Abstract
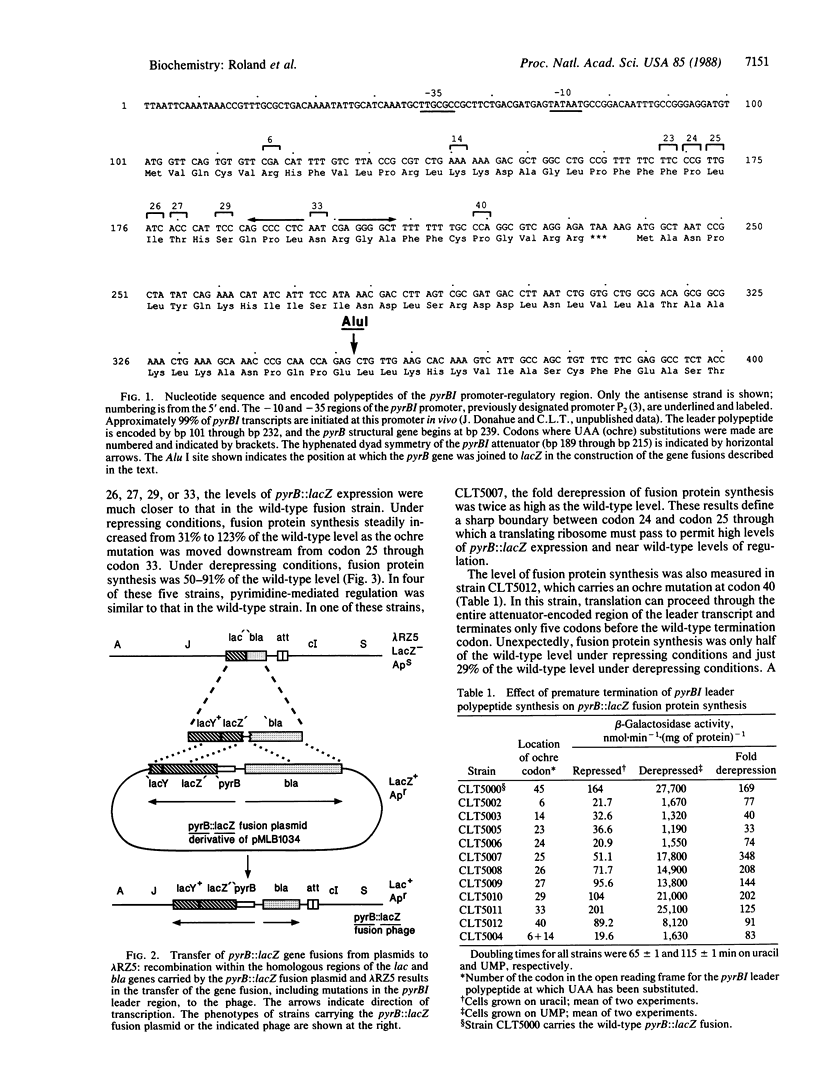
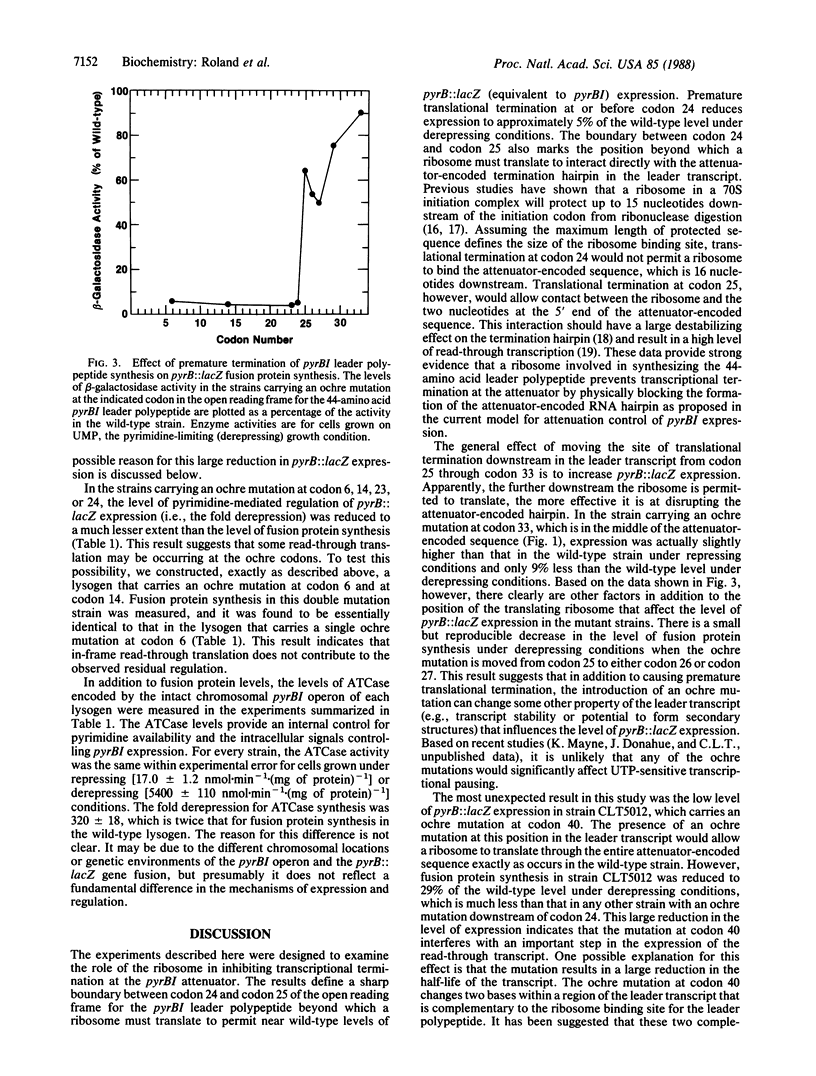
Pyrimidine-mediated regulation of pyrBI operon expression in Escherichia coli K-12 occurs primarily by an attenuation control mechanism. Previous studies have suggested a model for attenuation control in which low intracellular levels of UTP cause close coupling of transcription and translation within the pyrBI leader region. This close coupling apparently prevents transcriptional termination at an attenuator (a rho-independent transcriptional terminator) located 23 base pairs before the pyrBI structural genes within an open reading frame for a 44-amino acid leader polypeptide. Presumably, a ribosome involved in the synthesis of the leader polypeptide disrupts or precludes the formation of the attenuator-encoded RNA hairpin, which is required for transcriptional termination. In this study, we examined the role of the ribosome in inhibiting transcriptional termination at the pyrBI attenuator. Using oligonucleotide-directed mutagenesis, we systematically introduced termination codons into the reading frame for the leader polypeptide to determine the distance a ribosome must translate to suppress transcriptional termination. These mutations were incorporated individually into a pyrB::lacZ gene fusion, which was then introduced into the E. coli chromosome. The resulting fusion strains were used to measure the effect of each mutation on pyrB::lacZ expression. The results show that a ribosome must translate to within 14-16 nucleotides of the attenuator-encoded RNA hairpin to inhibit transcriptional termination efficiently, which indicates a direct interaction between the ribosome and the termination hairpin sequence as proposed in the present model. Additional results indicate that factors not included in the present model for attenuation control contribute to the expression and regulation of the pyrBI operon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistraro V. J., Kennell D. Escherichia coli lac operator mRNA affects translation initiation of beta-galactosidase mRNA. Nature. 1979 Feb 1;277(5695):407–409. doi: 10.1038/277407a0. [DOI] [PubMed] [Google Scholar]
- Christie G. E., Farnham P. J., Platt T. Synthetic sites for transcription termination and a functional comparison with tryptophan operon termination sites in vitro. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4180–4184. doi: 10.1073/pnas.78.7.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmesen K., Bonekamp F., Karlström O., Jensen K. F. Role of translation in the UTP-modulated attenuation at the pyrBI operon of Escherichia coli. Mol Gen Genet. 1985;201(2):247–251. doi: 10.1007/BF00425666. [DOI] [PubMed] [Google Scholar]
- Csonka L. N., Howe M. M., Ingraham J. L., Pierson L. S., 3rd, Turnbough C. L., Jr Infection of Salmonella typhimurium with coliphage Mu d1 (Apr lac): construction of pyr::lac gene fusions. J Bacteriol. 1981 Jan;145(1):299–305. doi: 10.1128/jb.145.1.299-305.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gay N. J. Construction and characterization of an Escherichia coli strain with a uncI mutation. J Bacteriol. 1984 Jun;158(3):820–825. doi: 10.1128/jb.158.3.820-825.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. F., Fast R., Karlström O., Larsen J. N. Association of RNA polymerase having increased Km for ATP and UTP with hyperexpression of the pyrB and pyrE genes of Salmonella typhimurium. J Bacteriol. 1986 Jun;166(3):857–865. doi: 10.1128/jb.166.3.857-865.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin H. L., Schachman H. K. Regulation of aspartate transcarbamoylase synthesis in Escherichia coli: analysis of deletion mutations in the promoter region of the pyrBI operon. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4643–4647. doi: 10.1073/pnas.82.14.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels G., Kelln R. A., Nargang F. E. Cloning, nucleotide sequence and expression of the pyrBI operon of Salmonella typhimurium LT2. Eur J Biochem. 1987 Jul 1;166(1):55–61. doi: 10.1111/j.1432-1033.1987.tb13483.x. [DOI] [PubMed] [Google Scholar]
- Navre M., Schachman H. K. Synthesis of aspartate transcarbamoylase in Escherichia coli: transcriptional regulation of the pyrB-pyrI operon. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1207–1211. doi: 10.1073/pnas.80.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Squires C., Yanofsky C. Ribosome-protected regions in the leader-trpE sequence of Escherichia coli tryptophan operon messenger RNA. J Mol Biol. 1976 May 15;103(2):411–420. doi: 10.1016/0022-2836(76)90320-x. [DOI] [PubMed] [Google Scholar]
- Poulsen P., Bonekamp F., Jensen K. F. Structure of the Escherichia coli pyrE operon and control of pyrE expression by a UTP modulated intercistronic attentuation. EMBO J. 1984 Aug;3(8):1783–1790. doi: 10.1002/j.1460-2075.1984.tb02046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland K. L., Powell F. E., Turnbough C. L., Jr Role of translation and attenuation in the control of pyrBI operon expression in Escherichia coli K-12. J Bacteriol. 1985 Sep;163(3):991–999. doi: 10.1128/jb.163.3.991-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Hicks K. L., Donahue J. P. Attenuation control of pyrBI operon expression in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1983 Jan;80(2):368–372. doi: 10.1073/pnas.80.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]


