Abstract
Mammalian meiotic recombination, which preferentially occurs at specialized sites called hotspots, assures the orderly segregation of meiotic chromosomes and creates genetic variation among offspring. A locus on mouse Chr 17, that controls activation of recombination at multiple distant hotspots, has been mapped within a 181 Kb interval, three of whose genes can be eliminated as candidates. The remaining gene, Prdm9, codes for a zinc finger containing histone H3K4 trimethylase that is uniquely expressed in early meiosis and whose deficiency results resulting in sterility in both sexes. Mus musculus exhibits five alleles of Prdm9; human populations exhibit two predominant alleles and several minor alleles. The discovery of Prdm9 as the first protein regulating mammalian recombination hotspots initiates molecular studies of this important biological control system.
Text
Genetic recombination is an essential biological process among eukaryotes. Mammalian meiotic recombination, which preferentially occurs at specialized sites, 1-2 Kb long, known as hotspots, assures the orderly segregation of meiotic chromosomes and creates genetic variation among offspring. However, despite their importance in determining the recombination landscape of the organism, we have little understanding of the elements determining the location and relative activity of hotspots. That the location of a hotspot is not determined simply by its internal DNA sequence is supported by both yeast 1 and mammalian studies 2,3. Two recent reports 4,5 have described the existence of trans-acting loci that control the activation of specific hotspots elsewhere. The loci, Dsbc1 and Rcr1, are located in overlapping 5.4 Mb and 6.3 Mb regions on mouse chromosome 17.
We have now extended the mapping of both Rcr1 and Dsbc1 using 1580 progeny of a cross between the C57BL/6J (B6) and CAST/EiJ (CAST) mouse strains differing in activity of the Rcr1/ Dsbc1 controlled hotspots Hlx1, Esrrg-1 and Psmb9 (Fig. S1). We located both loci to a common 181 Kb region containing four protein coding genes (Fig. 1): Psmb1, Tbp, Pdcd2, and Prdm9. We found no differences in expression levels of the transcripts of any of these proteins in testes when assayed by RT-PCR (Table S1).
Fig. 1.
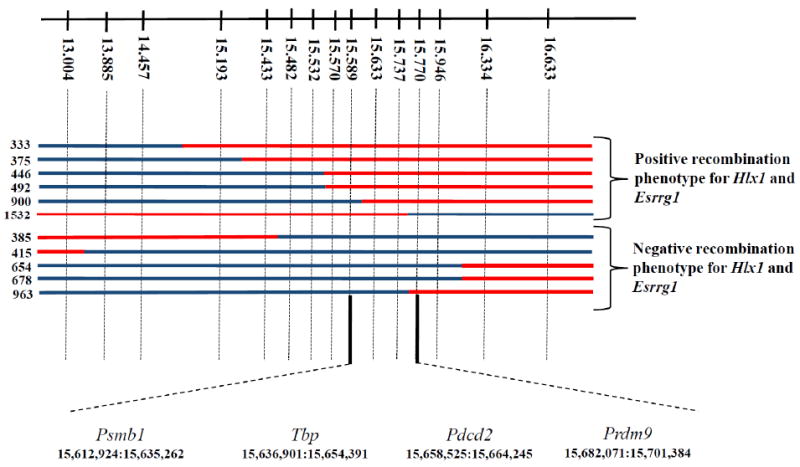
Fine mapping the location of Rcr1. Upper line shows the position, in Mb (Ensembl Build 37), of the markers used for genetic mapping. Crossovers collected in this region are marked with blue for B6 DNA and red for CAST. On the left are the serial numbers of the male progeny tested for recombination activity. The critical region containing Rcr1 is between markers at 15.589 and 15.770 Mb. Below are the order and coordinates of the four protein-coding genes in the critical region.
Psmb1, proteasome beta type 1 subunit, is a ubiquitously expressed protein that functions as a structural component of an organelle responsible for generalized protein degradation. Tbp, TATA binding protein, is an essential component of the TFIID transcription initiation complex which has considerable DNA binding specificity. Pdcd2, programmed cell death protein 2, is widely expressed, repressed during B cell lymphomagenesis. Based on DNA sequencing of the entire coding regions of these three proteins, we found only five coding SNPs, all synonymous, resulting in no amino acid differences between the B6 and CAST strains (Table S2). For the first exon of Pdcd2, we relied on the Sanger Institute sequence (http://www.sanger.ac.uk/modelorgs/mousegenomes/).
Prdm9, PR domain containing 9, is uniquely expressed during early meiosis in both males and females, and the knockout is blocked at the pachytene stage of meiosis I with reduction of the number of Dmc1 foci and a loss of Dmc1 and γ-H2AX colocalization, resulting in sterility in both sexes and azoospermia in males 6. It has three functional domains, a N terminal KRAB domain that can promote protein:protein binding and transcriptional repression when tethered to DNA by an adjacent DNA binding domain, a central PR/SET domain providing a histone methyl transferase activity capable of trimethylating H3K4 and thus altering chromatin configuration, and a terminal zinc finger domain of C2H2 type. The zinc finger domain contains tandem repeats, with one finger per repeat, and has 11 fingers in the CAST allele and 12 in the B6 allele, with a number of amino acid substitutions in the 6, 9 and 12 positions after the second cysteine of the fingers, the residues determining DNA binding specificity. (Fig. S2). Given the properties of Prdm9, including its requirement for meiosis, its histone 3 lysine-4 trimethylation activity and role in determining DNA DSBs, and the lack of a reasonable alternative candidate, we conclude that Prdm9 is Rcr1/Dsbc1. This is supported by the observation 7 that recombination at hotspots Psmb9 and Hlx1, regulated by Rcr1/Dsbc1, is preceded by histone 3 lysine-4 trimethylation.
We sequenced Prdm9 exon 12 containing the entire Zn finger domain in 20 mouse strains and found five alleles differing in the number of zinc finger repeats, ranging from 11 to 14, as well as in codons for the amino acids in 6th, 9th and 12th position of each finger responsible for DNA binding (Fig. S2). The alleles for C3H and PWD mouse strains are identical with those reported by Mihola et al. 8 who identified Prdm9 as the gene underlying the Hst1 locus determining male infertility in certain crosses between strains of M.m. musculus and M.m. domesticus.
We also sequenced exon 12 of human PRDM9 in 64 DNA samples from the Coriell Institute for Medical Research. We found two predominant alleles among these 128 chromosomes, containing a zinc finger domain of 12 tandem repeats, and several minor frequency alleles (Fig. S3A). African-Americans had eleven minor alleles with 11, 12 or 13 zinc fingers and a number of amino acid substitutions, Han Chinese three minor alleles, two with 12 and one with 13 fingers, and Mexican-Americans one, identical to the Chinese variant with 13 fingers, while Caucasians had none (Fig. S3B). These results conform well with presently accepted views of human evolution.
The discovery of Prdm9 as the first mammalian protein regulating meiotic recombination hotspots initiates studies of an important biological control system that has hitherto been inaccessible. A recent paper 9 identified Prdm9 as a speciation gene across diverse metazoans and hypothesized that its essential role in meiosis is directly related to its ability to bind rapidly evolving DNA sequences. Our results clearly show that these sequences represent recombination hotspots. An immediate compelling question is whether Prdm9 controls activation of all, or nearly all, recombination hotspots, or whether it is simply a member of a family of proteins, each controlling a sub-set of all hotspots.
Supplementary Material
References and Notes
- 1.Kon N, Krawchuk MD, Warren BG, Smith GR, Wahls WP. Transcription factor Mts1/Mts2 (Atf1/Pcr1, Gad7/Pcr1) activates the M26 meiotic recombination hotspot in Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1997;94:13765–70. doi: 10.1073/pnas.94.25.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiroishi T, Sagai T, Hanzawa N, Gotoh H, Moriwaki K. Genetic control of sex-dependent meiotic recombination in the major histocompatibility complex of the mouse. EMBO J. 1991;10:681–6. doi: 10.1002/j.1460-2075.1991.tb07997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann R, Jeffreys AJ. Polymorphism in the activity of human crossover hotspots independent of local DNA sequence variation. Hum Mol Genet. 2006;15:1401–1411. doi: 10.1093/hmg/ddl063. [DOI] [PubMed] [Google Scholar]
- 4.Parvanov ED, Ng SH, Petkov PM, Paigen K. Trans-regulation of mouse meiotic recombination hotspots by Rcr1. PLoS Biology. 2009;7:e1000036. doi: 10.1371/journal.pbio.1000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grey C, Baudat F, de Massy B. Long distance regulation of initiation of meiotic recombination. PLoS Biology. 2009;7 doi: 10.1371/journal.pbio.1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi K, Yoshida K, Matsui Y. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature. 2005;438:374–8. doi: 10.1038/nature04112. [DOI] [PubMed] [Google Scholar]
- 7.Buard J, Barthes P, Grey C, de Massy B. Distinct histone modifications define initiation and repair of meiotic recombination in the mouse. Embo J. 2009 doi: 10.1038/emboj.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihola O, Trachtulec Z, Vlcek C, Schimenti JC, Forejt J. A mouse speciation gene encodes a meiotic histone H3 methyltransferase. Science. 2009;323:373–5. doi: 10.1126/science.1163601. [DOI] [PubMed] [Google Scholar]
- 9.Oliver PL, et al. Accelerated Evolution of the Prdm9 Speciation Gene across Diverse Metazoan Taxa. PLoS Genet. 2009;5:e1000753. doi: 10.1371/journal.pgen.1000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The authors are indebted to Evie Sargent, Tim Billings and Gunjan Gilbert for technical assistance The work was supported in part by NIH grants GM 078643, 083408, 078452, 076468 and CA 34196.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


