Abstract
In many animal taxa, prior contest experience affects future performance such that winning increases the chances of winning in the future (winner effect) and losing increases the chances of losing in the future (loser effect). It is, however, not clear whether this pattern typically arises from experience effects on actual or perceived fighting ability (or both). In this study, we looked at winner and loser effects in the jumping spider Phidippus clarus. We assigned winning or losing experience to spiders and tested them against opponents of similar fighting ability in subsequent contests at 1-, 2-, 5-, and 24-h intervals. We examined the strength of winner and loser effects, how long effects persist, as well as how experience affected perceived and actual fighting ability. Our results demonstrate that winner and loser effects are of approximately the same magnitude, although loser effects last longer than winner effects. Our results also demonstrate that previous experience alters actual fighting ability because both the assessment and escalation periods were affected by experience. We suggest that the retention time of experience effects depends on expected encounter rates as well as other behavioral and ecological factors. In systems with short breeding seasons and/or rapidly fluctuating populations, context-dependent retention of experience effects may allow males to track their status relative to the fluctuating fighting ability of local competitors without paying the costs necessary to recall or assess individual competitors.
Keywords: contest experience, fighting ability, male–male competition, perceived RHP, Phidippus clarus, winner and loser effect
Prior contest experience alters future contest outcomes across taxa; winners are more likely to win future contests (winner effect), whereas losers are more likely to lose future contests (loser effects; for a review, see Hsu et al. 2006). However, the effect of prior experience is not fixed as the nature of winner and loser effects can vary between and within species (Hsu et al. 2006). For example, the magnitude of the winner and loser effect on subsequent contests may differ (e.g., Chase et al. 1994; Hsu and Wolf 1999). Furthermore, the retention times of winner and loser effects most often differ, with loser effects frequently lasting longer than winner effects (e.g., Beacham and Newman 1987; Schuett 1997). However, few studies have examined the relative magnitude and retention of winner and loser effects throughout multiple time frames as such studies require that experienced individuals fight a number of naive individuals (Hsu et al. 2006) and that individuals are tested at different naturally relevant time intervals (Hsu et al. 2006). Thus, examining such effects requires a large sample of animals, and this is prohibitive in many systems.
Apart from documentation of effects of prior contest experience, little is understood about why these effects exist. For example, it is not clear whether experience alters actual or perceived fighting ability. If experience alters actual fighting ability, then losers will continue to lose contests because their resource-holding potential (RHP) has decreased, making them poorer competitors (Parker 1974; Beaugrand et al. 1991). A second possibility is that prior experience alters how an individual perceives its own fighting ability relative to other individuals within a population (Whitehouse 1997; Mesterson-Gibbons 1999). Discriminating between these two possibilities requires an examination of aggressive behaviors along with contest outcomes as this will provide information on both motivation and fighting ability (Hsu et al. 2006). If prior contest experience affects perceived fighting ability, then aggressive behavior should only be affected during the assessment period of a contest. For example, winning experience should lead to elevated self-perception resulting in winners being more likely to initiate escalated contests. However, winners should be no more likely to persist and win escalated contests as this is determined by actual fighting ability. In contrast, if prior experience alters actual fighting ability, then fighting behavior should be altered throughout the entire fight as individual RHP has changed.
Our goal in this experiment was to determine the effect of prior contest experience on subsequent precontact contest behavior (perceived RHP) and postcontact contest behavior (actual RHP) as well as their overall effect on contest outcome. We used a jumping spider, Phidippus clarus, to examine 1) the magnitude of a loser versus a winner effect, 2) the retention of loser versus winner effects at different time intervals, and 3) potential effects on actual and perceived fighting ability in this species.
In P. clarus, the most recent contest experience can alter future contest outcomes (Kasumovic, Elias, et al. 2009), but the relative magnitude of winner versus loser effects and the retention of these effects are unknown. Moreover, this species is interesting to study as males follow self-assessment rules of conflict resolution during the assessment phase but may switch to partial opponent assessment when contests escalate to physical interactions (Elias et al. 2008). This system thus provides the opportunity to test whether perceived and/or actual fighting ability may be altered by examining the contest outcomes and dynamics during the assessment and escalation phases when different types of assessment are occurring. Studying mechanisms of the winner and loser effects in this species could contribute to our understanding of how social and ecological environments affect the evolution of experience effects.
MATERIALS AND METHODS
Housing and competitions
We collected adult males for this experiment from Koffler Scientific Reserve at Joker’s Hill, King City, Ontario, Canada (lat 44°03′N, long 79°29′W), from mid-June to mid-July 2008. All individuals were housed in individual 3 × 3 × 5 cm3 clear plastic cages on a 12:12 light:dark cycle and were fed small Acheta domesticus and Drosophila hydeii twice weekly. Because jumping spiders are known to possess well-developed vision (Forster 1982; Land 1985; Land and Nilsson 2002), we ensured that cages were separated by opaque barriers to minimize the potential effects of prior visual interactions on contest performance. All individuals were housed in this manner for at least 7 days to allow them to acclimate to laboratory conditions. We anesthetized all males used in this study 2 days before trials using CO2 and marked each individual with 2 spots of nontoxic fluorescent paint (Luminous paint; BioQuip Products, Inc., Rancho Dominguez, CA) on the abdomen to allow individual identification during contests. All individuals successfully captured prey after recovering from the anesthetic and behaved as other unmarked males in the population. This marking procedure has been successfully used in 2 previous studies examining intrasexual contests (Elias et al. 2008; Kasumovic, Elias, et al. 2009).
We staged 2 separate contests for each male, pairing males by weight (not body size) as weight is the only significant morphological predictor of contest outcomes in this species (Elias et al. 2008; Kasumovic, Elias, et al. 2009). There are 2 approaches used to provide individuals with an experience. The first is size-matching males and then allowing them to self-select the winner. Although this provides each individual with the same type of experience, experience effects become confounded with differences in intrinsic fighting ability, resulting in different predictions (Bégin et al. 1996). An alternative means is to predetermine the experience an individual will get by biasing the size difference between males (random selection procedure; Hsu et al. 2006). Although this removes any potential confounds with inherent fighting ability, it results in larger winners and smaller losers and individuals potentially receiving different “types” of experiences due to differences in relative sizes. Because our goal was to examine the effect of prior experience on contest outcomes, we used the random selection procedure because this ensured that inherent fighting ability played a minimum role. The mean weight difference was 19.85 ± 1.14% (mean ± standard error [SE]), and we attempted to control the effects of a size discrepancy in the first contest by ensuring that differences were not too large (17–22% size difference [95% confidence intervals]). To further alleviate the effect of differences in experience, each winner and loser from the first round were then paired against a naive weight-matched opponent (less than 5% difference in weight; mean weight difference = 2.85 ± 0.23% [mean ± SE]) for a second contest. To examine how long winner and loser effects last in this species, we placed winners and losers from the first contest in 1 of the 4 treatments defined by the interval between their first and second contest: 1) 1 h, 2) 2 h, 3) 5 h, and 4) 24 h.
We used multiple 5 × 5 × 4 cm3 plastic containers as the competitive arenas. All 4 walls were covered with petroleum jelly to prevent individuals from climbing the walls and leaving the arena. The base of each arena was covered with a sheet of paper that was changed between fights with new individuals to ensure there were no silk or pheromonal cues left by either the winner or the loser. To start each contest, we placed an opaque divider in the center of the arena and then placed 1 individual on either side of the divider to remove any potential ownership effects. Individuals were allowed 1 min to acclimate to their surroundings after which the divider was removed and the contest began.
During aggressive interactions, males perform a series of stereotyped behaviors that have been described elsewhere (Elias et al. 2008). Briefly, these behaviors can be divided into 2 phases: 1) a precontact phase in which males display toward one another and 2) an escalated contact phase in which males physically interact with one another. The precontact (assessment) phase begins when the 2 spiders orient toward one another, adopting a hunched posture. Males then approach or retreat from one another with their front legs outstretched horizontally. During these displays, males also produce a series of substrate-borne vibrations (Elias et al. 2008). The contact phase (escalation) begins when the 2 spiders are close to each other and begin to leg fence. Leg fencing consists of the 2 males touching each other’s horizontally outstretched legs whereby males attempt to push each other backward with their front legs and bodies. A subset of these interactions may escalate further to grappling, where males lock legs and chelicerae in relatively long bouts (Elias et al. 2008).
A contest lasted until an individual won, which was defined as the rival male turning away and retreating more than 2 body lengths. We timed all contests using a stopwatch, noting the time when both males first oriented toward each other, the time of first display, the individual that displayed first, the time of first contact, the time the contest ended, and the winner. After the outcome was decided, we removed each individual and placed it back into its cage. Males were not fed between the 2 contests. We weighed individuals before each fight using an Ohaus electronic balance.
Statistical analyses
Unless otherwise stated, we used a general linear model (GLM) with a binomial distribution and a logit link to examine the effect of prior experience and treatment on contest outcomes. We used individual binomial tests to test for experience effects at each time interval by comparing fight outcomes with random expectation (because size-matched males should have an equal chance of winning a fight; Elias et al. 2008); these tests were 1-tailed because we had a priori predictions about winner (increased winning) and loser (decreased winning) effects. We used a GLM with an exponential distribution and a reciprocal link to examine the effect of prior experience and time since prior experience on durations of various contest phases.
RESULTS
Contest outcomes
There were a total of 93 contests (using 186 males) with naive males in the first round, and heavier males won every contest as predicted (logistic: χ2 = 39.24, P < 0.0001). Winning a contest was not correlated with the first to display in these naive contests (binomial test: P = 0.12).
The 186 experienced males were paired with 186 naive males in the second round (N = 50 in each 1-, 2-, and 5-h treatments and N = 36 in the 24-h treatment). Contest outcome was significantly predicted by prior experience (χ2 = 5.84, degrees of freedom [df] = 1, 178, P = 0.016) and the interaction between prior experience and time interval (χ2 = 10.99, df = 3, 178, P = 0.012). There was no significant main effect of the time interval (χ2 = 4.01, df = 3, 178, P = 0.26). Individual binomial tests of winner or loser effects for each time since prior experience demonstrate that a loser effect was present after 1 h (18/25 losing males lost again; 1-tailed P = 0.021) and 2 h (18/25 losing males lost again; 1-tailed P = 0.021), with no significant effects at other times; a winner effect was present after 1 h only (19/25 winning males won again; 1-tailed P = 0.007; Figure 1). There was also an opposite winner effect present after 5 h where winners of the first contest were more likely to lose their subsequent contest than expected by chance (6/25 winning males won; 1-tailed P = 0.007; Figure 1).
Figure 1.
The proportion of male Phidippus clarus that won a contest with a naive size-matched rival at 4 time intervals after first winning (black) or losing (white) an initial contest. In P. clarus, relative size predicts competitive success, so if there is no experience effect, 50% of previous winners or losers should win subsequent contests (dotted line). Stars indicate proportions significantly different from 50% (see text).
Because winner and loser effects co-occurred only during the first hour, we used only contests in this treatment to estimate the magnitude of the winner relative to the loser effect in P. clarus. To quantify this effect, we compared the percent increase in the probability of winning the second contest (relative to 50%) for males that won the first contest and compared this with the percent decrease in the probability of winning for a male that lost the first contest. The probability of winning the second contest for a male with losing experience was 28% (7/25 losing males won), a decrement of 22% relative to random expectation. The probability of winning a subsequent contest for a male with a winning experience was 76% (19/25 males won), an increment of 26%. Thus, the magnitudes of each effect were roughly equal.
Contest behaviors
We examined whether a winner and loser effect altered 1) whether the focal (experienced) individual initiated signaling, 2) the time to signal initiation, 3) the duration of the assessment period, and 4) the duration of the escalation period.
Neither prior experience alone (χ2 = 0.53, df = 1, 178, P = 0.47) nor time since prior experience (χ2 = 1.19, df = 3, 178, P = 0.75) affected whether the focal individual initiated signaling. However, there was a significant interaction between prior experience and the time interval (χ2 = 8.61, df = 3, 178, P = 0.035), with winners being more likely to display first in subsequent contests after 1 h (Wald χ2 = 3.33, P = 0.068). Prior winners displayed more quickly in subsequent contests (χ2 = 8.62, df = 1, 178, P = 0.0033); however, there was no effect of time since prior experience (χ2 = 0.95, df = 3, 178, P = 0.81) or an experience × time interaction (χ2 = 1.62, df = 3, 178, P = 0.66) on the time to first display. The assessment period concluded more quickly in contests involving prior winners than contests involving prior losers (44.04 ± 7.86 s and 65.16 ± 7.86 s, respectively; χ2 = 6.96, df = 1, 178, P = 0.0083), whereas there was no effect of time since prior experience or an experience × time interaction on the assessment duration (χ2 = 0.46, df = 3, 178, P = 0.93 and χ2 = 6.79, df = 3, 178, P = 0.08, respectively). Prior experience predicted whether contests were escalated (χ2 = 12.23, df = 1, 178, P = 0.0005), with contests involving prior losers being less likely to escalate (62/93 escalated) than contests involving prior winners (81/93 escalated). The time since prior experience (χ2 = 2.38, df = 1, 178, P = 0.50) did not affect the likelihood of contest escalation nor did an experience × time interaction (χ2 = 4.39, df = 1, 178, P = 0.22).
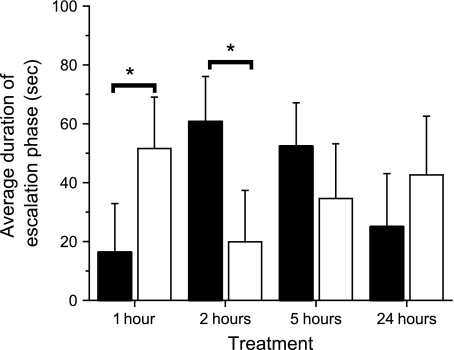
To examine whether prior winners were more likely to win escalated contests than prior losers, we compared the actual number of escalated contests won by winners and losers to the expected frequency of 50%. Although losers were more likely to lose escalated contests (losers won 19 of 62 escalated contests, P = 0.02), winners were no more likely to win escalated contests than expected (winners won 38 of 81 escalated contests, P = 0.42). Because not all prior winners won and not all prior losers lost their second contests, we only used escalated contests where winners won their second contest and losers lost their second contest (N = 77) to further examine whether there was a significant difference in the amount of time it took escalated contests with winners and losers to conclude. Prior experience affected the duration of the escalation period (χ2 = 8.04, df = 1, 69, P = 0.0046), with winners winning contests relatively more quickly than losers losing contests. There was also a significant experience × time interaction (χ2 = 36.73, df = 3, 69, P < 0.0001; Figure 2), with winners winning escalated contests relatively more rapidly at 1 h (Wald χ2 = 13.27, P < 0.0003) and losers losing escalated contests relatively more rapidly at 2 h (Wald χ2 = 16.82, P < 0.0001). There was no significant difference at 5 and 24 h. There was also no significant effect of the time since prior experience on the escalation duration (χ2 = 3.12, df = 3, 69, P = 0.37).
Figure 2.
Average duration of the escalation period of prior winners that won (black) and prior losers that lost (white) in their second contests with a naïve male.
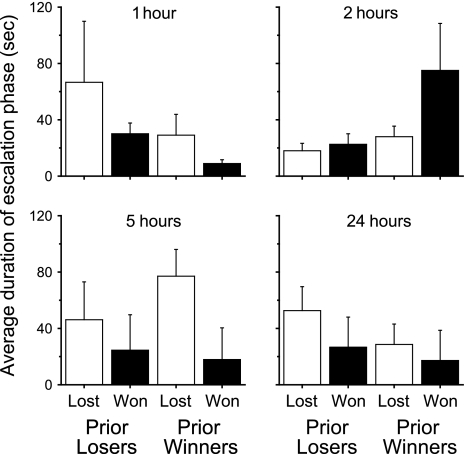
To examine whether this significant difference in the time to conclude escalated contests was because winners won more quickly or losers took longer to lose, we examined whether the length of the escalations periods of winners that won and losers that lost (individuals that should have the shortest and longest escalation periods, respectively) differed from escalation periods between winners that lost and losers that won (individuals that should have intermediate escalation periods) at the different time periods. Winners won more quickly and losers took longer to lose at 1 h (χ2 = 20.37, df = 3, 32, P < 0.0001), and winners took longer to win at 2 h (χ2 = 15.22, df = 3, 35, P = 0.0016; Figure 3). Winners also took longer to lose at 5 h (χ2 = 11.50, df = 3, 35, P = 0.0007), and losers took longer to lose at 24 h (χ2 = 3.98, df = 3, 35, P = 0.046; Figure 3).
Figure 3.
Average duration of the escalation period of prior losers that lost (white) and won (black) and prior winners that lost (white) and won (black) in their second contests at the different time frames.
DISCUSSION
In this study, we looked at overall winner and loser effects, perceived fighting ability (willingness to escalate contests), and actual fighting ability (ability of males to win escalated contests). For animals with fighting experience, any significant change in the probability of winning against weight-matched opponents will be a function of changes in perceived fighting ability, actual fighting ability, and costs associated with their previous encounter. Here, we were able to quantify changes in the frequency of escalated contest outcomes because in P. clarus, a naïve male’s chance of winning against an individual of the same weight is 50% (Hsu et al. 2006; Elias et al. 2008; Kasumovic, Elias, et al. 2009). We clearly demonstrate that there is both a winner and a loser effect in P. clarus. Experience effects, however, were not constant in our study and changed depending on both the form of experience (winner vs. loser experience) and the time between encounters. Males that lost a contest were more likely to lose a subsequent contest for a period of at least 2 h. In contrast, males that won a contest were more likely to win a subsequent contest for a period of at least 1 h, but also suffered a detrimental effect at 5 h. In addition, we demonstrate that experience can affect both perceived and actual fighting ability.
Our results demonstrate that winning and losing experiences have different retention times, similar to previous results in other taxa (e.g., Beacham and Newman 1987; Chase et al. 1994; but see Hsu and Wolf 1999). Although loser effects persisted for longer (in terms of the overall effect on contest outcome), both winner and loser effects reset after 24 h. We also show that overall winner and loser effects are of approximately the same magnitude after 1 h which is in contrast to other results (fish: Beacham and Newman 1987; Chase et al. 1994; snakes: Schuett 1997) but similar to a previous study that used naïve individuals as test subjects (Hsu and Wolf 1999). Previous work concluded that only the most recent experience was relevant for P. clarus (Kasumovic, Elias, et al. 2009). However, due to the large tournament-style design in the previous study, the mean intercontest duration exceeded 1 h (mean ± SE, 193.73 ± 3.23 min; Kasumovic, Elias, et al. 2009). Given the results presented here, it is difficult to surmise whether the most recent experience was important because of a time-related decay in experience effects or because the most recent experience replaced earlier experiences.
Experience can affect perceived fighting ability, actual fighting ability, or both. A male’s actual fighting ability is reflected in its willingness to engage and escalate a contest as well as how long males can persist in escalated interactions or in their ability to inflict costs on opponents (Hsu et al. 2006). In contrast, a male’s perceived fighting ability is reflected only in its willingness to engage and initiate contest escalation (Hsu et al. 2006). As previous experience affected both the willingness to escalate contests and how long males persist in contests, our results suggest that previous experience affects actual fighting ability in P. clarus. Our results thus provide indirect evidence against the social cue hypothesis (Rutte et al. 2006) that suggests that naïve opponents base their decision to escalate or retreat from experienced opponents based on cues from prior experience. Because prior experience altered the actual fighting ability of males, males would be signaling their actual fighting ability to rivals rather than prior contest experience. Although social cues may explain contest outcomes during eavesdropping (but see Earley et al. 2005; e.g., Earley and Dugatkin 2002), our study along with indirect and direct evidence from other (fish) species (Wallen and Wojciechowski-Metzlar 1985; Hsu et al. 2009) suggests that the social cue hypothesis cannot explain the winner–loser effect when experienced individuals encounter naïve individuals.
Our results demonstrate a time-dependent shift in fighting ability for both winners and losers. Prior winners won escalated contests more quickly after 1 h, whereas losers took longer to lose escalated contests after 1 h. Moreover, prior winning experience resulted in winners taking longer to win subsequent contests. These results suggest that prior experience positively affects actual fighting ability for both prior winners and losers in specific contexts. Our most interesting result, however, demonstrates a reduction in actual fighting ability for winners after 5 h (Figures 1 and 3), which suggests a time-dependent cost of winning. There are 2 possible explanations for this result. First, a decrease in fighting ability may be a result of physical injury (e.g., Neat et al. 1998) or increases in lactate accumulation, heart rate, or oxygen consumption (e.g., Hack 1997; Granter and Taborsky 1998; Neat et al. 1998; Sneddon et al. 1999). Although such responses could explain the differences seen between winners and losers (Neat et al. 1998; Schuett and Grober 2000), a response should occur immediately after a contest; a pattern not observed as the affect is seen after 5 h.
A secondary explanation is that decreases in fighting ability are due to physiological costs associated with the neuroendocrine system (see Hsu et al. 2006 for a review). Hormone titers (e.g., testosterone in vertebrates and serotonin in arthropods) initially increase after a fight and often lead to increases in contest success (e.g., Hannes et al. 1984; Edwards and Kravitz 1997; Huber and Delago 1998; Mehta and Josephs 1998; Earley and Hsu 2008). The differential decay of hormone titers to baseline levels may explain differences in winner and loser effects (Hannes et al. 1984; Summers et al. 2003; Overli et al. 2004; Hsu et al. 2006). Furthermore, as increases in hormone levels are associated with costs in both vertebrate and invertebrate systems (e.g., Marler and Moore 1988; Folstad and Karter 1992; Sapolsky 1996), these costs may manifest as a decrease in fighting ability after 5 h. Our results suggest a precise time line of hormonal effects in P. clarus, and future work is needed to examine this effect. The fast time line of experience effects suggests that the mechanism behind experience effects in P. clarus could be purely hormonal and need not be based on changes in synaptic wiring (i.e., learning) that are usually involved in longer “memory” time frames.
The retention time of experience effects should depend on behavioral and ecological factors that affect whether the future likelihood of winning or losing contests is more reliably predicted by early experiences or by information gleaned from competitors in each interaction. For example, short-lived organisms like arthropods should benefit from “remembering” only the most recent experiences, whereas long-lived organisms should benefit from longer-term integration of experience effects. Another important consideration is the reliability of information that recent experiences were based on. In P. clarus, competitive signals relay information about cephalothorax (head) width, but this does not predict fighting success (Elias et al. 2008); in fact, the only significant phenotypic predictor of contest success is weight (Elias et al. 2008; Kasumovic, Elias, et al. 2009)—even when a principal component analysis is used to remove correlations between traits (Kasumovic MM, Elias DO, unpublished data). Unlike all measures of size, weight is likely to rapidly fluctuate throughout a breeding season, both in terms of the individual’s absolute weight (RHP) and in terms of the individual’s weight relative to a constantly shifting distribution of competitors. As weight predicts contest success and weight fluctuates, the information from recent experience is likely unreliable. Under this scenario, one would predict that experience effects should be integrated over a relatively short time window (Hsu et al. 2006).
In addition, as males and females are maturing throughout the breeding season (Hoefler 2007) and females multiply mate (Sivalinghem S, Kasumovic MM, Elias DO, in preparation), competitive challenges can shift throughout a breeding season (Kasumovic et al. 2008; Kasumovic, Bruce, et al. 2009). Thus, as actual fighting ability in P. clarus can change as a result of experience, coupled with fluctuations in weight, and unreliability of signals, long-term experience effects would not yield optimum fighting strategies. Males could, however, determine their fighting ability relative to other individuals within the population through single encounters and adjust their competitive strategy (threshold for engagement and escalation) according to their most recent experience and, in the absence of a recent fight or after gaining/losing weight, could reset themselves to some average strategy (e.g., Whitehouse 1997). Under these conditions, poor competitors would typically avoid costs associated with fights, whereas good competitors would initiate and win more fights. Our data (and see Kasumovic, Elias, et al. 2009) suggest this type of experience effect in P. clarus, supporting the idea of adaptation to rapidly changing competitive conditions.
Our results provide empirical data for the model of experience integration proposed by Hsu et al. (2006). In this model, Hsu and collaborators propose a “leaky integrator” model of contest experience where additive experiences alter fighting ability, which decay through time to some baseline “experience” level. Over repeated encounters, an individual’s estimate of his own fighting ability changes as due to successful (incrementing) and unsuccessful (decrementing) encounters (Hsu et al. 2006). Our results show the time course of the predicted experience “decay” and suggest that the decay of experience effects can be complex and context dependent. Furthermore, we suggest that the Hsu and Wolf model of integrating experience (Hsu et al. 2006) may be a particularly relevant and elegant solution for contests in situations where competitive environments rapidly fluctuate and/or competitive traits/signals are unreliable. Over repeated encounters, an individual’s estimate of his own fighting ability should float to a level relative to that of his pool of competitors as the ratio of successful (incrementing) and unsuccessful (decrementing) encounters. Under this situation, individuals can keep a “running average” of their competitive ability relative to those around without having to pay the costs associated with long-term memory storage.
FUNDING
Natural Sciences and Engineering Research Council (NSERC) postdoctoral fellowship to M.M.K.; National Institute of Health National Research Service Award (1F32GM076091-01A1) to D.O.E.; NSERC Discovery Grants (229029-2004 to M.C.B.A., 238882 241419 to A.C.M.); Canadian Foundation for Innovation and Ontario Innovation Trust to M.C.B.A. and A.C.M.
Supplementary Material
Acknowledgments
We would like to thank Tessa Wallace for invaluable field and laboratory assistance. We would also like to thank R. Elwood, R. Dukas, M. Hall and two anonymous reviewers that provided comments that improved the manuscript, and the University of Toronto, Scarborough and the Integrative Behaviour and Neuroscience Group for useful discussions.
References
- Beacham JL, Newman JA. Social experience and the formation of dominance relationships in the pumpkinseed sunfish, Lepomis gibbosus. Anim Behav. 1987;35:1560–1562. [Google Scholar]
- Beaugrand J, Goulet C, Payette D. Outcome of dyadic conflict in male green swordtail fish, Xiphophorus helleri: effects of body size and prior dominance. Anim Behav. 1991;41:417–424. [Google Scholar]
- Bégin J, Beaugrand JP, Zayan R. Selecting dominants and subordinates at conflict outcome can confound the effects of prior dominance or subordination experience. Behav Process. 1996;36:219–226. doi: 10.1016/0376-6357(95)00031-3. [DOI] [PubMed] [Google Scholar]
- Chase ID, Bartolomeo C, Dugatkin LA. Aggressive interactions and inter-contest interval: how long do winners keep winning? Anim Behav. 1994;48:393–400. [Google Scholar]
- Earley RL, Druen M, Dugatkin LA. Watching fights does not alter a bystander’s response towards naïve conspecifics in male green swordtail fish, Xiphophorus helleri. Anim Behav. 2005;69:1139–1145. [Google Scholar]
- Earley RL, Dugatkin LA. Eavesdropping on visual cues in green swordtail (Xiphophorus helleri) fights: a case for networking. Anim Behav. 2002;39:442–451. doi: 10.1098/rspb.2002.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley RL, Hsu Y. Reciprocity between endocrine state and contest behavior in the killifish, Kryptolebias marmoratus. Horm Behav. 2008;53:442–451. doi: 10.1016/j.yhbeh.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Kravitz EA. Serotonin, social status, and aggression. Curr Opin Neurobiol. 1997;7:812–819. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- Elias DO, Kasumovic MM, Punzalan D, Andrade MCB, Mason AC. Male assessment during aggressive contests in jumping spiders. Anim Behav. 2008;76:901–910. doi: 10.1016/j.anbehav.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am Nat. 1992;139:603–622. [Google Scholar]
- Forster L. Visual communication in jumping spiders (Salticidae) In: Witt PN, Rovner JS, editors. Spider communication: mechanisms and ecological significance. Princeton (NJ): Princeton University Press; 1982. pp. 161–212. [Google Scholar]
- Granter A, Taborsky M. The metabolic rates associated with resting, and with the performance of agonistic, submissive and digging behaviours in the cichlid fish Neolamprologus pulcher (Pisces: Cichlidae) J Comp Physiol B. 1998;186:427–433. [Google Scholar]
- Hack MA. The energetic costs of fighting in the house cricket, Acheta domesticus L. Behav Ecol. 1997;8:28–36. [Google Scholar]
- Hannes RP, Franck D, Liemann F. Effects of rank order fights on whole-body and blood concentrations of androgens and corticosteroids in the male swordtail (Xiphophorus helleri) Z Tierpsychol. 1984;65:53–65. [Google Scholar]
- Hoefler CD. Male mate choice and size-assortative pairing in a jumping spider, Phidippus clarus. Anim Behav. 2007;73:943–954. [Google Scholar]
- Hsu Y, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Lee IH, Lu CK. Prior contest information: mechanisms underlying winner and loser effects. Behav Ecol Sociobiol. 2009;63:1247–1257. [Google Scholar]
- Hsu Y, Wolf LL. The winner and loser effect: integrating multiple experiences. Anim Behav. 1999;57:903–910. doi: 10.1006/anbe.1998.1049. [DOI] [PubMed] [Google Scholar]
- Huber R, Delago A. Serotonin alters decisions to withdraw in fighting crayfish, Astacus astacus: motivational concept revisited. J Comp Physiol A. 1998;182:573–583. [Google Scholar]
- Kasumovic MM, Bruce MJ, Andrade MCB, Herberstein ME. Spatial and temporal demographic variation drives within-season fluctuations in sexual selection. Evolution. 2008;62:2316–2325. doi: 10.1111/j.1558-5646.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- Kasumovic MM, Bruce MJ, Herberstein ME, Andrade MCB. Evidence for developmental plasticity in response to demographic variation in nature. Ecology. 2009;90:2287–2296. doi: 10.1890/08-1540.1. [DOI] [PubMed] [Google Scholar]
- Kasumovic MM, Elias DO, Punzalan D, Andrade MCB, Mason AC. Experience affects the outcome of agonistic contests without affecting the selective advantage of size. Anim Behav. 2009;77:1533–1538. doi: 10.1016/j.anbehav.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land MF. The morphology and optics of spider eyes. In: Barth FG, editor. Neurobiology of arachnids. New York: Springer-Verlag; 1985. pp. 53–78. [Google Scholar]
- Land MF, Nilsson DE. Animal eyes. Oxford: Oxford University Press; 2002. [Google Scholar]
- Marler CA, Moore MC. Evolutionary costs of aggression revealed by testosterone manipulations in free-living male lizards. Behav Ecol Sociobiol. 1988;23:21–26. [Google Scholar]
- Mehta PH, Josephs RA. Testosterone change after losing predicts the decision to compete again. Horm Behav. 1998;50:684–692. doi: 10.1016/j.yhbeh.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Mesterson-Gibbons M. On the evolution of pure winner and loser effects: a game theoretic model. Bull Math Biol. 1999;61:1151–1186. doi: 10.1006/bulm.1999.0137. [DOI] [PubMed] [Google Scholar]
- Neat FC, Taylor AC, Huntingford FA. Proximate costs of fighting in male cichlid fish: the role of injuries and energy metabolism. Anim behav. 1998;55:875–882. doi: 10.1006/anbe.1997.0668. [DOI] [PubMed] [Google Scholar]
- Overli O, Korzan WJ, Hoglund E, Winberg S, Bollig H, Watt M, Forster GL, Barton BA, Overlie E, Renner KJ, et al. Stress coping style predicts aggression and social dominance in rainbow trout. Horm Behav. 2004;45:235–241. doi: 10.1016/j.yhbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Parker GA. Assessment strategy and the evolution of fighting behaviour. J Theor Biol. 1974;47:223–243. doi: 10.1016/0022-5193(74)90111-8. [DOI] [PubMed] [Google Scholar]
- Rutte C, Taborsky M, Brinkhof MWG. What sets the odds of winning and losing? Trends Ecol Evol. 2006;21:16–21. doi: 10.1016/j.tree.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Schuett GW. Body size and agonistic experience affect dominance and mating success in male copperheads. Anim Behav. 1997;54:213–224. doi: 10.1006/anbe.1996.0417. [DOI] [PubMed] [Google Scholar]
- Schuett GW, Grober MS. Post-fight levels of plasma lactate and corticosterone in male copperheads, Agkistrodon contortrix (Serpentes, Viperidae): differences between winners and losers. Physiol Behav. 2000;71:335–341. doi: 10.1016/s0031-9384(00)00348-6. [DOI] [PubMed] [Google Scholar]
- Sneddon LU, Taylor AC, Huntingford FA. Metabolic consequences of agonistic behaviour: crab fights in declining oxygen tension. Anim Behav. 1999;57:353–363. doi: 10.1006/anbe.1998.0982. [DOI] [PubMed] [Google Scholar]
- Summers CH, Summers TR, Moore MC, Korzan WJ, Woodley SK, Ronan PJ, Hoglund E, Watt MJ, Greenberg N. Temporal patterns of limbic monoamine and plasma corticosterone response during social stress. Neuroscience. 2003;116:553–563. doi: 10.1016/s0306-4522(02)00708-x. [DOI] [PubMed] [Google Scholar]
- Wallen K, Wojciechowski-Metzlar CI. Social conditioning and dominance in male Betta splendens. Behav Process. 1985;11:181–188. doi: 10.1016/0376-6357(85)90059-2. [DOI] [PubMed] [Google Scholar]
- Whitehouse MEA. Experience influences male-male contests in the spiders Argyrodes antipodiana (Theridiidae: Aranea) Anim Behav. 1997;53:913–923. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.