Abstract
Fibrosis of the glomerulus and the tubulointerstitium occurs in patients with hypertension. Studies have shown that renal oxidative stress appears in hypertensive kidney disease. The potential role of oxidative stress in renal fibrogenesis remains to be elucidated. Herein, we tested the hypothesis that oxidative stress contributes to the development of renal fibrosis during hypertension. Sprague-Dawley rats received angiotensin II (AngII; 9 μg/h s.c.) for 4 weeks with/without co-treatment of antioxidants, apocynin and tempol (120 mg/kg/day each, p.o.). Untreated rats served as controls. Appearance of renal oxidative stress and its effect on the expression of transforming growth factor (TGF)-ß1, population of myofibroblasts, collagen synthesis/degradation and fibrosis in kidneys were examined. Chronic AngII infusion elevated systemic blood pressure (228 ± 6 mm Hg), which was accompanied with extensive renal fibrosis and oxidative stress represented as upregulated NADPH oxidase and suppressed superoxide dismutase (SOD). Co-treatment with antioxidants led to: (1) markedly decreased renal NADPH oxidase; (2) significantly attenuated gene expression of TGF-ß1, type I collagen, and tissue inhibitors of matrix metalloproteinase (TIMP)-I/-II in the kidney; (3) largely reduced population of myofibroblasts in both the cortex and medulla; (4) significantly reduced renal collagen volume, and (5) partially suppressed blood pressure (190 ± 8 mm Hg). Thus, prolonged AngII administration promotes renal oxidative stress, which is associated with hypertensive renal disease. AngII induces renal oxidative stress by increasing NADPH oxidase and reducing SOD in the kidney, which, in turn, upregulates collagen synthesis, while suppressing collagen degradation, thereby promoting the development of fibrosis in kidneys of hypertensive rats.
Key Words: Hypertension, Angiotensin II, Oxidative stress, Kidney fibrosis
Introduction
Fibrosis of the glomerulus and tubulointerstitium is developed in patients and animals with hypertension, which impairs renal function, finally leading to organ failure [1, 2]. Multiple lines of mechanisms have been demonstrated to be involved in the development of hypertensive kidney disease. In addition to elevated blood pressure, numerous local factors, including angiotensin II (AngII), are known to be contributory to the formation of renal fibrosis [3, 4]. AngII, an effector hormone of the circulating renin-angiotensin system, has well-known endocrine properties in regulating cardiovascular homeostasis. A broader perspective of this peptide is emerging as ongoing research uncovers an expanding portfolio of the pathophysiologic significance of AngII on tissue repair/remodeling [5,6,7]. Experimental evidence has demonstrated that AngII stimulates the expression of NADPH oxidase, the major enzyme for reactive oxygen species (ROS) production in the repairing tissue, thereby contributing to the appearance of oxidative stress in various organs [8,9,10,11]. Renal oxidative stress has been demonstrated in several hypertension models [12,13,14,15]. Numerous studies have shown that chronic antioxidant treatment can suppress renal oxidative stress and improve renal function [13, 16]. Experimental studies have also demonstrated that oxidative stress can induce most of the changes that are thought to contribute to hypertensive kidney disease including inflammation, endothelial dysfunction, tissue damage and hypertension [16, 17]. However, the potential role of oxidative stress on renal fibrogenesis remains to be elucidated. In the current study, we sought to determine whether oxidative stress is involved in the development of renal fibrosis that appears in hypertensive kidney disease.
The expression of profibrotic cytokines, particularly transforming growth factor (TGF)-ß1, the growth of extracellular matrix-producing cells, and the balance between collagen synthesis and degradation are the major determinants for fibrous tissue formation in the kidney. Myofibroblasts are phenotypically-transformed fibroblasts, which appear in the repairing kidney, including in hypertensive kidney disease, where they play a major role in collagen synthesis [15]. Collagen degradation involves degradative enzymes, e.g. matrix metalloproteinases (MMPs), whose activity is inhibited by tissue inhibitors of matrix metalloproteinase (TIMPs). By using a hypertension model created by chronic AngII infusion, we studied potential regulation of oxidative stress on the molecular and cellular events related to renal fibrosis, including the population of myofibroblasts, expression of TGF-ß1, type I collagen, and TIMPs, and collagen volume in the kidney.
Material and Methods
Animal Model
Eight-week-old male Sprague-Dawley rats (Harlan, Indianapolis, Ind., USA) were used in this study. Three animal groups were included (n = 8 in each group): (1) untreated age-matched rats serving as controls; (2) rats receiving AngII (9 μg/h) given by implanted minipump for 4 weeks, and (3) rats on the same dose of AngII also receiving a combination of antioxidants, i.e. apocynin (NADPH oxidase inhibitor) and tempol (radical scavenger; 120 mg/kg/day each) given in drinking water for 4 weeks. Before sacrifice, systolic blood pressure was measured by tail-cuff method. Kidneys were then removed, frozen in isopentane with dry ice, and kept at −80°C. This study was approved by the University of Tennessee Health Science Center Animal Care and Use Committee.
In situ Hybridization
The localization and optical density of TGF-ß1, type I collagen, and TIMP-I/II mRNAs in the kidney were detected by quantitative in situ hybridization. In brief, cryostat kidney sections (16 μm) were fixed in 4% formaldehyde for 10 min, washed with phosphate-buffered saline (PBS, pH 7.4), and incubated in 0.25% acetic anhydride in 0.1 M TE-HCl for 10 min. Sections were then hybridized overnight with [35S]dATP-labeled DNA probes for TGF-ß1, type I collagen, and TIMP-1/II at 45°C. The hybridized sections were then washed, dried, and subsequently exposed to Kodak Biomax X-ray film. After exposure, the film was developed. Quantitation of mRNA optical density (4 sections/kidney) was performed using a computer image analysis system (NIH Image, 1.60) [18].
Immunohistochemistry
NADPH oxidase (gp91phox) and myofibroblasts in the kidney were detected by immunohistochemistry. Cryostat kidney sections (6 μm) were air-dried, fixed in 10% buffered formalin for 5 min, and washed in PBS for 10 min. Sections were then incubated with the primary antibody against gp91phox and α-smooth muscle actin (SMA; Sigma, St Louis, Mo., USA) for 1 h at room temperature. Sections were then incubated with IgG peroxidase-conjugated secondary antibody (Sigma) for 1 h at room temperature, washed in PBS for 10 min, and incubated with 0.5 mg/ml diaminobenzidine tetrahydrochloride 2-hydrate + 0.05% H2O2 for 2 min. Negative control sections were incubated with secondary antibody alone. All sections were counterstained with hematoxylin, dehydrated, mounted, and viewed by light microscopy [19].
Morphology
Cryostat kidney sections (6 μm) were prepared to determine the fibrillar collagen accumulation by collagen-specific picrosirius red staining and observed by light microscopy as previously reported. Collagen volume fraction was determined using a computer image analysis system (NIH image, 1.60) and was calculated as the sum of connective tissue areas, divided by the sum of connective tissue area and non-connective tissue area in all fields of the kidney section (4 sections/kidney) [20].
Western Blot
Manganese superoxide dismutase (MnSOD) levels in the kidney were measured by Western blot. Briefly, the kidney was homogenized in lysis buffer and then separated by 12% SDS-PAGE. After electrophoresis, samples were transferred to PVDF membranes and incubated with antibody against MnSOD. Blots were subsequently incubated with peroxidase-conjugated secondary antibody. After washing, the blots were developed with enhanced chemiluminescence method. The amount of protein detected by each antibody was measured by a computer image analysis system.
Statistical Analysis
Statistical analysis of systemic blood pressure, in situ hybridization, Western blot, and collagen volume fraction data was performed using analysis of variance. Values are expressed as mean ± SEM with p < 0.05 considered significant. Multiple group comparisons among controls and each group were made by Scheffé's F-test.
Results
Systolic Blood Pressure
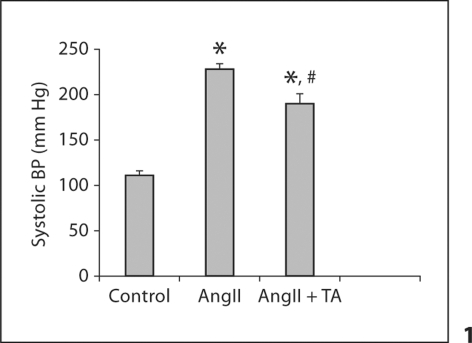
Compared to controls, systolic blood pressure was markedly elevated in rats that received AngII. Co-treatment with antioxidants partially suppressed systolic blood pressure compared to the AngII group, but it remained significantly higher than controls (fig. 1).
Fig. 1.
Systolic blood pressure in response to AngII and antioxidant treatment. ∗ p < 0.05 vs. controls; # p <0.05 vs. AngII group. TA = Tempol and apocinin.
NADPH Subunit, gp91phox Expression
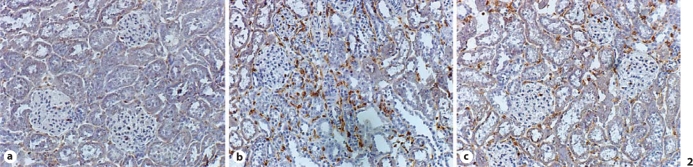
Expression of gp91phox in the kidney was detected by immunohistochemistry. In the normal kidney, positively labeled cells were rarely seen in both the cortex and medulla (fig. 2a). In rats that received AngII, abundant positively labeled cells were observed within the interstitial space (fig. 2b). Cells expressing gp91phox were primarily inflammatory cells. Co-treatment of antioxidants largely reduced gp91phox expression in the kidney compared to rats that received AngII (fig. 2c).
Fig. 2.
Expression of gp91phox in the kidney. Immunohistochemical gp91phox labeling was not seen in the normal kidney (a). In AngII-treated animals, extensive gp91phox labeling was observed in the interstitial space (b). Co-treatment of antioxidants largely reduced gp91phox labeling in the kidney (c). ×200.
MnSOD Protein Levels
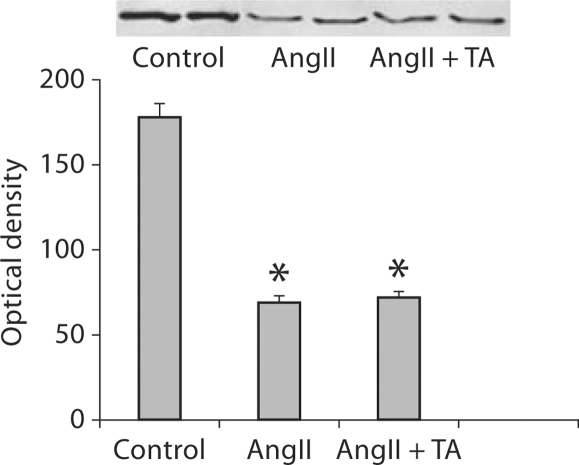
Detected by Western blot, renal MnSOD levels in rats that received AngII were significantly reduced compared to controls (fig. 3). Co-treatment with antioxidants, however, did not alter renal MnSOD levels in the AngII group (fig. 3).
Fig. 3.
MnSOD levels in the kidney. Detected by Western blot, MnSOD protein levels in the kidney were significantly reduced in AngII-treated rats compared to controls. Co-treatment of antioxidants did not recover renal MnSOD levels in AngII-treated rats.
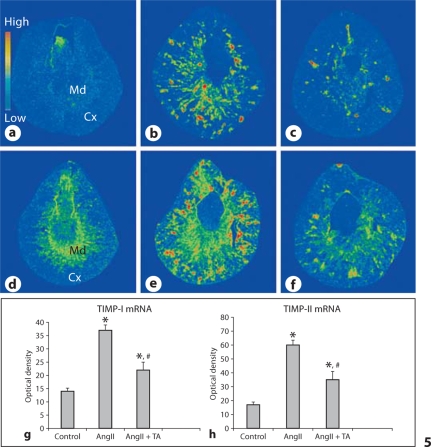
TGF-ß1, Type I and TIMP-I/II Gene Expression
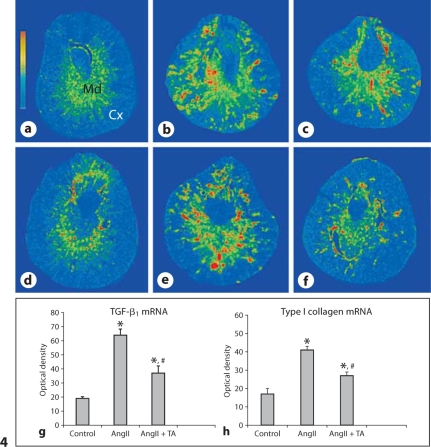
As detected by quantitative in situ hybridization, the density of TGF-ß1 mRNA was low in the cortex and medium in the medulla of the normal kidney (fig. 4a). In rats that received AngII infusion, TGF-ß1 mRNA was significantly increased in both the cortex and the medulla (fig. 4b). Co-treatment of antioxidants significantly suppressed renal TGF-ß1 mRNA levels compared to the AngII group (fig. 4c). The quantitative data on TGF-ß1 mRNA density in kidneys are shown in figure 4g.
Fig. 4.
TGF-ß1 and type-I collagen mRNAs in the kidney. Detected by in situ hybridization, TGF-ß1 and type-I collagen mRNAs were largely increased in the kidney at both the cortex (Cx) and medulla (Md) (b and e, respectively) compared to controls (a and d, respectively). Co-treatment of antioxidants largely attenuated renal TGF-ß1 and type-I collagen mRNA levels (c and f, respectively). Quantitative TGF-ß1 and type-I collagen mRNA levels are shown in g and h.
In the normal kidney, the cortex contained low levels of type I collagen mRNA, while the medulla expressed medium levels of type I collagen mRNA (fig. 4d). In rats given AngII, type I collagen mRNA level was significantly elevated at sites of injury/fibrosis in both the cortex and medulla (fig. 4e). It was significantly reduced in rats receiving co-treatment of antioxidants (fig. 4f). The quantitative data on type I collagen mRNA density in kidneys are shown in figure 4h.
A low density of TIMP-I mRNA was observed in the normal kidney (fig. 5a). In rats that received AngII infusion, TIMP-1 mRNA was significantly elevated in the cortex and medulla (fig. 5b). Co-treatment of antioxidants significantly suppressed renal TIMP-I mRNA levels compared to the AngII group (fig. 5c).
Fig. 5.
TIMP-I and TIMP-II mRNAs in the kidney. TIMP-I and TIMP-II mRNAs were markedly elevated in the kidney at both cortex and medulla (b and e, respectively) compared to controls (a and d, respectively). Co-treatment of antioxidants largely attenuated renal TIMP-I and TIMP-II mRNA levels (c and f, respectively). Quantitative TIMP-I and TIMP-II mRNA levels are shown in g and h.
In the normal kidney, the cortex contained low levels of TIMP-II mRNA, while the medulla expressed medium levels of TIMP-II mRNA (fig. 5d). Compared to controls, renal TIMP-II mRNA was significantly increased in animals that received AngII (fig. 5e), which was largely suppressed by co-treatment of antioxidants (fig. 5f). The quantitative data on TIMP-I/II mRNA density in kidneys are shown in figure 5g and h, respectively.
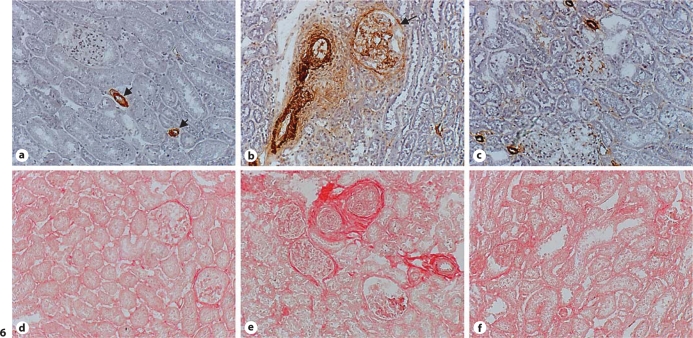
Myofibroblasts
A hallmark of myofibroblasts is the expression of α-SMA. In the normal kidney, vascular smooth muscle cells are positively labeled, while myofibroblasts were not observed in both the cortex and medulla (fig. 6a). In rats that received AngII, abundant myofibroblasts were seen in the glomeruli, interstitial and perivascular space (fig. 6b). Co-treatment with antioxidants largely attenuated the population of myofibroblasts at these sites compared to the AngII group (fig. 6c).
Fig. 6.
Myofibroblasts in the kidney. α-SMA-positive myofibroblasts were not observed in the normal kidney (a). Vascular smooth muscle cells were positively labeled (a, arrows). In AngII-treated animals, myofibroblasts were accumulated in the glomeruli (arrow) and interstitial space (b, brown). Co-treatment of antioxidants largely reduced the population of myofibroblasts in the kidney (c). Normal kidney contained small amount of collagen in the interstitial space (d, red). In AngII-treated rats, collagen was accumulated within the glomeruli, interstitial and perivascular space (e), which was largely reduced by co-treatment of antioxidants (f). ×200.
Kidney Fibrosis
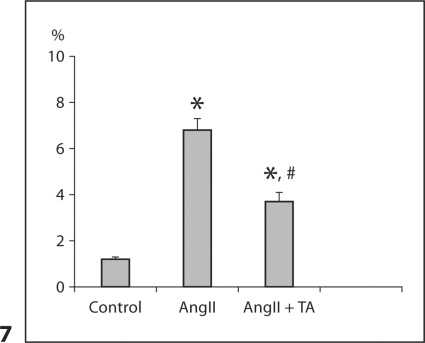
By collagen-specific picrosirius red staining, we observed a small amount of collagen in the renal interstitial space (fig. 6d). After AngII infusion, accumulated collagen was observed in the glomeruli and interstitial and perivascular space (fig. 6e). Collagen volume at these sites was markedly reduced in rats receiving antioxidant co-treatment (fig. 6f). Renal collagen volume fraction data are shown in figure 7.
Fig. 7.
Renal collagen volume fraction in response to AngII infusion with or without co-treatment of antioxidants.
Discussion
Hypertensive kidney disease is characterized by extensive fibrosis in the cortex and medulla. Increasing evidence from both animal and human studies has demonstrated that renal oxidative stress plays an important role in the pathogenesis of renal injury/repair and progression of renal dysfunction. The current study addressed the potential regulation of oxidative stress in molecular and cellular events related to renal fibrosis that appears in hypertension.
Firstly, this study has demonstrated the occurrence of renal oxidative stress in rats given AngII. The expression of gp91phox was significantly increased in both the cortex and medulla of rats with AngII infusion, indicating elevated NADPH oxidase in the kidney. This observation has confirmed the previous findings from other laboratories [21, 22] and suggests that ROS production is upregulated in the kidney in response to AngII infusion. Enhanced gp91phox expression is co-localized with sites of renal fibrosis, suggesting the potential involvement of oxidative stress in AngII-induced renal fibrogenic responses. In addition to enhanced NADPH oxidase, we further observed reduced renal SOD protein levels in rats that received AngII infusion. This finding suggests that antioxidant capacity in the kidney is also impaired. The mechanisms for the changes in SOD levels in the repairing kidney remain to be elucidated. Studies have shown that SOD is decreased in the repairing heart and kidney [23, 24]. Thus, renal oxidative stress in this model of hypertension is induced by both upregulated ROS production and reduced antioxidant enzyme in the kidney. In addition to elevated circulating AngII levels, local AngII production in the kidney is enhanced in this model, suggesting that locally generated AngII may contribute to renal oxidative stress in an autocrine/paracrine manner [25]. Oxidative stress has been observed in other kidney diseases, including diabetic nephropathy [26], and repairing tissue in various organs [8, 19], demonstrating that oxidative stress is coincident with tissue repair irrespective of its etiological and anatomical basis.
Secondly, we studied molecular and cellular changes related to renal fibrosis in response to AngII infusion. TGF-ß1 is a locally generated profibrogenic cytokine. It primarily suppresses collagen degradation and stimulates matrix-producing cell proliferation and collagen synthesis in the repairing tissue, thereby leading to fibrous tissue formation. Excessive TGF-ß1 contributes to a pathologic excess of tissue fibrosis in various diseases [18, 27]. In the current study, we observed significantly elevated TGF-ß1 gene expression particularly at the sites of fibrosis in the cortex and medulla, supporting the previous findings of the involvement of TGF-ß1 in renal fibrogenesis [28].
Interstitial fibroblasts are responsible for collagen synthesis in the normal kidney. However, studies have shown that myofibroblasts are the major cells responsible for fibrous tissue formation in hypertensive kidney disease. Myofibroblasts possess the features of both fibroblasts and smooth muscle cells and actively produce collagen in the repairing tissue [29, 30]. These cells are primarily differentiated from interstitial fibroblasts under the stimulation of TGF-ß1[31]. In the current study, myofibroblasts became abundant in the kidney of rats that received AngII infusion and were co-localized with accumulated collagen, indicating that they are responsible for fibrous tissue formation in the kidney with hypertensive disease.
Type I collagen is the major extracellular component in the kidney. The current study shows markedly increased type I collagen gene expression in glomeruli and interstitial space in AngII-treated rats. Enhanced renal collagen mRNA is anatomically coincident with myofibroblasts and accumulated collagen. These observations indicate that collagen synthesis is upregulated in the kidney after AngII infusion.
Collagen synthesis and degradation coexist in the kidney and their balance determines renal collagen volume. Collagen degradation involves MMPs and their activity is controlled by TIMPs. TIMPs function as an important regulatory brake on MMP activity by inhibition of the active species, thereby suppressing collagen degradation. TIMP expression is tightly controlled at the transcription level, which is induced by TGF-ß [32, 33]. In animals given AngII, gene expression of TIMP-I and TIMP-II are significantly increased in the kidney, indicating that collagen degradation is suppressed. Thus, in the model of hypertension, the imbalance of renal collagen synthesis and degradation results in renal fibrosis.
Finally, we studied the importance of oxidative stress on the development of renal fibrosis in hypertensive kidney disease. Rats were co-treated with AngII and a combination of antioxidants (i.e. apocynin, a NADPH oxidase inhibitor, and tempol, a radical scavenger) to investigate the potential regulation of oxidative stress in renal fibrogenesis. We found that antioxidants attenuated renal NADPH oxidase expression, which could, in turn, suppress ROS production in the kidney. Even though reduced renal SOD levels in rats that received AngII were not recovered by co-treatment of antioxidants, tempol served as a radical scavenger to catalyze ROS, which could suppress renal oxidative stress. Our study also shows that antioxidants significantly reduced TGF-ß1 expression, which, in turn, attenuated the population of myofibroblasts, collagen synthesis, and MMP inhibition, thereby suppressing renal fibrosis. These observations indicate that oxidative stress stimulates renal profibrogenic phenotype in hypertensive kidney disease. Pathways for the regulation of oxidative stress on TGF-ß1 synthesis and its related fibrogenic responses in kidneys remain to be determined. It has been demonstrated that oxidative stress activates a major transcription factor, nuclear factor-κB (NF-κB), which stimulates TGF-ß1 expression [34]. Studies have also shown that AngII stimulates TGF-ß1 expression, which can be prevented by AngII receptor antagonist [18, 35]. The stimulatory role of AngII on TGF-ß1 production can be mediated through ROS [36]. In the current study, antioxidant treatment partially suppressed blood pressure, which may also contribute to improved renal remodeling in rats with AngII infusion.
In summary, chronic AngII infusion leads to hypertension, which is accompanied by a fibrogenic phenotype involving the cortex and medulla in the kidney. Renal TGF-ß1 expression is enhanced, which is co-localized with appearance of myofibroblasts and activated collagen synthesis, while collagen degradation is suppressed in rats that received AngII infusion. Antioxidant co-treatment suppressed TGF-ß1 expression and its related fibrogenic responses in the kidney, indicating that oxidative stress contributes to the development of renal fibrosis in hypertensive kidney disease. Thus, prolonged AngII administration promotes renal oxidative stress, which is associated with hypertensive renal disease. These changes, induced by prolonged AngII infusion and prevented by antioxidants, provide important data incriminating AngII as one of the putative underlying mechanisms associated with renal structural derangements with hypertensive disease.
References
- 1.Griffin KA. Hypertension and kidney damage. J Clin Hypertens (Greenwich) 2006;8:209–214. doi: 10.1111/j.1524-6175.2005.05111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finco DR. Association of systemic hypertension with renal injury in dogs with induced renal failure. J Vet Intern Med. 2004;18:289–294. doi: 10.1892/0891-6640(2004)18<289:aoshwr>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Wolf G. Link between angiotensin II and TGF-ß in the kidney. Miner Electrolyte Metab. 1998;24:174–180. doi: 10.1159/000057367. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992;19:464–474. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- 5.Sun Y, Weber KT. Tissue angiotensin II and myocardial infarction. EXS. 1996;76:479–488. doi: 10.1007/978-3-0348-8988-9_29. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez A, Lopez B, Diez J. Fibrosis in hypertensive heart disease: role of the renin-angiotensin-aldosterone system. Med Clin North Am. 2004;88:83–97. doi: 10.1016/s0025-7125(03)00125-1. [DOI] [PubMed] [Google Scholar]
- 7.Varagic J, Frohlich ED. Local cardiac renin-angiotensin system: hypertension and cardiac failure. J Mol Cell Cardiol. 2002;34:1435–1442. doi: 10.1006/jmcc.2002.2075. [DOI] [PubMed] [Google Scholar]
- 8.Higashi Y, Yoshizumi M. Oxidative stress, reactive oxygen species (in Japanese) Nippon Rinsho. 2004;62:49–55. [PubMed] [Google Scholar]
- 9.Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol. 2003;30:860–866. doi: 10.1046/j.1440-1681.2003.03930.x. [DOI] [PubMed] [Google Scholar]
- 10.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S241–S245. doi: 10.1097/01.asn.0000077410.66390.0f. [DOI] [PubMed] [Google Scholar]
- 12.Biswas SK, de Faria JB. Which comes first: Renal inflammation or oxidative stress in spontaneously hypertensive rats? Free Radic Res. 2007;41:216–224. doi: 10.1080/10715760601059672. [DOI] [PubMed] [Google Scholar]
- 13.Tian N, Rose RA, Jordan S, Dwyer TM, Hughson MD, Manning RD., Jr N-acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J Hypertens. 2006;24:2263–2270. doi: 10.1097/01.hjh.0000249705.42230.73. [DOI] [PubMed] [Google Scholar]
- 14.Wesseling S, Ishola DA, Jr, Joles JA, Bluyssen HA, Koomans HA, Braam B. Resistance to oxidative stress by chronic infusion of angiotensin II in mouse kidney is not mediated by the AT2 receptor. Am J Physiol Renal Physiol. 2005;288:F1191–F1200. doi: 10.1152/ajprenal.00322.2004. [DOI] [PubMed] [Google Scholar]
- 15.Jin L, Beswick RA, Yamamoto T, Palmer T, Taylor TA, Pollock JS, Pollock DM, Brands MW, Webb RC. Increased reactive oxygen species contributes to kidney injury in mineralocorticoid hypertensive rats. J Physiol Pharmacol. 2006;57:343–357. [PubMed] [Google Scholar]
- 16.Manning RD, Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol. 2005;25:311–317. doi: 10.1159/000086411. [DOI] [PubMed] [Google Scholar]
- 17.Vaziri ND. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens. 2004;13:93–99. doi: 10.1097/00041552-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Zhang JQ, Zhang J, Ramires FJ. Angiotensin II, transforming growth factor-ß1 and repair in the infarcted heart. J Mol Cell Cardiol. 1998;30:1559–1569. doi: 10.1006/jmcc.1998.0721. [DOI] [PubMed] [Google Scholar]
- 19.Zhao W, Ahokas RA, Weber KT, Sun Y. Ang II-induced cardiac molecular and cellular events: role of aldosterone. Am J Physiol. 2006;291:H336–H343. doi: 10.1152/ajpheart.01307.2005. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Ratajska A, Weber KT. Inhibition of angiotensin-converting enzyme and attenuation of myocardial fibrosis by lisinopril in rats receiving angiotensin II. J Lab Clin Med. 1995;126:95–101. [PubMed] [Google Scholar]
- 21.Lopez B, Salom MG, Arregui B, Valero F, Fenoy FJ. Role of superoxide in modulating the renal effects of angiotensin II. Hypertension. 2003;42:1150–1156. doi: 10.1161/01.HYP.0000101968.09376.79. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal R, Campbell RC, Warnock DG. Oxidative stress in hypertension and chronic kidney disease: role of angiotensin II. Semin Nephrol. 2004;24:101–114. doi: 10.1016/j.semnephrol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Khaper N, Kaur K, Li T, Farahmand F, Singal PK. Antioxidant enzyme gene expression in congestive heart failure following myocardial infarction. Mol Cell Biochem. 2003;251:9–15. [PubMed] [Google Scholar]
- 24.Moreno I, Pichardo S, Jos A, Gomez-Amores L, Mate A, Vazquez CM, Camean AM. Antioxidant enzyme activity and lipid peroxidation in liver and kidney of rats exposed to microcystin-LR administered intraperitoneally. Toxicon. 2005;45:395–402. doi: 10.1016/j.toxicon.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Hartner A, Porst M, Klanke B, Cordasic N, Veelken R, Hilgers KF. Angiotensin II formation in the kidney and nephrosclerosis in REN-2 hypertensive rats. Nephrol Dial Transplant. 2006;21:1778–1785. doi: 10.1093/ndt/gfl065. [DOI] [PubMed] [Google Scholar]
- 26.Anjaneyulu M, Chopra K. Diltiazem attenuates oxidative stress in diabetic rats. Ren Fail. 2005;27:335–344. [PubMed] [Google Scholar]
- 27.Sun Y, Zhang J, Zhang JQ, Ramires FJ. Local angiotensin II and transforming growth factor-ß1 in renal fibrosis of rats. Hypertension. 2000;35:1078–1084. doi: 10.1161/01.hyp.35.5.1078. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto T, Noble NA, Miller DE, Border WA. Sustained expression of TGF-ß1 underlies development of progressive kidney fibrosis. Kidney Int. 1994;45:916–927. doi: 10.1038/ki.1994.122. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Weber KT. Angiotensin converting enzyme and myofibroblasts during tissue repair in the rat heart. J Mol Cell Cardiol. 1996;28:851–858. doi: 10.1006/jmcc.1996.0080. [DOI] [PubMed] [Google Scholar]
- 30.Badid C, Mounier N, Costa AM, Desmouliere A. Role of myofibroblasts during normal tissue repair and excessive scarring: interest of their assessment in nephropathies. Histol Histopathol. 2000;15:269–280. doi: 10.14670/HH-15.269. [DOI] [PubMed] [Google Scholar]
- 31.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-ß1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li WQ, Qureshi HY, Liacini A, Dehnade F, Zafarullah M. Transforming growth factor-ß1 induction of tissue inhibitor of metalloproteinases 3 in articular chondrocytes is mediated by reactive oxygen species. Free Radic Biol Med. 2004;37:196–207. doi: 10.1016/j.freeradbiomed.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 33.Wright JK, Cawston TE, Hazleman BL. Transforming growth factor-ß stimulates the production of the tissue inhibitor of metalloproteinases (TIMP) by human synovial and skin fibroblasts. Biochim Biophys Acta. 1991;1094:207–210. doi: 10.1016/0167-4889(91)90010-u. [DOI] [PubMed] [Google Scholar]
- 34.Komura E, Tonetti C, Penard-Lacronique V, Chagraoui H, Lacout C, Lecouedic JP, Rameau P, Debili N, Vainchenker W, Giraudier S. Role for the nuclear factor-κB pathway in transforming growth factor-ß1 production in idiopathic myelofibrosis: possible relationship with FK506-binding protein 51 overexpression. Cancer Res. 2005;65:3281–3289. doi: 10.1158/0008-5472.CAN-04-2339. [DOI] [PubMed] [Google Scholar]
- 35.Shihab FS, Bennett WM, Tanner AM, Andoh TF. Angiotensin II blockade decreases TGF-ß1 and matrix proteins in cyclosporine nephropathy. Kidney Int. 1997;52:660–673. doi: 10.1038/ki.1997.380. [DOI] [PubMed] [Google Scholar]
- 36.Noh H, Ha H, Yu MR, Kim YO, Kim JH, Lee HB. Angiotensin II mediates high glucose-induced TGF-ß1 and fibronectin upregulation in HPMC through reactive oxygen species. Perit Dial Int. 2005;25:38–47. [PubMed] [Google Scholar]