Abstract
Purpose
To provide a current perspective on the management of CI in children by summarizing the findings and discussing the clinical implications from three recent randomized clinical trials in which we evaluated various treatments for children with symptomatic CI. We then present an evidence-based treatment approach for symptomatic CI based upon the results of these trials. Finally, we discuss unanswered questions and suggest directions for future research in this area.
Methods
We reviewed 3 multi-center randomized clinical trials comparing treatments for symptomatic CI in children 9 to 17 years old (one study 9 to 18 years old). Two trials evaluated active therapies for CI. These trials compared the effectiveness of office-based vergence/accommodative therapy, office-based placebo therapy, and home-based therapy (pencil push-ups alone [both trials], home-based computer vergence/accommodative therapy and pencil push-ups [large-scale study]). One trial compared the effectiveness of base-in prism reading glasses to placebo reading glasses. All studies included well-defined criteria for the diagnosis of CI, a placebo group, and masked examiners. The primary outcome measure was the Convergence Insufficiency Symptom Survey score. Secondary outcomes were near point of convergence and positive fusional vergence at near.
Results
Office-based vergence/accommodative therapy was significantly more effective than home-based or placebo therapies. Base-in prism reading glasses were no more effective than placebo reading glasses for the treatment of symptomatic CI in children.
Conclusions
Recent clinical trials showed that office-based vision therapy was successful in about 75% of patients (resulting in normal or significantly improved symptoms and signs) and was the only treatment studied which was more effective than placebo treatments for children with symptomatic CI. Eye care providers who do not currently offer this treatment may consider referring these patients to a doctor who provides this treatment or consider expanding the treatment options available within their practice to manage this condition.
Keywords: convergence insufficiency, vision therapy, orthoptics, vergence/accommodative therapy, computer orthoptics, pencil push-ups, base-in prism reading glasses, exophoria, eyestrain, symptoms
Convergence insufficiency (CI) is a common binocular vision disorder that affects approximately 4% of the population1–3 and is often associated with symptoms such as frequent loss of place, loss of concentration, having to re-read, reading slowly, trouble remembering what was read, sleepiness, blurred vision, diplopia, headaches, and/or eyestrain during reading or other near work.4–12 Clinical signs of CI include an exodeviation that is greater at near than at distance, a receded near point of convergence (NPC), and reduced positive fusional vergence at near (PFV).4,13–14
The overall objective of this paper is to provide a current perspective on the management of CI in children. First, we summarize the findings and discuss the clinical implications from three recent randomized clinical trials in which we evaluated various treatments for children with symptomatic CI. Second, we propose an evidence-based treatment approach for symptomatic CI based upon the results of these trials. Finally, we discuss unanswered questions and suggest directions for future research in this area.
Treatment of Convergence Insufficiency: Previous Research
Historically, there has been a lack of consensus regarding the most effective treatment for CI. Various therapeutic options are available9,15–24 including passive treatment with base-in prism reading glasses, and active treatments such as office-based vergence/accommodative vision therapy/orthoptics or home-based therapy with pencil push-ups alone or pencil push-up therapy plus other vergence/accommodative procedures. The lack of agreement among eye care providers may in part be due to a perception that there are considerable differences in terms of cost and the ease of implementation of these treatments.
Recent studies surveying eye care providers suggest that home-based pencil push-up therapy and base-in prism reading glasses are the most commonly prescribed treatments by both optometrists and ophthalmologists, with 87% prescribing these two treatment modalities fairly often, often or always for young patients with symptomatic CI.25–27 The clinical popularity of these treatments, however, has been based mainly upon observations and clinical impressions rather than evidence-based medicine. The one study that has evaluated pencil push-ups24 and the few that have evaluated base-in prism treatment for children with CI have suffered from significant design flaws.24,28–31
Because of the limited quality evidence to guide eye care providers in their clinical decision making for school-age children with symptomatic CI, the CITT Investigator Group systematically addressed this void by completing three multi-center, randomized clinical trials with the goal of evaluating the effectiveness of the commonly prescribed treatments for CI in children with symptomatic CI.
Recent Investigations of the Treatments of Convergence Insufficiency in Children
All three CITT studies were randomized clinical trials; each included a placebo control group and used masked examiners for the assessment of outcome measures. Symptomatic CI was defined as: 1) an exodeviation at near at least 4 prism diopters (Δ) greater than at far, 2) a receded NPC break (6 cm or greater), 3) insufficient PFV (i.e., failing Sheard’s criterion or minimum PFV of ≤15Δ base-out blur or break), and 4) a symptomatic score on the Convergence Insufficiency Symptom Survey (CISS). Major eligibility criteria for the studies were essentially the same (Table 1).32–34
Table 1.
Eligibility and exclusion criteria.
| Eligibility Criteria |
| Age 9 to 17 years |
| Best-corrected visual acuity of 20/25 or better in both eyes at distance and near |
| Willingness to wear eyeglasses or contact lenses to correct refractive error, if necessary |
| Exodeviation at near at least 4Δ greater than at far |
| Insufficient positive fusional convergence (i.e., failing Sheard’s criterion35 or ≤15Δ blur or break) on positive fusional vergence testing using a prism bar* |
| Receded near point of convergence of ≥6 cm break |
| Appreciation of at least 500 seconds of arc on the forms part of the Randot Stereotest |
| Symptomatic CI Symptom Survey Score (13 question version ≥ 9 [pilot study]; 15 question version ≥16 [Base-in prism study; large-scale study]) |
| Informed consent and willingness to participate in the study and be randomized |
| Exclusion Criteria |
| CI previously treated with pencil push-up therapy (more than 2 weeks of treatment) |
| CI previously treated with home- or office-based VT/orthoptics |
| Amblyopia (≥2 line difference in best-corrected visual acuity between the two eyes) |
| Constant strabismus |
| History of strabismus surgery |
| High refractive error: Myopia ≥6.00D sphere (in any meridian), hyperopia ≥5.00D sphere (in any meridian), astigmatism ≥4.00D |
| Anisometropia ≥2.00D spherical equivalent |
| Prior refractive surgery |
The primary outcome measure for all studies was the CISS, a 15-item questionnaire that measures symptoms experienced when reading or doing other close work. The instrument has been shown to be a reliable and valid measure of symptoms in children with CI,12,35 with a symptom score ≥16 differentiating children with symptomatic CI from those with normal binocular vision. (12) Secondary outcome measures were the NPC and PFV at near.
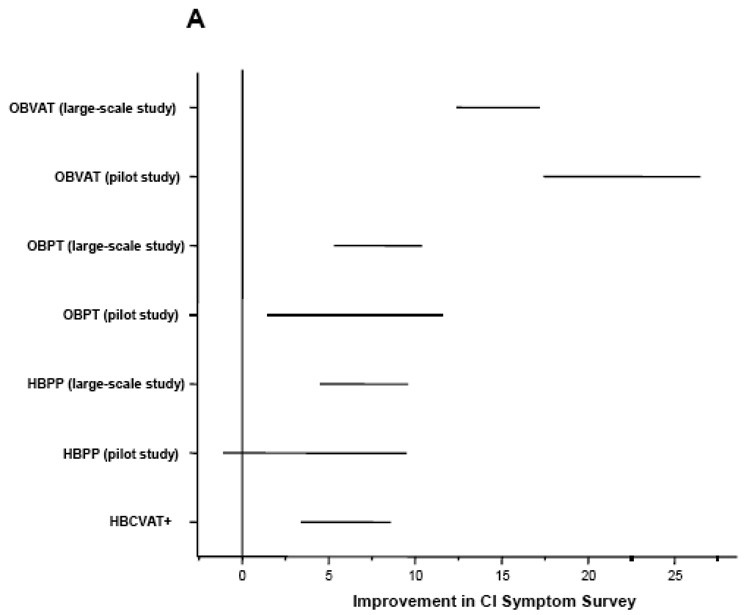
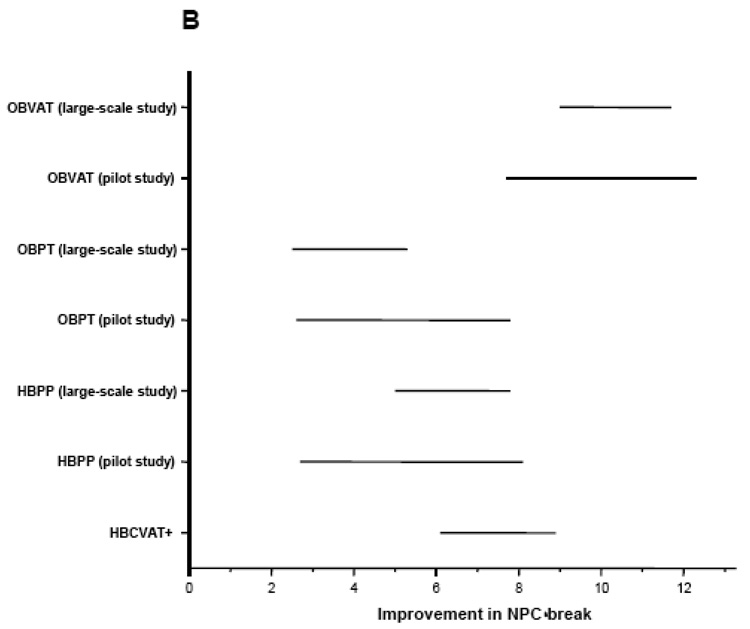
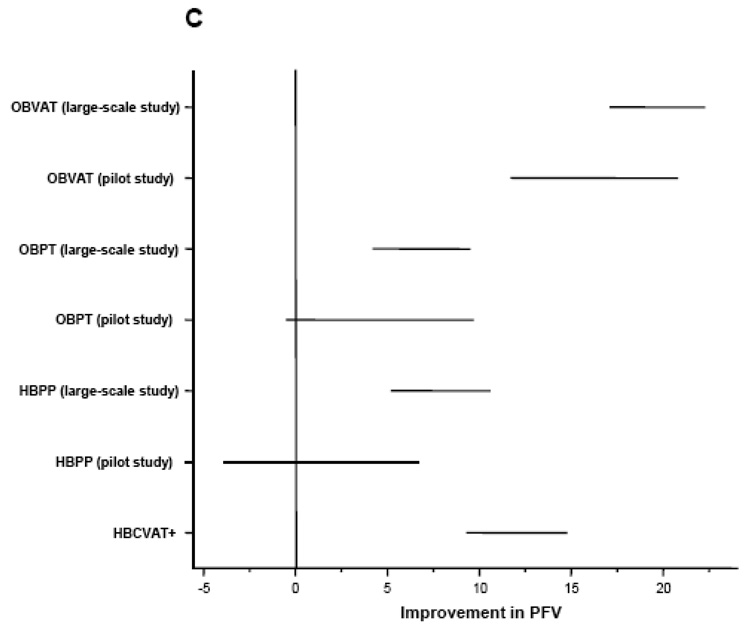
Outcome measures were assessed at baseline and again by a masked examiner at the outcome examination? Table 2 summarizes the study design and results of the three CITT trials and Figure 1 provides a graphical comparison of the results from the three studies.
Table 2.
Summary of CITT randomized clinical trials.
| Study | Year Journal | Age (Years) | # Patients | Treatment Groups | Key Results |
|---|---|---|---|---|---|
| A Randomized Clinical Trial of Treatments for Convergence Insufficiency in Children | 2005 Arch Ophthalmol | 9–18 | 47 | Home-Based Pencil Push-ups Office-Based VT/Orthoptics Office-Based Placebo VT/Orthoptics | Office-based VT/Orthoptics was more effective than home-based pencil push-ups and office-based placebo VT/Orthoptics in reducing symptoms and improving signs of CI in children 97#x02013;18 years of age |
| Neither pencil push-ups nor office-based placebo VT/Orthoptics was effective in improving either symptoms or signs associated with CI | |||||
| A Randomized Clinical Trial of the Effectiveness of Base-in Prism Reading Glasses vs. Placebo Reading Glasses for Symptomatic Convergence Insufficiency in Children | 2005 Br J Ophthalmol | 9–17 | 72 | Base-in Prism Reading Glasses Placebo Reading Glasses | Base-in prism reading glasses were found to be no more effective in alleviating symptoms, improving the NPC, or improving PFV at near than placebo reading glasses for the treatment of symptomatic CI in children 9 to <18 years of age |
| A Randomized Clinical Trial of Treatments for Symptomatic Convergence Insufficiency in Children | 2008 Arch Ophthalmol | 9–17 | 221 | Home-based pencil push-ups | 12 weeks of office-based vergence/accommodative therapy results in a significantly greater improvement in symptoms and clinical measures of NPC and PFV and a greater percentage of patients reaching pre-determined criteria of success when compared with home-based pencil push-ups, home-based computer vergence/accommodative therapy and pencil push-ups, and office-based placebo therapy. |
| Home-based computer vergence/accommodative therapy and pencil push-ups | |||||
| Office-based vergence/accommodative therapy with home reinforcement | |||||
| Office-based placebo therapy | |||||
Figure 1.
Mean improvement adjusted for baseline in (A) CI Symptom Survey, (B) Near Point of Convergence Break (cm), and (C) Positive Fusional Vergence (Δ).OBVAT: Office-based vergence/accommodative therapy with home reinforcement; OBPT: Office-based placebo therapy with home reinforcement; HBPP: Home-based pencil push-up therapy; HBCVAT+: Home-based computer vergence/accommodative therapy and pencil push-ups.
CITT Pilot Study: A Randomized Clinical Trial of Treatments for Convergence Insufficiency in ChildreN
This study was a multi-center clinical trial of 47 children aged 9–18 years with symptomatic CI who were randomly assigned to receive a 12-week program of home-based pencil push-ups, office-based VT/orthoptics, office-based placebo therapy. All methods have been described previously in detail.32 In brief, the home-based pencil push-ups group were prescribed 15 minutes of pencil push-ups for 5 days per week using small letters on a pencil as the target and a physiological diplopia awareness control. The office-based VT/orthoptics group received a weekly 60-minute in-office therapy visit with additional home therapy procedures prescribed for 15 minutes a day, 5 days per week. Therapy consisted of a specific sequence of standard vergence and accommodative procedures.22,32 Patients in the office-based placebo therapy group also received therapy during a weekly 60-minute office visit and were prescribed procedures to be performed at home for 15 minutes per day, 5 days per week; however, their therapy procedures were designed to look like real vergence/accommodative therapy procedures yet not stimulate vergence, accommodation, or fine saccadic eye movement skills beyond normal daily visual activities.36
RESULTS
There were no significant differences at baseline between the groups. At the 12-week outcome examination, the CISS score was significantly reduced in the VT/orthoptics group (mean score decreased from 32.1 to 9.5), but not in the pencil push-ups (mean score decreased from 29.3 to 25.9) or placebo VT/orthoptics (mean score decreased from 30.7 to 24.2) groups. Only patients in the VT/orthoptics group demonstrated both statistically and clinically significant changes in NPC (decreased from 13.7cm to 4.5cm, p=0.0001) and PFV (increased from 12.5Δ to 31.8Δ, p=0.0004).32
In this study, office-based VT/orthoptics was found to be more effective than home-based pencil push-ups or office-based placebo therapy in reducing symptoms and improving signs of CI. Moreover, it was the only treatment that resulted in normalization of CI-related symptoms and signs. Interestingly, pencil push-up therapy was found to be no more effective than the placebo therapy.
CITT Base-In Reading Glasses Study: A Randomized Clinical Trial of the Effectiveness of Base-in Prism Reading Glasses vs. Placebo Reading Glasses for Symptomatic Convergence Insufficiency in Children
In this clinical trial,33 72 children 9 to <18 years of age with symptomatic CI were randomly assigned to wear either base-in prism glasses or placebo reading glasses for all reading and near tasks requiring more than 5 minutes for 6 weeks. Eligibility criteria were similar to those listed in Table 1. Patients in the base-in prism group received a refractive correction for distance (if necessary) or plano-powered lenses when refractive error was minimal with base-in prism prescribed according to Sheard (i.e., prism equal to 2/3 the phoria minus 1/3 the PFV)37 and prism rounded up to the nearest 0.5Δ. Patients assigned placebo reading glasses received glasses that corrected their refractive error (if necessary), or plano lenses when no refractive correction was required. The primary outcome examination was conducted after 6 weeks of glasses wear.
RESULTS
In the group receiving base-in prism, the mean prism prescription was 4.14Δ (SD=2.4, range 1Δ to 10Δ). The majority (79–90%) of children and parents in both groups reported excellent (>75% of time) compliance with glasses wear. Reported wearing time was not statistically different between the two groups for the children’s (p=0.18) or parents’ (p=0.24) responses. The mean CISS score decreased (i.e., became less symptomatic) from 31.6 (±10.4) to 16.5 (±9.2) in the base-in prism group and from 28.4 (±8.8) to 17.5 (±12.3) in the placebo reading glasses group. The changes in the CISS scores (p=0.33), NPC (p=0.91), and PFV at near (p=0.59) were not significantly different between the two groups after 6 weeks of glasses wear. Thus, base-in prism reading glasses were found to be no more effective than placebo reading glasses for the treatment of symptomatic CI in children 9 to <18 years of age.
CITT Large-Scale Study: A Randomized Clinical Trial of Treatments for Convergence Insufficiency in Children
This study34 was a multi-center clinical trial of 221 children aged 9–17 years with symptomatic CI who were randomly assigned to receive a 12-week program of home-based pencil push-ups, home-based computer vergence/accommodative therapy and pencil push-ups, office-based vergence/accommodative therapy with home reinforcement, and office-based placebo therapy. The purpose of this large-scale, randomized clinical trial was to further evaluate commonly used active treatments for CI.for improving symptoms and signs associated with CI in children. The sample size was considerably larger than the first trial and we included a second home-based therapy group that performed computerized therapy and pencil push-up therapy to represent a more intensive, stepwise, home-based therapy regimen rather than pencil push-ups alone. This was added in response to a suggestion that some eye care providers recommend more intensive home-based therapy than pencil push-ups alone38 and because this therapy is being prescribed increasingly by both ophthalmologists and optometrists. We also followed the patients for 1 year after treatment completion to determine the long-term effectiveness of treatments. All methods have been described in detail in previous publications.32,33,39
Treatment Protocols
The treatment groups of home-based pencil push-ups, office-based vergence/accommodative therapy with home reinforcement, and office-based placebo therapy were essentially the same as those in the aforementioned clinical trial. Patients assigned to the home-based computer vergence/accommodative therapy and pencil push-ups therapy group were prescribed 25 minutes of therapy per day on the Home Therapy System (HTS/CVS) (www.visiontherapysolutions.com) computer software and 5 minutes per day of pencil push-ups for 5 days per week. The computerized therapy consisted of fusional vergence and accommodative therapy procedures including accommodative rock, vergence base in, vergence base out, auto-slide vergence, and jump ductions vergence programs using random dot stereopsis targets.
Therapists contacted the patients in the home-based groups by phone on a weekly basis to review the therapy procedures and to motivate the patients to adhere to treatment. In addition, the children attended monthly in-office assessment visits where the therapists answered questions, reviewed the home therapy procedures, and estimated adherence (compliance). Patients in all 4 groups were instructed to maintain a home therapy log and record their performance for each home therapy session.
RESULTS
After 12 weeks of treatment, the office-based vergence/accommodative therapy group’s CISS score (15.1) was significantly lower than the home-based pencil push-ups therapy, home-based computer vergence/accommodative therapy and pencil push-ups, and office-based placebo therapy groups’ scores of 21.3, 24.7, and 21.9, respectively (p < 0.001 for each comparison). Although symptoms improved somewhat with the two home-based therapies, these treatments were no more effective in improving symptoms than office-based placebo therapy (p>0.38 for both comparisons). After treatment, 73% of patients assigned to office-based vergence/accommodative therapy achieved a normal or improved (10 point or more decrease) symptom score on the CISS, in contrast to 47% assigned to home-based pencil push-ups, 39% assigned to home-based computer vergence/accommodative therapy and pencil push-ups, and 43% assigned to office-based placebo therapy (p= 0.006, 0.0004 and 0.0014, respectively).
Many clinicians evaluate changes in NPC and PFV when judging the success of therapy for CI. The office-based vergence/accommodative therapy group demonstrated a significantly improved NPC and PFV compared with the other groups (p <= 0.005). While the mean NPC of both home-based groups measured significantly closer than that of the office-based placebo therapy group (pair-wise p-values all ≤ 0.013), there were no statistically significant differences (P = 0.33) between the two home-based therapy groups. The mean PFV for patients in the office-based vergence/accommodative therapy group was significantly greater than all other groups (pair-wise p-values all < 0.001) with that in the home-based computer vergence/accommodative therapy and pencil push-ups group being significantly better (higher) than in the home-based pencil push-ups (p = 0.037) and office-based placebo therapy groups (p = 0.008). The proportion of patients who achieved a clinically normal level for both measures was 73% in the office-based vergence/accommodative therapy group versus no more than 40% in each of the other three treatment groups (p<0.001 for each comparison).
Finally, patients were classified as “successful” or “improved” using a composite outcome classification. This composite outcome classification considered the change in all three outcome measures from baseline to the outcome examination. A “successful” outcome was a score of <16 on the CISS, a normal NPC (i.e., less than 6 cm), and normal PFV (i.e., greater than 15Δ and passing Sheard’s criterion). “Improved” was defined as a score of <16 or a 10 point decrease in the CISS score, and at least one of the following: normal NPC, improvement in NPC of more than 4 cm, or normal PFV or an increase in PFV of more than 10Δ. Patients who did not meet the criteria for “successful” or “improved” were considered “non-responders.” While 73% of patients in the office-based vergence/accommodative therapy group were either "successful" or "improved", 45% of patients in the home-based pencil push-ups group, 33% of the patients in the home-based computer vergence/accommodative therapy group, and 35% of the office-based placebo group (35%) were similarly classified (p<0.002 for each comparison). There were no significant differences between the two home-based therapy groups and the placebo therapy group (p≥0.39 for both).
Analysis of effect size can be used to determine whether changes are clinically meaningful. According to Cohen’s40 guidelines for interpretation of effect size a SD of 0.2 is small, 0.5 is medium, and 0.8 is large. Sloan et al.41 have asserted that an effect size of 0.5 is a conservative estimate of a clinically meaningful difference that is scientifically supportable and unlikely to be one that can be ignored. Between the office-based vergence/accommodative therapy group and the placebo therapy group, we found large effect sizes (0.77 SD for the CISS, 0.81 SD for NPC, and 3.43 SD for PFV.) Therefore, in addition to being statistically significant, these changes are considered be clinically meaningful. On the other hand, the effect sizes between the home-based therapy groups and the placebo therapy group were small for all outcome measures (pencil push-ups: 0.11 SD for CISS, 0.32 SD for NPC, 0.3 SD for PFV; home-based computer therapy: 0.18 SD for CISS, 0.45 SD for NPC) except PFV for the home-based computer vergence/accommodative therapy and pencil push-ups group (1.41 SD).
These results showed that 12 weeks of office-based vergence/accommodative therapy resulted in a greater percentage of patients reaching pre-determined success criteria when compared with home-based pencil push-ups, home-based computer vergence/accommodative therapy and pencil push-ups, and office-based placebo therapy, and a clinically meaningful and statistically significantly greater improvement in symptoms and clinical measures of NPC and PFV.
DISCUSSION
Until recently, clinicians seeking evidence regarding the effectiveness of treatments for children with symptomatic CI had limited quality data to support any available treatment option. This probably explains the reason why there has been no consensus regarding the most effective treatment approach; albeit 87% of optometrists and ophthalmologists reported they prescribed either home-based pencil push-ups or base-in prism reading glasses.26 It is easy to understand the clinical popularity of home-based pencil push-ups and base-in prism reading glasses because of their simplicity and low cost. These attributes most likely account for why home-based computer software for the treatment of CI appears to be a growing trend in both eye care fields.
The aforementioned randomized clinical trials demonstrated that base-in prism reading glasses were no more effective than placebo glasses, and that home-based pencil push-up therapy and computerized therapy combined with pencil push-ups are significantly less effective than office-based vergence-accommodative therapy. Patients in both home-based therapy groups in the large-scale trial were contacted on a weekly basis by a therapist, completed a home log, and returned for office visits every fourth week. Because this is considerably closer follow-up than is typical in clinical practice, it is likely that this treatment would be less effective if prescribed according to usual clinical practice of no weekly telephone calls and less frequent follow-up. The results of the CITT pilot study,32 in which the home-based pencil push-ups group did not receive weekly phone calls, provide some support for this hypothesis for the pencil push-ups group as none of the these patients were classified as successful or improved.
Evidence-Based Guidelines for the Treatment of Children with Symptomatic Convergence Insufficiency
Eye care providers wishing to make an evidence-based recommendation regarding the most effective treatment for children with symptomatic CI can use the results from the clinical trials described herein. Because office-based vision therapy was found to be the most effective treatment, many will feel that this form of therapy should be the first-line treatment. We recognize that this creates challenges for many clinicians because only about 15% of optometrists and 3% of ophthalmologists currently offer office-based vision therapy for CI.26 However, if eye care providers do not offer this treatment modality, they can either co-manage these patients with a provider who does or alternatively consider incorporating this form of treatment within their practice. We recognize that there are a number of obstacles associated with the latter option including education and training, equipment, and space.
An additional concern is the increased cost associated with office-based treatment.38,42 Certainly when the service is not available locally or the parents are unable to afford office-based vision therapy, parents may opt for home-based therapy initially despite the lower success rates. In such instances, we suggest the use of the home-based computer software plus pencil push-ups because this treatment approach was more effective than pencil push-ups alone in improving positive fusional vergence, is more engaging for the child, and provides an automated, stepwise treatment approach. Monitoring of compliance is suggested in these cases, and might include a weekly call from the doctor or assistant or the use of the computer software Internet monitoring feature. If the patient attempts this home-based treatment and is not successful, there would be no other alternative than to refer the patient for office-based vision therapy. Nevertheless, we believe that in all instances patients should be informed of the significant differences in effectiveness of office vs. home-based vision therapy based on current evidence.
In regard to base-in prism reading glasses our data do not support their use. However, we recognize that we used a single criterion for determining the magnitude of the prism prescription (Sheard’s criterion). Further research is indicated to determine if base-in prism reading glasses prescribed using other criteria, such as fixation disparity measurements, may be an effective treatment option.
New Questions and Future Challenges
The aforementioned clinical trials answer a number of important questions about the effectiveness of various treatments for symptomatic CI in children. However, the results of these studies also raise new questions and suggest additional challenges that need to be addressed in future studies of CI.
Wallace42 expressed uncertainty as to whether these home-based treatment groups were ideal comparison groups to study. He suggested that an ideal comparison group would have received the same amount of therapy at home as the office-based therapy groups received in the office as well as equal contact time with the therapist. However, this was not the intent of the trial.
Active treatment approaches for CI differ in several ways including dosage and mode of administration, and the objective of the CITT was to compare the effectiveness of three commonly prescribed treatments. Effectiveness refers to whether an intervention has benefit as used in actual clinical practice. This is in contrast to the efficacy of treatment, which refers to whether an intervention can be successful when it is properly implemented under highly controlled conditions. Thus, the home-based pencil push-ups and the computerized therapy groups were prescribed treatment regimens that closely approximated how these treatment modalities are currently used in clinical practice – that is, prescribed to be performed at home for 15 and 20 minutes per day, respectively (albeit patients also received weekly phone calls and attended monthly follow-up visits in the office with the therapist).
The only way to have ensured equalization of “treatment dosage” and face-to-face “office contact time” with the therapist would have been for the children in the home-based groups to have attended 12 weeks of 60-minute, therapist-supervised therapy sessions in the office. Although this treatment protocol might indeed have greater efficacy than the prescribed 15 to 20 minutes of 5 days per week home-based therapy, it is unlikely that many eye care providers would prescribe 60-minute in-office therapy sessions of therapist-supervised pencil push-ups or computerized therapy. Alternatively, 12 more minutes of pencil push-ups for the pencil push-up therapy group or 7 more minutes of computer therapy for the computerized therapy group performed at home on each of the 5 therapy days would equalize therapy dosage (presuming the children performed the therapy exactly as prescribed); however, it would not address the issue of equal face-to-face therapist contact time unless the child also came to the office and interacted with the therapist in some way for 60 minutes each week. We believe that these hypothetical treatment approaches are untenable and unlikely to be prescribed or successfully completed. More importantly, however, because they are not representative of what happens in clinical practice and therefore would have precluded us from evaluating the effectiveness of the treatments. While it may be of scientific interest whether the same number of minutes of home-based pencil push-ups or computerized therapy combined with the same face-to-face therapist contact time can produce an outcome equivalent to that found in the office-based accommodative/vergence therapy group, it would have limited clinical utility. Moreover, equalizing in-office therapist contact time would negate the primary advantages of home-based treatment, which are its simplicity and low cost.
One might argue that home-based therapy should be the first-line treatment for children with symptomatic CI and that office-based vision therapy should be reserved only for unresponsive or poorly responsive cases. This argument, however, is based solely on convenience and cost because there are no data to support this approach and there is no method to predict who will respond to home-based therapy. Medical decisions should not be based on cost alone. Rather, there should be effectiveness data to support any recommended therapy.
In our studies, home-based pencil push-ups and home-based computer vergence/accommodative therapy with pencil push-ups were no more effective than placebo treatment in normalizing symptoms and signs of CI (composite outcome of 33%, 43%, and 35% respectively). Of note, the home-based therapy programs required 15 to 20 hours of therapy, weekly phone calls from the therapist, and 4 office visits over a 12-week period. Recommending a procedure that has been shown to be effective only one-third of the time as a first-line treatment for a child who is symptomatic while reading would be delaying another treatment shown to be effective 75% of the time for 3 months. However, the cost of treatment is an important issue and in some cases will be a barrier to treatment. While it is incumbent upon all of us to educate parents regarding the success rates and advantages and disadvantages for all available treatment options so that parents can make a truly informed consent. Parents must make their own decisions based upon the information given to them, their perception and concern regarding their child’s symptoms, their own personal goals and values, and their financial situation.
One might wonder if the improvement in the office-based vision therapy group could be from a combined effect of patient-provider interaction and/or the patient’s belief in the effectiveness of the treatment plus the effect of the home-based vision therapy alone rather than from the therapy performed in the office and at home. This would assume, however, that the 2 home-based therapy groups experienced no effects of patient-provider interaction or belief in the effectiveness of the treatment when, in fact, there is no reason to think that the home-based therapy groups would be immune to these effects. Indeed, patients in the home-based therapy groups had less overall contact time with the therapist, but patients in all groups of the large-scale clinical trial had weekly contact with the therapist to encourage motivation. Furthermore, because the patients in the home-based therapy groups were not masked, they were aware that the therapy they were prescribed was not placebo therapy. We do not know if the improvements found in the home-based treatment groups were due to a real treatment effect or a placebo effect.
In addition, the effect sizes for each outcome measure were large between the office-based vergence/accommodative therapy and placebo groups, but small between home-based therapy and placebo therapy groups. Therefore, the incorporation of an office-based placebo group in both of our studies definitively demonstrates a real treatment effect with office-based vision therapy.
The placebo effect is viewed as a change in a patient’s illness attributable to the symbolic aspect of a treatment and not to any specific pharmacologic or physiologic properties.43 Placebo response rates for a variety of medical conditions have been reported to range from15% to 58% with an average placebo effectiveness of 35%.44 While this is similar to the effectiveness rates found in our office-based placebo therapy and placebo glasses groups, it is not certain how much of the effect in these groups was from the placebo effect versus regression to the mean and/or natural history of the disease because a no-treatment control group was not included. Nevertheless, any such effects should have affected all treatment groups similarly. In addition, the effect sizes for all three outcome measures were large between the office-based vergence/accommodative therapy and placebo groups, but small between the home-based therapy and placebo therapy groups. Therefore, we feel that the presence of the office-based placebo group definitively demonstrates there was a real treatment effect with office-based vergence/accommodative therapy.
Directions for Future Research
Our CITT Investigator Group continues to be committed to investigating CI and some of the key questions we hope to study in the future include:
Would a longer duration of office- and home-based therapies have been effective in a higher percentage of children?
Are certain office-based vergence/accommodative therapy procedures more effective than others in treating CI? Is there an office-based therapy program that would be equally as effective or perhaps even more effective but could be administered for a shorter duration?
Would a protocol that more closely monitors and encourages adherence affect the outcome for home-based computer vergence/accommodative therapy group?
Are there different home-based therapy combinations (e.g., computer therapy combined with therapy procedures such as loose prism or free-space fusion cards rather than pencil push-ups) and/or a modified computer therapy program that might be more effective than the combined computerized therapy and pencil push-up approach that we prescribed?
Is there a better method of prescribing prism, such as based on fixation disparity testing, that might be more effective in reducing symptoms of CI?
What effect does successful treatment of symptomatic CI have on various aspects of reading performance?
What effect does the successful treatment of CI have on behavior rating scales in children with CI and Attention-Deficit Hyperactivity Disorder whose behaviors are still an issue despite medical management for the latter?
What exactly is the cost utility of the various treatments for CI?
Do low plus lenses that are anecdotally reported by some clinicians to be useful for the treatment of CI in children have any beneficial treatment effect?
ACKNOWLEDGMENTS
To successfully plan and implement a randomized clinical trial requires many clinical investigators, a significant time commitment, and funding. The success of the randomized trials described in this paper was based on the participation of a large number of dedicated, investigators from many different institutions around the country and funding from the National Eye Institute. The names of the CITT investigators are listed in previous publications.32–34
The CITT Pilot study and the CITT large-scale trial were supported by grants from National Eye Institute of the National Institutes of Health, Department of Health and Human Services. The Base-in Prism study was funded by grants from the Pennsylvania and Ohio Lions along with internal institutional support from participating colleges and schools of optometry.
REFERENCES
- 1.Rouse MW, Borsting E, Hyman L, Hussein M, Cotter SA, Flynn M, Scheiman M, Gallaway M, De Land PN The Convergence Insufficiency and Reading Study (CIRS) group. Frequency of convergence insufficiency among fifth and sixth graders. Optom Vis Sci. 1999;76:643–649. doi: 10.1097/00006324-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Letourneau JE, Ducic S. Prevalence of convergence insufficiency among elementary school children. Can J Optom. 1988;50:194–197. [Google Scholar]
- 3.Letourneau JE, Lapierre N, Lamont A. The relationship between convergence insufficiency and school achievement. Am J Optom Physiol Opt. 1979;56:18–22. doi: 10.1097/00006324-197901000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Daum KM. Convergence insufficiency. Am J Optom Physiol Opt. 1984;61:16–22. doi: 10.1097/00006324-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Cooper J, Duckman R. Convergence insufficiency: incidence, diagnosis, and treatment. J Am Optom Assoc. 1978;49:673–680. [PubMed] [Google Scholar]
- 6.Kent PR, Steeve JH. Convergence insufficiency, incidence among military personnel and relief by orthoptic methods. Milit Surgeon. 1953;112:202–205. [PubMed] [Google Scholar]
- 7.Poynter HL, Schor C, Haynes HM, Hirsch J. Oculomotor functions in reading disability. Am J Optom Physiol Opt. 1982;59:116–127. doi: 10.1097/00006324-198202000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Mazow ML. The convergence insufficiency syndrome. J Pediatr Ophthalmol. 1971;8:243–244. [Google Scholar]
- 9.Duke-Elder S, Wybar K. Ocular motility and strabismus. In: Duke-Elder S, editor. System of Ophthalmology. Vol 6. St Louis: Mosby; 1973. pp. 547–551. [Google Scholar]
- 10.Pickwell LD, Hampshire R. The significance of inadequate convergence. Ophthalmic Physiol Opt. 1981;1:13–18. [PubMed] [Google Scholar]
- 11.Borsting E, Rouse MW, Deland PN, Hovett S, Kimura D, Park M, Stephens B. Association of symptoms and convergence and accommodative insufficiency in school-age children. Optometry. 2003;74:25–34. [PubMed] [Google Scholar]
- 12.Borsting EJ, Rouse MW, Mitchell GL, Scheiman M, Cotter SA, Cooper J, Kulp MT, London R. Validity and reliability of the revised convergence insufficiency symptom survey in children aged 9 to 18 years. Optom Vis Sci. 2003;80:832–838. doi: 10.1097/00006324-200312000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Duane A. A new classification of the motor anomalies of the eye, based upon physiological principles. Ann Ophthalmol. 1897;6:84–122. 247–260. [Google Scholar]
- 14.White JW, Brown HW. Occurrence of vertical anomalies associated with convergent and divergent anomalies - a clinical study. Arch Ophthalmol. 1939;21:999–1009. [Google Scholar]
- 15.von Noorden GK. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. 5 ed. St Louis: Mosby; 1996. [Google Scholar]
- 16.Abrams D, Duke-Elder S. Duke-Elder’s Practice of Refraction. 10th ed. New York: Churchill-Livingstone; 1993. [Google Scholar]
- 17.Tongue AC. Evaluation of asthenopia in childhood. In: Cibis GW, Tongue AC, Stass-Isern ML, editors. Decision Making in Pediatric Ophthalmology. St. Louis: B.C. Decker; 1993. pp. 208–209. [Google Scholar]
- 18.Pratt-Johnson JA, Tillson G. Management of Strabismus and Amblyopia: A Practical Guide. 2nd ed. New York: Thieme; 2001. [Google Scholar]
- 19.von Noorden GK, Helveston EM. Strabismus: A Decision Making Approach. St. Louis: Mosby; 1994. [Google Scholar]
- 20.Griffin JR, Grisham JD. Binocular Anomalies: Diagnosis and Vision Therapy. 4th ed. Philadelphia: Butterworth-Heinemann; 2002. [Google Scholar]
- 21.Press LJ. Applied Concepts in Vision Therapy. St. Louis: Mosby; 1997. [Google Scholar]
- 22.Scheiman M, Wick B. Clinical Management of Binocular Vision: Heterophoric, Accommodative and Eye Movement Disorders. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 23.Hugonnier R, Clayette-Hugonnier S, Veronnearu-Troutman S. Strabismus, Heterophoria, Ocular Motor Paralysis: Clinical Ocular Muscle Imbalance. St Louis: Mosby; 1969. [Google Scholar]
- 24.Gallaway M, Scheiman M, Malhotra K. The effectiveness of pencil pushups treatment for convergence insufficiency: a pilot study. Optom Vis Sci. 2002;79:265–267. doi: 10.1097/00006324-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Chin FH, Faibish B, Hisaka C, Thal L, Tsuda K. A survey of the treatment of convergence insufficiency. J Behav Optom. 1995;6:91–92. 109. [Google Scholar]
- 26.Scheiman M, Cooper J, Mitchell GL, de Land P, Cotter S, Borsting E, London R, Rouse M. A survey of treatment modalities for convergence insufficiency. Optom Vis Sci. 2002;79:151–157. doi: 10.1097/00006324-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Scheiman M, Mitchell GL, Cotter S, Cooper J, Kulp M, Rouse M, Borsting E, London R, Wensveen J. Convergence Insufficiency Randomized Clinical Trial. Arch Ophthalmol. 2005;123:1760–1761. doi: 10.1001/archopht.123.1.14. [DOI] [PubMed] [Google Scholar]
- 28.Worrell BE, Jr, Hirsch MJ, Morgan MW. An evaluation of prism prescribed by Sheard's criterion. Am J Optom Arch Am Acad Optom. 1971;48:373–376. doi: 10.1097/00006324-197105000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Stavis M, Murray M, Jenkins P, Wood R, Brenham B, Jass J. Objective improvement from base-in prisms for reading discomfort associated with mini-convergence insufficiency type exophoria in school children. Binocul Vis Strabismus Q. 2002;17:135–142. [PubMed] [Google Scholar]
- 30.Haase HJ. Binocular testing and distance correction with the Berlin Polatest. J Am Optom Assn. 1962;34:115–125. [Google Scholar]
- 31.Grisham JD. Visual therapy results for convergence insufficiency: a literature review. Am J Optom Physiol Opt. 1988;65:448–454. doi: 10.1097/00006324-198806000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Scheiman M, Mitchell GL, Cotter S, Cooper J, Kulp M, Rouse M, Borsting E, London R, Wensveen J. A randomized clinical trial of treatments for convergence insufficiency in children. Arch Ophthalmol. 2005;123:14–24. doi: 10.1001/archopht.123.1.14. [DOI] [PubMed] [Google Scholar]
- 33.Scheiman M, Cotter S, Rouse M, Mitchell GL, Kulp M, Cooper J, Borsting E. Randomised clinical trial of the effectiveness of base-in prism reading glasses versus placebo reading glasses for symptomatic convergence insufficiency in children. Br J Ophthalmol. 2005;89:1318–1323. doi: 10.1136/bjo.2005.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Convergence Insufficiency Treatment Trial Investigator Group. Randomized clinical trial of treatments for symptomatic convergence insufficiency in children. Arch Ophthalmol. 2008;126:1336–1349. doi: 10.1001/archopht.126.10.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borsting E, Rouse MW, De Land PN The Convergence Insufficiency and Reading Study (CIRS) Group. Prospective comparison of convergence insufficiency and normal binocular children on CIRS symptom surveys. Optom Vis Sci. 1999;76:221–228. doi: 10.1097/00006324-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 36.Kulp MT, Borsting E, Mitchell GL, Scheiman M, Cotter S, Cooper J, Rouse M, London R, Wensveen J. Feasibility of using placebo vision therapy in a multicenter clinical trial. Optom Vis Sci. 2008;85:255–261. doi: 10.1097/OPX.0b013e318169288a. [DOI] [PubMed] [Google Scholar]
- 37.Sheard C. Zones of ocular comfort. Am J Optom Arch Am Acad Optom. 1930;7:9–25. [Google Scholar]
- 38.Kushner B. The treatment of convergence insufficiency. Arch Ophthalmol. 2005;123:100–101. doi: 10.1001/archopht.123.1.100. [DOI] [PubMed] [Google Scholar]
- 39.Convergence Insufficiency Treatment Trial Investigator Group. The convergence insufficiency treatment trial: design, methods, and baseline data. Ophthalmic Epidemiol. 2008;15:24–36. doi: 10.1080/09286580701772037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- 41.Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: another step toward consensus. J Clin Epidemiol. 2005;58:1217–1219. doi: 10.1016/j.jclinepi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Wallace DK. Treatment options for symptomatic convergence insufficiency. Arch Ophthalmol. 2008;126:1455–1456. doi: 10.1001/archopht.126.10.1455. [DOI] [PubMed] [Google Scholar]
- 43.Brody H. Placebo effect: an examination of Grunbaum's definition. In: White L, Tursky B, Schwartz GE, editors. Placebo: Theory, Research, and Mechanisms. New York: Guilford Press; 1985. pp. 37–58. [Google Scholar]
- 44.Beecher HK. The powerful placebo. JAMA. 1955;159:1602–1606. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]