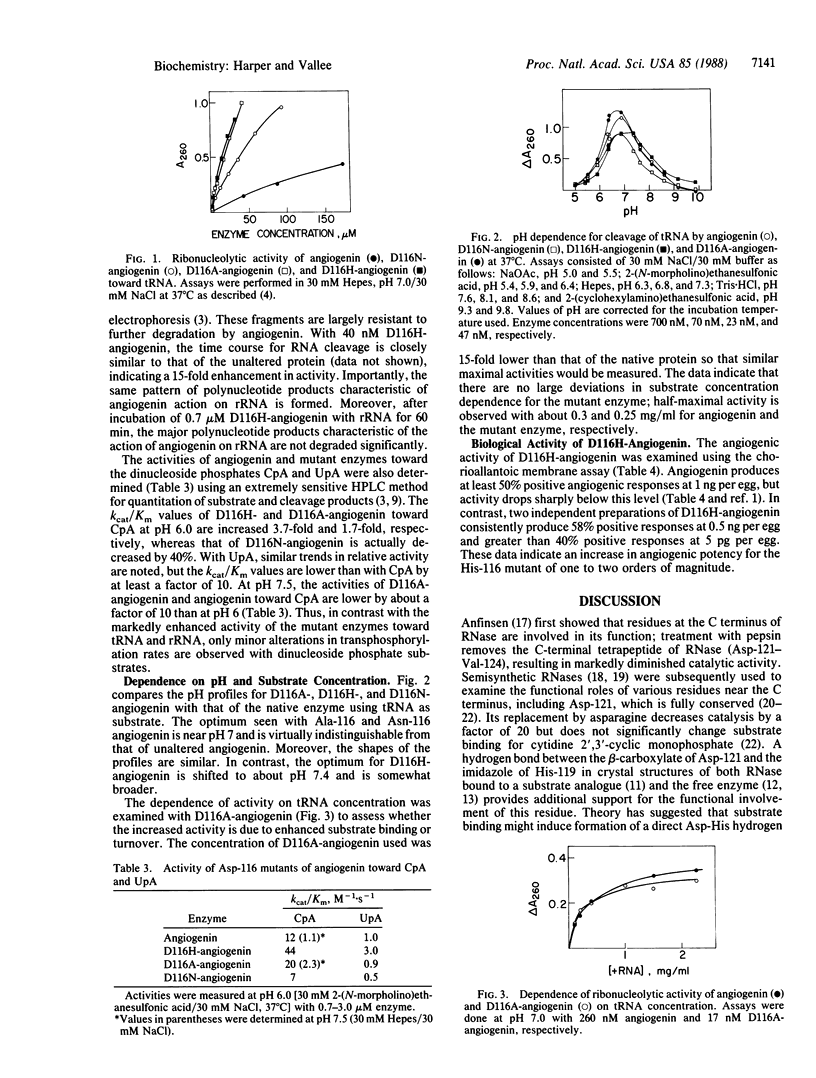
Site-specific mutagenesis of the blood vessel-inducing protein angiogenin has been used to further explore both its homology to pancreatic ribonuclease and the functional roles of particular residues. Replacement of Asp-116 in angiogenin by either asparagine (D116N), alanine (D116A), or histidine (D116H) markedly enhances both its ribonucleolytic activity and angiogenic potency. Activity toward tRNA is 8-, 15-, and 18-fold greater than native angiogenin for D116N-, D116A-, and D116H-angiogenin, respectively. The enzymatic specificity of angiogenin, however, has been maintained. Thus, cleavage of 18S and 28S rRNA by the most active His-116 mutant yields the same pattern of polynucleotide products as from angiogenin, whereas there are only minor alterations in activity with cytidylyl(3',5')adenosine and uridylyl(3',5')-adenosine. Extensive biological assays on the chicken embryo chorioallantoic membrane demonstrate that D116H-angiogenin is one to two orders of magnitude more potent in inducing neovascularization than native angiogenin, which correlates well with enhanced enzymatic action. These results support the proposition that the enzymatic and angiogenic activities on angiogenin are interrelated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B. The limited digestion of ribonuclease with pepsin. J Biol Chem. 1956 Jul;221(1):405–412. [PubMed] [Google Scholar]
- Bicknell R., Vallee B. L. Angiogenin activates endothelial cell phospholipase C. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5961–5965. doi: 10.1073/pnas.85.16.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünger A. T., Brooks C. L., 3rd, Karplus M. Active site dynamics of ribonuclease. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8458–8462. doi: 10.1073/pnas.82.24.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. L., Petsko G. A. Ribonuclease structure and catalysis: crystal structure of sulfate-free native ribonuclease A at 1.5-A resolution. Biochemistry. 1987 Dec 29;26(26):8579–8584. doi: 10.1021/bi00400a013. [DOI] [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Gutte B., Lin M. C., Caldi D. G., Merrifield R. B. Reactivation of des(119-, 120-, or 121-124) ribonuclease A by mixture with synthetic COOH-terminal peptides of varying lengths. J Biol Chem. 1972 Aug 10;247(15):4763–4767. [PubMed] [Google Scholar]
- Hodges R. S., Merrifield R. B. The role of serine-123 in the activity and specificity of ribonuclease. Reactivation of ribonuclease 1-118 by the synthetic COOH-terminal tetradecapeptide, ribonuclease 111-124, and its O-methylserine and alanine analogs. J Biol Chem. 1975 Feb 25;250(4):1231–1241. [PubMed] [Google Scholar]
- Knighton D., Ausprunk D., Tapper D., Folkman J. Avascular and vascular phases of tumour growth in the chick embryo. Br J Cancer. 1977 Mar;35(3):347–356. doi: 10.1038/bjc.1977.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. C., Gutte B., Caldi D. G., Moore S., Merrifield R. B. Reactivation of des (119-124) ribonuclease A by mixture with synthetic COOH-terminal peptides; the role of phenylalanine-120. J Biol Chem. 1972 Aug 10;247(15):4768–4774. [PubMed] [Google Scholar]
- Lin M. C., Gutte B., Moore S., Merrifield R. B. Regeneration of activity by mixture of ribonuclease enzymically degraded from the COOH terminus and a synthetic COOH-terminal tetradecapeptide. J Biol Chem. 1970 Oct 10;245(19):5169–5170. [PubMed] [Google Scholar]
- Shapiro R., Riordan J. F., Vallee B. L. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry. 1986 Jun 17;25(12):3527–3532. doi: 10.1021/bi00360a008. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Human placental ribonuclease inhibitor abolishes both angiogenic and ribonucleolytic activities of angiogenin. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2238–2241. doi: 10.1073/pnas.84.8.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. S., Doscher M. S. Aspartic acid-121 functions at the active site of bovine pancreatic ribonuclease. FEBS Lett. 1984 Jun 11;171(2):253–256. doi: 10.1016/0014-5793(84)80498-6. [DOI] [PubMed] [Google Scholar]
- Strydom D. J., Fett J. W., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985 Sep 24;24(20):5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodawer A., Svensson L. A., Sjölin L., Gilliland G. L. Structure of phosphate-free ribonuclease A refined at 1.26 A. Biochemistry. 1988 Apr 19;27(8):2705–2717. doi: 10.1021/bi00408a010. [DOI] [PubMed] [Google Scholar]
- Wodak S. Y. The structure of cytidilyl(2',5')adenosine when bound to pancreatic ribonuclease S. J Mol Biol. 1977 Nov;116(4):855–875. doi: 10.1016/0022-2836(77)90275-3. [DOI] [PubMed] [Google Scholar]



