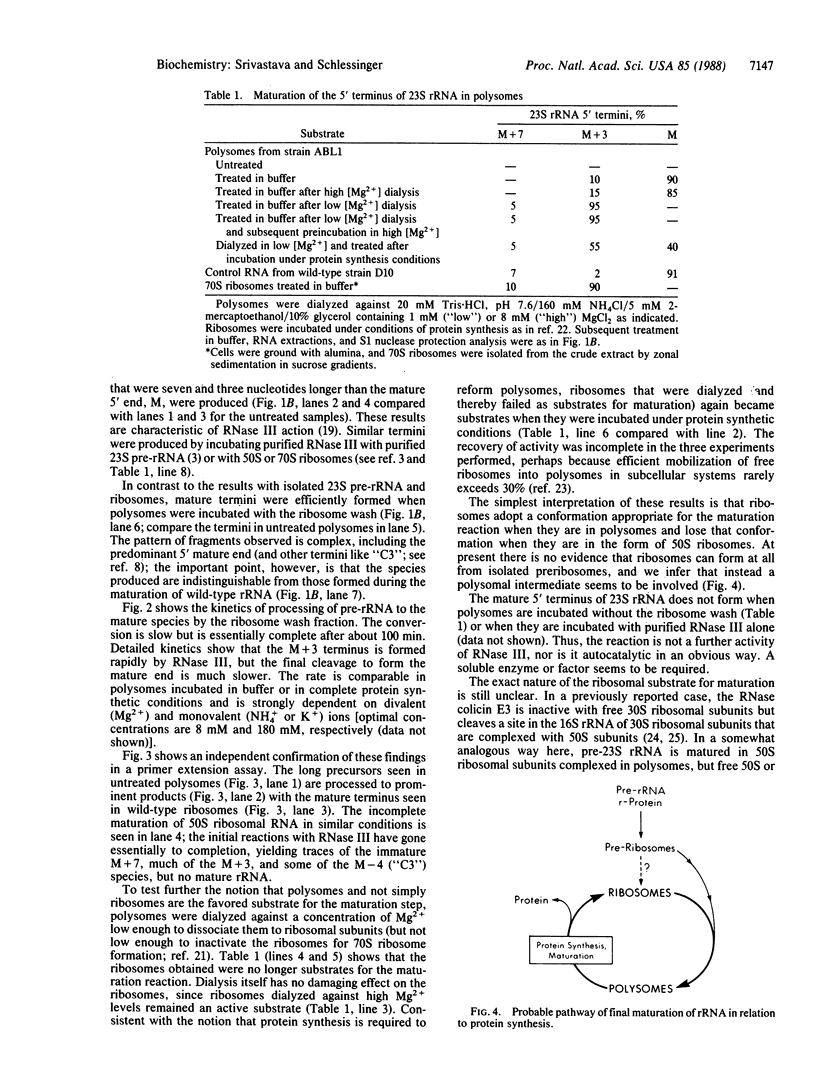
Abstract
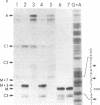
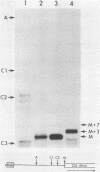
In Escherichia coli, the final maturation of rRNA occurs in precursor particles, and recent experiments have suggested that ongoing protein synthesis may somehow be required for maturation to occur. The protein synthesis requirement for the formation of the 5' terminus of 23S rRNA has been clarified in vitro by varying the substrate of the reaction. In cell extracts, pre-23S rRNA in free ribosomes was not matured, but that in polysomes was efficiently processed. The reaction occurred in polysomes without the need for an energy source or other additives required for protein synthesis. Furthermore, when polysomes were dissociated into ribosomal subunits, they were no longer substrates for maturation; but the ribosomes became substrates again when they once more were incubated in the conditions for protein synthesis. All of these results are consistent with the notion that protein synthesis serves to form a polysomal complex that is the true substrate for maturation. Ribosomes in polysomes, possibly in the form of 70S initiation complexes, may more easily adopt a conformation that facilitates maturation cleavage. As a result, the rates of ribosome formation and protein synthesis could be coregulated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amils R., Matthews E. A., Cantor C. R. Reconstitution of 50 S ribosomal subunits from Escherichia coli. Methods Enzymol. 1979;59:449–461. doi: 10.1016/0076-6879(79)59107-1. [DOI] [PubMed] [Google Scholar]
- Boon T. Inactivation of ribosomes in vitro by colicin E 3 and its mechanism of action. Proc Natl Acad Sci U S A. 1972 Mar;69(3):549–552. doi: 10.1073/pnas.69.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M. Inactivation of ribosomes by colicin E3 in vitro: Requirement for 50 S ribosomal subunits. FEBS Lett. 1972 Apr 15;22(1):73–75. doi: 10.1016/0014-5793(72)80222-9. [DOI] [PubMed] [Google Scholar]
- Bram R. J., Young R. A., Steitz J. A. The ribonuclease III site flanking 23S sequences in the 30S ribosomal precursor RNA of E. coli. Cell. 1980 Feb;19(2):393–401. doi: 10.1016/0092-8674(80)90513-9. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Ceccarelli A., Dotto G. P., Altruda F., Perlo C., Silengo L., Turco E., Mangiarotti G. Immature 50 S subunits in Escherichia coli polyribosomes. FEBS Lett. 1978 Sep 15;93(2):348–350. doi: 10.1016/0014-5793(78)81137-5. [DOI] [PubMed] [Google Scholar]
- Feunteun J., Jordan B. R., Monier R. Study of the maturation of 5 s RNA precursors in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):465–474. doi: 10.1016/0022-2836(72)90553-0. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Gierer L., Gierer A. Synthesis of ribosomal proteins and formation of ribosomes in Escherichia coli. J Mol Biol. 1968 Jul 14;34(2):293–303. doi: 10.1016/0022-2836(68)90254-4. [DOI] [PubMed] [Google Scholar]
- Gitelman D. R., Apirion D. The synthesis of some proteins is affected in RNA processing mutants of Escherichia coli. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1063–1070. doi: 10.1016/0006-291x(80)90060-1. [DOI] [PubMed] [Google Scholar]
- Hayes F., Vasseur M. Processing of the 17-S Escherichia coli precursor RNA in the 27-S pre-ribosomal particle. Eur J Biochem. 1976 Jan 15;61(2):433–442. doi: 10.1111/j.1432-1033.1976.tb10037.x. [DOI] [PubMed] [Google Scholar]
- King T. C., Schlessinger D. S1 nuclease mapping analysis of ribosomal RNA processing in wild type and processing deficient Escherichia coli. J Biol Chem. 1983 Oct 10;258(19):12034–12042. [PubMed] [Google Scholar]
- King T. C., Sirdeshmukh R., Schlessinger D. RNase III cleavage is obligate for maturation but not for function of Escherichia coli pre-23S rRNA. Proc Natl Acad Sci U S A. 1984 Jan;81(1):185–188. doi: 10.1073/pnas.81.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. C., Sirdeskmukh R., Schlessinger D. Nucleolytic processing of ribonucleic acid transcripts in procaryotes. Microbiol Rev. 1986 Dec;50(4):428–451. doi: 10.1128/mr.50.4.428-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Turco E., Ponzetto A., Altruda F. Precursor 16S RNA in active 30S ribosomes. Nature. 1974 Jan 18;247(5437):147–148. doi: 10.1038/247147a0. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Eldridge J. D. alpha-Cardiac actin is the major sarcomeric isoform expressed in embryonic avian skeletal muscle. Science. 1984 Jun 29;224(4656):1436–1438. doi: 10.1126/science.6729461. [DOI] [PubMed] [Google Scholar]
- Schlessinger D., Ono M., Nikolaev N., Silengo L. Accumulation of 30S preribosomal ribonucleic acid in an Escherichia coli mutant treated with chloramphenicol. Biochemistry. 1974 Oct 8;13(21):4268–4271. doi: 10.1021/bi00718a004. [DOI] [PubMed] [Google Scholar]
- Silengo L., Nikolaev N., Schlessinger D., Imamoto F. Stabilization of mRNA with polar effects in an Escherichia coli mutant. Mol Gen Genet. 1974;134(1):7–19. doi: 10.1007/BF00332808. [DOI] [PubMed] [Google Scholar]
- Sirdeshmukh R., Krych M., Schlessinger D. Escherichia coli 23S ribosomal RNA truncated at its 5' terminus. Nucleic Acids Res. 1985 Feb 25;13(4):1185–1192. doi: 10.1093/nar/13.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirdeshmukh R., Schlessinger D. Ordered processing of Escherichia coli 23S rRNA in vitro. Nucleic Acids Res. 1985 Jul 25;13(14):5041–5054. doi: 10.1093/nar/13.14.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirdeshmukh R., Schlessinger D. Why is processing of 23 S ribosomal RNA in Escherichia coli not obligate for its function? J Mol Biol. 1985 Dec 5;186(3):669–672. doi: 10.1016/0022-2836(85)90139-1. [DOI] [PubMed] [Google Scholar]
- Talkad V., Achord D., Kennell D. Altered mRNA metabolism in ribonuclease III-deficient strains of Escherichia coli. J Bacteriol. 1978 Aug;135(2):528–541. doi: 10.1128/jb.135.2.528-541.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Stone P. J. Proofreading of the codon-anticodon interaction on ribosomes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):198–202. doi: 10.1073/pnas.74.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udem S. A., Warner J. R. The cytoplasmic maturation of a ribosomal precursor ribonucleic acid in yeast. J Biol Chem. 1973 Feb 25;248(4):1412–1416. [PubMed] [Google Scholar]
- Wireman J. W., Sypherd P. S. In vitro assembly of 30S ribosomal particles from precursor 16S RNA of Escherichia coli. Nature. 1974 Feb 22;247(5442):552–554. doi: 10.1038/247552a0. [DOI] [PubMed] [Google Scholar]
- Wishnia A., Boussert A. S. The non-specific role of Mg2+ in ribosomal subunit association: kinetics and equilibrium in the presence of other divalent metal ions. J Mol Biol. 1977 Nov 5;116(3):577–591. doi: 10.1016/0022-2836(77)90085-7. [DOI] [PubMed] [Google Scholar]