Abstract
Background/Aims
Preeclampsia is a hypertensive disorder which develops de novo in women during pregnancy. The urinary excretion of the cardiotonic steroid, marinobufagenin (MBG), is increased prior to the development of hypertension. Preeclamptic patients are volume expanded but much of the excess salt and water appears to be located primarily in the interstitial space. Therefore, ‘capillary leak’ syndrome has been postulated in this disorder.
Methods
We evaluated the vascular leakage in normal rats following MBG injection and in a rat model of human preeclampsia. We measured the changes in light intensity comparing that in the intravascular to the extravascular space by assessing ‘leak’ of fluorescein-labeled albumin (FITC-albumin) from mesenteric postcapillary venules.
Results
FITC-albumin extravasation continued to increase in a time-dependent fashion after MBG infusion and was significant (p < 0.05) at 60 min of observation when compared to sham rats. We also observed a significant difference in ‘vascular leakage’ in preeclamptic rats compared to control non-pregnant and normal pregnant groups starting at 20 min after the FITC-albumin infusion.
Conclusion
We propose that MBG is involved in the production of a ‘vascular leak’ in our rat model of preeclampsia.
Key Words: Marinobufagenin, Capillary leak, Preeclampsia, Vascular permeability
Introduction
During pregnancy an increase in extracellular fluid (ECF) volume occurs which reaches a 40–50% increase by the end of gestation [1]. Red cell mass also increases but is less extensive than the increment in the volume of the intravascular compartment, so that hematocrit and hemoglobin concentration fall [1]. This results in the so-called ‘physiologic’ anemia of pregnancy. In normal pregnancy, expansion of the ECF volume appears to be distributed rather evenly between the intravascular and interstitial compartments [2]. In preeclampsia, however, the hematocrit rises, and although the patient is, indeed, volume expanded, the fluid seems to be redistributed more extensively to the interstitial than the intravascular compartment [2, 3]. Accordingly, the preeclamptic patient has been suspected of demonstrating an increased vascular permeability or ‘capillary leak’ syndrome [4, 5].
A rat model which has many of the phenotypic characteristics of human preeclampsia has been developed in this laboratory [6]. It consists of the expansion of the ECF volume in pregnant rats with the administration of desoxycorticosterone acetate (DOCA) and the replacement of their drinking water with 0.9% saline. The rats develop hypertension, proteinuria and intrauterine growth restriction. The hematocrits of these animals, as is the case in humans, are elevated compared to those of normal pregnant rats [6]. Furthermore, their excretion of the bufodienolide, marinobufagenin (MBG), a cardiac glycoside, is increased. Additionally, this increased MBG excretion occurs prior to the animals becoming hypertensive or proteinuric [7]. Circulating levels of MBG have been reported to be increased in patients with volume expansion-mediated hypertension and preeclampsia [8, 9].
We have demonstrated that MBG impairs both the proliferation and growth factor-induced migration of first trimester human extravillous cytotrophoblast (CTB) cells, which are important for normal placental development [10, 11]. We determined that the MBG-induced impairment of CTB cell function is accompanied by the modulation of mitogen-activated protein kinase and also by the stimulation of apoptosis [12]. Additionally, in a preliminary report, we have recently demonstrated that MBG triggers enhanced monolayer permeability in rat lung microvascular endothelial cells [13].
There has been no previous evaluation of the possibility that there is an increased vascular leak in our rat model of preeclampsia. Furthermore, the possibility that MBG could be involved in the pathophysiology of this abnormal ‘capillary leak’ has not been evaluated. Accordingly, the studies presented in this report were performed for the following purposes: (1) to determine if MBG causes increased vascular leakage; (2) to determine if increased vascular leakage exists in our rat model of preeclampsia, and, if so, (3) to investigate the signaling mechanisms by which this occurs.
Materials and Methods
Animals
The surgical procedures and experimental protocol were conducted at Texas A&M University Health Science Center/Scott and White Hospital after approval by the Institutional Animal Care and Use Committee. The facility is approved by the American Association for Accreditation of Laboratory Animal Care in accordance with National Institutes of Health guidelines.
Animal Preparation
Male (275–325 g) and female (200–250 g) Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, Mass., USA). These animals were housed in the institutional animal facility and allowed free access to standard rat chow (Lab Diet 5001 Laboratory Rodent Diet) and tap water. They were maintained on a 12:12 h dark/light cycle. The room temperature and humidity were maintained at 25 ± 2°C and 55%, respectively. Two groups of animals were utilized for studies of vascular leakage following MBG injection. The sham rats (male, n = 5) were injected with DMSO and the MBG rats (male n = 5 and female n = 2, total n = 7) were injected with a bolus of 200 nM MBG (kind gift of Dr. G.R. Pettit, Arizona State University, USA). All injections were made into the jugular vein.
Three groups of female animals were utilized for studies of vascular leakage in our rat model. These were: group 1: control (C), non-pregnant animals (n = 5); group 2: normal pregnant (NP) animals (n = 9), and group 3: pregnant DOCA + saline (PDS) animals (rat model of preeclampsia; n = 9) [4]. Systolic blood pressure was measured by the tail-cuff method (IITC Inc., LifeScience Instruments, Model 59). At 18–19 days of pregnancy (or at a similar time period in the control rats), 24-hour urine was collected in the absence of food (this was done to eliminate contamination of the urinary protein determination by any fallen food particles). Each animal was housed separately in a metabolic cage. Blood was drawn from the carotid artery.
Animal Surgery and Intravital Microscopy
Prior to each experiment the rats were fasted for 18 h and given water ad libitum. The animals were anesthetized by a single intramuscular injection of 50% urethane (1.5 g/kg). Polyethylene tubing (PE-50, 0.58 mm ID) was placed in the right internal jugular vein to give fluid intravenously (normal saline) at 2 ml/h by continuous infusion pump (Harvard Apparatus, South Natick, Mass., USA) and in the right carotid artery for blood withdrawal. The mean arterial pressure was monitored continuously using a PE-50 cannula placed in the left femoral artery connected to a blood pressure analyzer (Dig-Med, BPA 400A, Micromed, Louisville, Ky., USA). A midline laparotomy incision was performed to expose a section of mesentery from the proximal ileum for exteriorization. The rats were placed in a lateral decubitus position on a temperature-controlled Plexiglas platform mounted to an intravital upright microscope (Nikon E600, Tokyo, Japan). The mesentery was superfused with normal saline at 2 ml/min and covered with plastic wrap to reduce evaporation. Venules with diameters of 20–35 μm were selected for study with a Nikon 20× objective, 0.45–2.16 mm working distance (Nikon Instruments, Inc., Natick, Mass., USA). Images were obtained with a Photometric Cascade Camera (Roper Scientific, Tucson, Ariz., USA). A video time and date generator (WJ-810; Panasonic, Secaucus, N.J., USA) provided on screen time, date, and stopwatch functions. The images were projected onto a computer monitor (Trinitron 20-inch monitor; Sony, New York, N.Y.) and were captured digitally on computer disc. Fluorescein isothiocyanate-bovine albumin (FITC-albumin; Sigma, St. Louis, Mo., USA) was dissolved in saline and injected into the jugular vein at a dose of 50 mg/kg to measure permeability. Data were analyzed using MetaMorph 4.5/4.6 (Universal Imaging Corp., Downingtown, Pa., USA).
Urine and Blood Analyses
The 24-hour protein excretion was measured using the pyrogallol red method (Total Protein Kit, Micro Pyrogallol Red Method, Sigma). Creatinine was measured in the blood and urine on a Nova 16 Analyzer (Waltham, Mass., USA) and the creatinine clearance was calculated. Hematocrit was measured using a StatSpin MP Multipurpose Centrifuge (Norwood, Mass., USA).
Measurement of Vascular Leakage
The extravasation of FITC-albumin from the intravascular space was measured by determining the changes in integrated optical intensity by image analysis. The appearance of the labeled albumin (FITC) was employed to represent the relative change in vascular leakage. Areas in the small bowel mesentery, postcapillary venules, and the adjacent extravascular space, were selected for study. ΔI = 1 – (Ii– Io)/Ii, where ΔI is the change in light intensity; Ii is the light intensity inside the vessel, and Io is the light intensity outside the vessel. Each experimental frame was digitized into a 512 × 512 charged coupled device that yielded 16 bits of data/pixel. Gray scale values were measured in the postcapillary venules and in the extravascular space around the venules per unit area throughout the experiment and at selected times using the MetaMorph image analysis systems. The images were standardized to images taken at the beginning of each experiment within the same animal and at selected timed intervals between different animals. This method of standardization was selected to minimize the bias incurred with changes in room lighting and hematocrit concentrations. This method of measuring vascularleakage using image analysis was validated by Bekker et al. [14].
Harvesting of Mesentery Tissues and Preparation of Tissue Homogenates
Mesenteric vessels were collected from C, NP and PDS rats after sacrificing the rats. Mesenteric vessels were dissected from the rat, weighed and homogenized in a cold buffer preparation (10 mM Tris-HCl pH = 7.5, 0.3 M sucrose, 10 μM apoptinin, 10 μM pepstatin, 10 μM leupeptin, 1 mM PMSF). The tissue homogenates were centrifuged (10,000 g for 60 min at 4°C) and the supernatant (cytosol fraction) was collected and subjected to protein estimation using the pyrogallol red method (Total Protein Kit, Micro Pyrogallol Red Method, Sigma).
Caspase-8 and -3 Activities in the Mesentery Tissue Homogenates
Each experimental group consisted of 5 female rats. Caspase-8 and -3 activities were determined using caspase-8 and -3 assay kits (Calbiochem, La Jolla, Calif., USA). The tissue homogenate was treated with the substrate conjugate and incubated for 2 h at 37°C. The IETD substrate (caspase-8 assay) and DEVD substrate (caspase-3 assay) provided in the assay kits were already labeled with a fluorescent molecule, 7-amino-4-trifluoromethyl coumarin. The resulting fluorescence was measured in a fluorescent plate reader capable of measuring excitation at 400 nm and emission at 505 nm.
Statistical Analysis
Data are presented as mean ± SEM. Statistical comparison for multiple determinations was performed using a one-way ANOVA analysis of variance with Tukey's post hoc t test. Caspase-8 and -3 activities were assessed by using Student's t test. A p value <0.05 was considered significant.
Results
Blood Pressure, Urine and Blood Analyses
Data for blood pressure measurements, the urinary excretion of protein, creatinine clearance, hematocrit levels and weight gain are presented in table 1. Blood pressure (BP) in the control, non-pregnant animals (C) did not change over the course of the experiments (18–20 days). Mean BP in the normal pregnant (NP) animals was 109 ± 5 mm Hg at baseline and 105 ± 6 mm Hg at the end of the experiment (p > 0.05). BP rose in the pregnant animals given saline + DOCA from 107 ± 5 to 146 ± 4 mm Hg (p < 0.001; compared to NP; table 1). The PDS animals showed a statistically significant increase in protein excretion when compared with the NP group: NP: 2.6 ± 0.8 mg/24 h; PDS: 5.2 ± 0.6 mg/24 h; p < 0.05. Both of the latter groups had a significantly higher protein excretion when compared with the non-pregnant animals: C: 1.2 ± 0.4 mg/24 h; p < 0.05 in each case (table 1). Both the NP and PDS rats showed a statistically significant increase in creatinine clearance when compared with the C group: C: 0.41 ± 0.17 ml/min; NP: 0.81 ± 0.29 ml/min; PDS: 1.05 ± 0.46 ml/min; p < 0.05 in each case (table 1). The mean hematocrit value of 0.38 ± 0.02 for the PDS group was statistically significantly different from those for the NP group (0.34 ± 0.02, p = 0.02) and for the C animals (0.44 ± 0.01, p < 0.001). Furthermore, C differed from NP (p < 0.001; table 1). Data for weight gain for the three groups of animals is also presented in table 1. The mean value of 57 ± 5 g for the PDS group was significantly different from those for the NP group (32 ± 12 g, p < 0.05) and for the C animals (14 ± 4 g, p < 0.05). Furthermore, NP differed from C (p < 0.05).
Table 1.
Blood pressure, urinary protein excretion, creatinine clearance, hematocrit values and weight gain in control, normal pregnant and pregnant animals treated with DOCA and saline
| Animal group |
C (n = 5) |
NP (n = 9) |
PDS (n = 9) |
|---|---|---|---|
| Baseline BP, mm Hg | 105±2 | 109±5 | 107±5 |
| Final BP, mm Hg | 106±3 | 105±6 | 146±41 |
| Urinary protein excretion mg/24 h | 1.2±0.4 | 2.6±0.82 | 5.2±0.63 |
| Creatinine clearance ml/min | 0.41±0.17 | 0.81±0.294 | 1.05±0.464 |
| Hematocrit | 0.44±0.01 | 0.34±0.022 | 0.38±0.023 |
| Weight gain, g | 14±4 | 32±122 | 57±53 |
Values are mean ± SD; n = number of rats. C = Control, non-pregnant animals; NP = normal pregnant animals; PDS = pregnant animals receiving DOCA and saline.
There was a statistically significant difference between baseline BP and final BP in PDS rats as well as a difference between final BP in PDS rats and final BP in both C and NP rats (p < 0.05).
NP group is different from both C and PDS, p < 0.05.
PDS group is different from both C and NP, p < 0.05.
Both NP and PDS groups are different from C, p < 0.05.
Taken together, these data verify the observations made concerning the BP, protein excretion, creatinine clearance and hematocrit changes obtained in previous studies of this animal model of preeclampsia [6]. Thus, the PDS animals became hypertensive and demonstrated an increase in protein excretion which exceeded that seen in the other two groups of animals. As is the case in normal human pregnancy, the hematocrit values in our NP rats fell as compared to non-pregnant female rats (C). These data presumably reflect the physiological anemia resulting from a proportionally greater increase in plasma volume than in red cell mass [1]. Also, as is the case in preeclamptic patients, the hematocrit value in our PDS rats was higher than that for NP (table 1). The weight gain in PDS compared to NP rats reflects the fact that volume expansion in PDS (‘preeclamptic’) rats exceeds that in the normal pregnant animals.
MBG Injection Induced Vascular Leakage
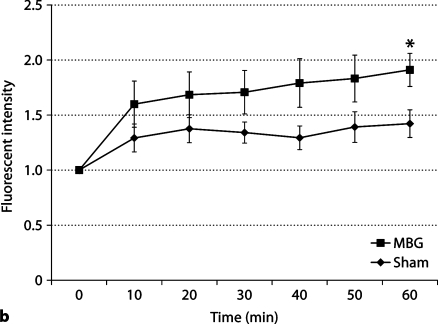
Composite images obtained in a rat mesenteric postcapillary venule from a representative animal at 30 and 60 min after the intravenous injection of a 200-nM bolus of MBG are presented in figure 1a. Sham animals received an injection of DMSO, the diluent utilized for MBG. The images show the progressive leakage of FITC-albumin from the venule. Figure 1b provides the mean data for the changes in microvascular permeability in a single rat mesenteric postcapillary venule obtained in 7 animals at 10, 20, 30, 40, 50, and 60 min after the intravenous injection of a 200 nM bolus of the steroid. Dye extravasation is expressed as a ratio of the extra- to intravascular appearance of the dye. FITC-albumin extravasation continued to increase in a time-dependent fashion after MBG infusion and was significant (p < 0.05) at 60 min of observation when compared to sham rats.
Fig. 1.
a Representative study demonstrating the effect of MBG on vascular leakage in a single rat mesentery postcapillary venule. Images shown were obtained prior to the injection (0 min) and at 30 and 60 min after the bolus injection of 200 nM MBG. FITCalbumin extravasation into the extravascular space is virtually complete by 60 min after MBG injection. b Mean values for the effects of MBG on vascular leakage in mesenteric postcapillary venules compared to rats infused with the vehicle (DMSO) only (sham) at 10, 20, 30, 40, 50 and 60 min of observation. Vascular leakage is expressed as the change in fluorescent intensity inside the vessel compared to that outside the vessel. ∗ p < 0.05: n = 5 for sham and n = 7 for MBG.
Increased Vascular Leakage Was Observed in PDS Rats Compared to Control and Normal Pregnant Animals
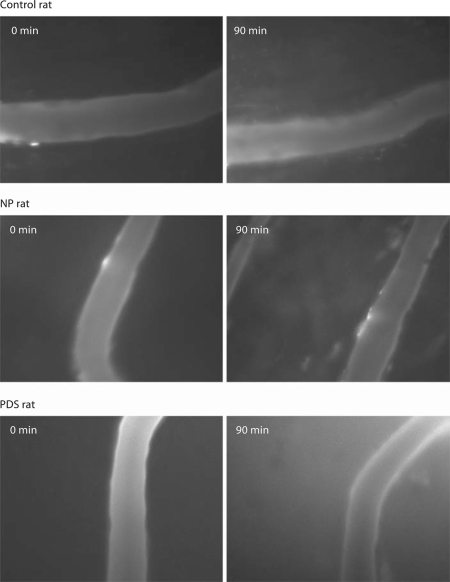
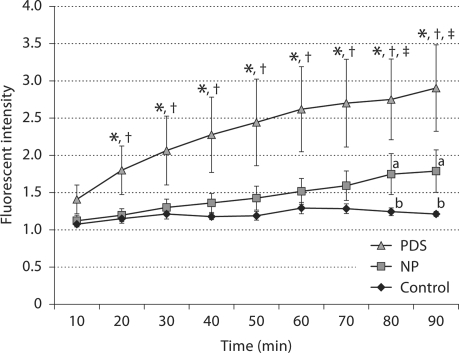
Figure 2 shows images of a rat mesenteric postcapillary venule at 90 min for female control, normal pregnant, and PDS rats. There was negligible leakage of FITC- albumin into the extravascular space in the control female rat. The normal pregnant rat showed minimal leakage while the PDS rat demonstrated significant leakage indicating the extravasation of most of the FITC-albumin into the extravascular space. These qualitative observations of vascular leakage were verified by analyses of mean data and are presented in figure 3. In this figure the data obtained for permeability from a postcapillary venule in the three groups of animals are presented. NP rats showed no extravasation of FITC-albumin into the extravascular space at 10 min, but there was a small but significant leakage observed at 80–90 min (p < 0.05). FITC-albumin extravasation was significantly higher (p < 0.05) in PDS rats beginning at 20 min when compared to C and NP rats and continued to increase throughout the study.
Fig. 2.
Images from representative studies of rat mesenteric postcapillary venules in a female non-pregnant control rat, a normal pregnant animal (NP), and a pregnant rat given DOCA and saline (PDS). The images on the left are at time 0 and on the right at time 90 min. The normal pregnant rat shows minimal leakage while the PDS rat demonstrates a marked increase in vascular leak.
Fig. 3.
Comparison of vascular leakage in mesenteric postcapillary venules in three groups of female rats: control = non-pregnant female animals (n = 5); normal pregnant (NP) rats (n = 9); pregnant animals administered DOCA and saline (PDS) (n = 9). NP rats showed leakage of dye at 80–90 min (p < 0.05). PDS rats showed significant leakage beginning at 20 min (p < 0.05) when compared to control and NP rats. ∗ p < 0.05 vs. control, † p < 0.05 vs. NP, and ‡ p < 0.006 vs. control.
Apoptotic Signaling Was Activated in the Mesenteric Tissues of PDS Rats
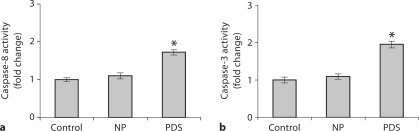
The mesenteric vascular tissues include a mixture of arterioles and venules with a few associated mesenteric support structure cells. However, care was taken to delicately dissect the vascular structures from the surrounding tissue. Representative tissues of female control, NP and PDS rats were scanned for the pro-apoptotic factor BAK, release of cytochrome c, and caspase-8 and -3 activities. No differences were observed in BAK expression and cytochrome c release between these groups (data not shown). However, there was a marked elevation of both active caspase-8 and -3 observed in PDS rats when compared to control non-pregnant and normal pregnant rats (p < 0.05; fig. 4a, b).
Fig. 4.
Caspase-8 (a) and caspase-3 (b) activities in mesenteric vasculature. The caspase-8 and -3 activities were significantly increased in PDS rats when compared to control and NP rats. ∗ p < 0.05 in each case; n = 5.
Discussion
The results indicate that in the rat model of preeclampsia induced by excessive volume expansion [6] an increase in vascular leak is demonstrable. Furthermore, we noted a much smaller but finite ‘leak’ in normal pregnant animals compared to their non-pregnant counterparts. Pregnancy is a state of volume expansion [1]. Volume expansion is the stimulus for the secretion of MBG [15, 16]. In our rat model of preeclampsia [6], we noted an increase in the excretion of MBG prior to the development of hypertension and proteinuria [7]. These data suggest that this circulating bufadienolide may play a pathogenetic role. The data presented in this communication indicate that MBG may play a role in the vascular ‘leak’ noted in our experimental animals as well. Thus, persistent secretion of MBG resulting from sustained expansion in animals that are unable to rid themselves of the excess salt and water seems linked both to the development of the preeclamptic syndrome and to the establishment of increased vascular leakage. These observations may have relevance to the human preeclamptic syndrome in which elevated blood levels of MBG have been reported [8, 9]. Endothelial cell dysfunction has been shown to be a central event in the pathophysiology of preeclampsia [4, 5, 17]. We have recently demonstrated that MBG impairs both the proliferation- and growth-factor-induced migration of first trimester CTB cells, which are critical for placental development [11].
The molecular mechanism by which MBG produces its vasoactive effects is unknown. Recently, data have accumulated that suggest that MBG may have profound effects on the mitogen-activated protein kinase pathway and may stimulate apoptosis through the activation of caspases [12]. In the instance of hemorrhagic shock, for example, in which microvascular hyperpermeability has been described [18], activation of the mitochondrial intrinsic pathway occurs. This is evidenced by an increase in the pro-apoptic Bcl-2 family member BAK, release of mitochondrial cytochrome c into the cytoplasm and activation of caspase-3 [18]. Whether MBG is involved in the microvascular hyperpermeability of hemorrhagic shock has not yet been evaluated. Neither has it been determined if MBG affects vascular permeability via Bcl-2. However, those studies are planned. Finally, whether MBG is involved in other situations in which ‘vascular leak’ is a prominent characteristic (e.g. ARDS, burns, sepsis, endotoxemia, etc.) is unknown at present.
Our results show that apoptotic signaling factors such as active caspase-8 and -3 significantly increased in the mesenteric vasculature of PDS rats compared to control and NP rats. These results suggest a relationship between the activation of the apoptotic cascade and a vascular leak in PDS rats. There were no differences observed in BAK protein expression and cytochrome c release (data not shown). Apoptosis is known to alter cell morphology by interrupting the cell-cell and cell-matrix interaction resulting in complete removal of endothelial cells from their underlying basement membrane. Regardless of the causative agents, this characteristic series of morphological changes is consistently observed. This suggests the existence of a common pathway by which an increase in the activation of a family of proteolytic enzymes (the caspases) may occur [19]. Hemorrhagic shock following trauma has been shown by several investigators to activate mediators of apoptosis including the caspases [20,21,22,23]. Tinsley et al. [24] reported burn-plasma induced hyperpermeability in monolayers of rat lung microvascular endothelial cells. Additionally, endothelial hyperpermeability resulting from cell exposure to various agonists has previously been reported [11,25,26,27].
In summary, we have documented the development of microvascular leakage in a rat model of preeclampsia. In addition, we have demonstrated that MBG is capable of causing abnormal vascular leakage in the rat. We propose, therefore, that MBG plays a role, perhaps a major one, in the development of leak from the vascular tree into the interstitium in preeclampsia. These data provide additional evidence for the view that MBG participates importantly in the pathogenesis of the preeclamptic syndrome. We recognize that the pathophysiology of the latter disorder no doubt involves multiple etiologic factors [28, 29] and that not every patient with preeclampsia is likely to demonstrate this pathogenetic sequence. To our knowledge, this communication provides the first evidence that MBG increases vascular leakage. These observations may have important implications with regard to the involvement of MBG in the causation of abnormal vascular permeability in other syndromes characterized by ‘capillary leak’.
Acknowledgements
These studies were supported, in part, by a research grant-in-aid from Dialysis Clinic, Inc., and by the Department of Medicine, Texas A&M College of Medicine and the Scott & White Memorial Hospital Temple, Tex., and by a grant from the National Heart, Lung, and Blood Institute (1K01-HL-07815-01A1; E.W. Childs). We thank Dr. G. R. Pettit (Arizona State University, USA) for his gift of the MBG, and Dr. Brett Mitchell for his critical review of the manuscript.
References
- 1.Scott DE. Anemia in pregnancy. Obstet Gynecol Annu. 1972;1:219–244. [PubMed] [Google Scholar]
- 2.Chesley LC. Plasma and red cell volumes during pregnancy. Am J Obstet Gynecol. 1972;112:440–450. doi: 10.1016/0002-9378(72)90493-0. [DOI] [PubMed] [Google Scholar]
- 3.Hays PM, Cruikshank DP, Dunn LJ. Plasma volume determination in normal and preeclamptic pregnancies. Am J Obstet Gynecol. 1985;151:958–966. doi: 10.1016/0002-9378(85)90675-1. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 6.Ianosi-Irimie M, Vu HV, Whitbred JM, Pridjian CA, Nadig JD, Williams MY, Wrenn DC, Pridjian G, Puschett JB. A rat model of preeclampsia. Clin Exp Hypertens. 2005;27:605–617. doi: 10.1080/10641960500298608. [DOI] [PubMed] [Google Scholar]
- 7.Vu HV, Ianosi-Irimie MR, Pridjian CA, Whitbred JM, Durst JM, Bagrov AY, Fedorova OV, Pridjian G, Puschett JB. Involvement of marinobufagenin in a rat model of human preeclampsia. Am J Nephrol. 2005;25:520–528. doi: 10.1159/000088461. [DOI] [PubMed] [Google Scholar]
- 8.Lopatin DA, Ailamazian EK, Dmitrieva RI, Shpen VM, Fedorova OV, Doris PA, Bagrov AY. Circulating bufadienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens. 1999;17:1179–1187. doi: 10.1097/00004872-199917080-00018. [DOI] [PubMed] [Google Scholar]
- 9.Gonick HC, Ding Y, Vaziri ND, Bagrov AY, Fedorova OV. Simultaneous measurement of marinobufagenin, ouabain, and hypertension-associated protein in various disease states. Clin Exp Hypertens. 1998;20:617–627. doi: 10.3109/10641969809053240. [DOI] [PubMed] [Google Scholar]
- 10.LaMarca HL, Morris CA, Pettit GR, Nagowa T, Puschett JB. Marinobufagenin impairs first trimester cytotrophoblast differentiation. Placenta. 2006;27:984–988. doi: 10.1016/j.placenta.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Uddin MN, Horvat D, Glaser SS, Danchuk S, Mitchell BM, Sullivan DE, Morris CA, Puschett JB. Marinobufagenin inhibits proliferation and migration of cytotrophoblast and CHO cells. Placenta. 2008;29:266–273. doi: 10.1016/j.placenta.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Uddin MN, Horvat D, Glaser SS, Mitchell BM, Puschett JB. Examination of the cellular mechanisms by which marinobufagenin inhibits cytotrophoblast function. J Biol Chem. 2008;283:17946–17953. doi: 10.1074/jbc.M800958200. [DOI] [PubMed] [Google Scholar]
- 13.Uddin MN, Hunter FA, Glaser SS, Horvat D, Childs EW, Puschett JB. Marinobufagenin impairs proliferation and triggered enhanced vascular permeability in rat lung microvascular endothelial cells. J Invest Med. 2008;56:481. [Google Scholar]
- 14.Bekker AY, Ritter AB, Duran WN. Analysis of microvascular permeability to macromolecules by video image digital processing. Microvasc Res. 1989;38:200–216. doi: 10.1016/0026-2862(89)90028-9. [DOI] [PubMed] [Google Scholar]
- 15.Fedorova OV, Doris PA, Bagrov AY. Endogenous marinobufagenin-like factor in acute plasma volume expansion. Clin Exp Hypertens. 1998;20:581–591. doi: 10.3109/10641969809053236. [DOI] [PubMed] [Google Scholar]
- 16.Bagrov AY, Fedorova OV, Dmitrieva RI, French AW, Anderson DE. Plasma marinobufagenin-like and ouabain-like immunoreactivity during saline volume expansion in anesthetized dogs. Cardiovasc Res. 1996;31:296–305. [PubMed] [Google Scholar]
- 17.Wang Y, Gu Y, Granger DN, Roberts JM, Alexander JS. Endothelial junctional protein redistribution and increased monolayer permeability in human umbilical vein endothelial cells isolated during preeclampsia. Am J Obstet Gynecol. 2002;186:214–220. doi: 10.1067/mob.2002.119638. [DOI] [PubMed] [Google Scholar]
- 18.Childs EW, Tharakan B, Hunter FA, Tinsley JH, Cao X. Apoptotic signaling induces hyperpermeability following hemorrhagic shock. Am J Physiol. 2007;292:H3179–H3189. doi: 10.1152/ajpheart.01337.2006. [DOI] [PubMed] [Google Scholar]
- 19.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 20.Davidson MT, Deitch EA, Lu Q, Hasko G, Abungu B, Nemeth ZH, Zaets SB, Gaspers LD, Thomas AP, Xu DZ. Trauma-hemorrhagic shock mesenteric lymph induced endothelial apoptosis that involves both caspase-dependent and caspase-independent mechanisms. Ann Surg. 2004;240:123–131. doi: 10.1097/01.sla.0000129341.94219.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauriz JL, Gonzalez P, Jorquera F, Olcoz JL, Gonzalez-Gallego J. Caspase inhibition does not protect against liver damage in hemorrhagic shock. Shock. 2003;19:33–37. doi: 10.1097/00024382-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Murao Y, Hata M, Ohinishi K, Okuchi K, Nakajima Y, Hiasa Y, Junger WG, Hoyt DB, Ohinishi T. Hypertonic saline resuscitation reduces apoptosis and tissue damage of the small intestine in a mouse model of hemorrhagic shock. Shock. 2003;20:23–28. doi: 10.1097/01.shk.0000078832.57685.6c. [DOI] [PubMed] [Google Scholar]
- 23.Watts JA, Grattan RM, Whitlow BS, Kline JA. Activation of poly (ADP-ribose) polymerase in severe hemorrhagic shock and resuscitation. Am J Physiol. 2001;281:G498–G506. doi: 10.1152/ajpgi.2001.281.2.G498. [DOI] [PubMed] [Google Scholar]
- 24.Tinsley JH, Breslin JW, Teasdale NR, Yuan SY. PKC-dependent, burn-induced adherens junction reorganization and barrier dysfunction in pulmonary microvascular endothelial cells. Am J Physiol. 2005;289:L217–L223. doi: 10.1152/ajplung.00248.2004. [DOI] [PubMed] [Google Scholar]
- 25.Tinsley JH, Ustinova EE, Xu W, Yuan SY. Src-dependent, neutrophil-mediated vascular hyperpermeability and β-catenin modification. Am J Physiol. 2002;283:C1745–C1751. doi: 10.1152/ajpcell.00230.2002. [DOI] [PubMed] [Google Scholar]
- 26.Tinsley JH, Wu MH, Ma W, Taulman AC, Yuan SY. Activated neutrophils induce hyperpermeability and phosphorylation of adherens junction proteins in coronary venular endothelial cells. J Biol Chem. 1999;274:24930–24934. doi: 10.1074/jbc.274.35.24930. [DOI] [PubMed] [Google Scholar]
- 27.Yuan SY, Wu MH, Ustinova EE, Guo M, Tinsley JH, De Lanerolle P, Xu W. Myosin light chain phosphorylation in neutrophil-stimulated coronary microvascular leakage. Circ Res. 2002;90:1214–1221. doi: 10.1161/01.res.0000020402.73609.f1. [DOI] [PubMed] [Google Scholar]
- 28.Pridjian G, Puschett JB. Preeclampsia. Part 1: Clinical and pathophysiological considerations. Obstet Gynecol Surv. 2002;57:598–618. doi: 10.1097/00006254-200209000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Pridjian G, Puschett JB. Preeclampsia. Part 2: Experimental and genetic considerations. Obstet Gynecol Surv. 2002;57:619–640. doi: 10.1097/00006254-200209000-00024. [DOI] [PubMed] [Google Scholar]