Abstract
Objective
To determine if prostate tumour volume is an independent prognostic factor in a contemporary cohort of men who had a radical prostatectomy (RP) for clinically localized disease, as the effect of tumour volume on prostate cancer outcomes has not been consistently shown in the era of widespread screening with prostate-specific antigen (PSA).
Patients and methods
The study included 856 men who had RP from 1998 to 2007 for localized prostate cancer. Tumour volume based on pathology was analysed as a continuous and categorized (< 0.26, 0.26–0.50, 0.51–1.00, 1.01–2.00, 2.01–4.00, > 4.00 mL) variable using Cox proportional hazards regression and Kaplan-Meier analysis. A multivariable analysis was also conducted controlling for PSA level, Gleason grade, surgical margins, and pathological stage.
Results
Tumour volume had a positive association with grade and stage, but did not correlate with biochemical recurrence-free survival on univariate analysis as a continuous variable (hazard ratio 1.00, P = 0.09), and was only statistically significant for volumes of > 4 mL as a categorical variable. No tumour volume was an independent predictor of prostate cancer recurrence on multivariate analysis. There was no difference between tumour volume and time to cancer recurrence for organ-confined tumours using Kaplan-Meier analysis. In low-risk patients (PSA level < 10 ng/mL, Gleason score ≤□ 6, clinical stage T1c/T2a) tumour volume did not correlate with biochemical recurrence-free survival in univariate or multivariable analysis.
Conclusions
There is no evidence that tumour volume is an independent predictor of prostate cancer outcome and it should not be considered as a marker of tumour risk, behaviour or prognosis.
Keywords: prostate cancer, prognosis, localized, volume, radical prostatectomy
Introduction
The general principles of tumour biology stress the importance of tumour volume and its role in predicting clinical oncological behaviour [1]. Historically, this relationship was thought to apply to prostate cancer. Indeed, tumour volume has been shown to be proportional to well established predictive indicators including PSA level, Gleason grade, surgical margin (SM) status and pathological stage [2]. Despite these associations, tumour volume has not been consistently shown to be an independent predictor of the outcome after radical prostatectomy (RP), especially when pathological grade and stage are taken into account.
Two opposing studies are often cited for the importance of tumour volume in predicting biochemical failure after RP. Stamey et al. [2,3] showed that cancer volume was independently associated with prostate cancer progression, when controlling for PSA level, grade and stage, in men who had surgery from 1983 to 1992. Epstein et al. [4] subsequently reported that tumour volume did not provide additional prognostic information beyond Gleason grade and surgical margin status in men treated with RP from 1982 to 1988. Notably, both studies were conducted before the widespread use of PSA screening.
The effect of tumour volume on prostate cancer outcomes as an independent predictor continues to be controversial in the PSA era, in which ever more low-volume, low-grade cancers are diagnosed [5,6]. A few recent studies have sought to answer this question, but no consensus has been reached. Thus, our objective was to determine if prostate tumour volume is an independent prognostic factor in a contemporary cohort of men who had RP for clinically localized disease.
Patients and methods
All men undergoing RP at our institution are entered prospectively into the University of California, San Francisco Urologic Oncology Data Base, which gathers staging/risk assessment, intraoperative, pathological, health-related quality of life and outcomes data. Between 1998 and 2007, 2138 patients had RP as monotherapy for localized prostate cancer and consented to have their clinical data included in the registry. Of these men, 738 had missing or unknown tumour volume and 486 had < 6 months of documented follow-up; 914 patients were therefore eligible for the current analysis. Also, 58 men had missing data on one or more tumour characteristics, leaving 856 patients for inclusion in the multivariate analysis. None of the included patients received neoadjuvant or adjuvant therapy. Preoperative PSA levels were obtained for all patients. Participating men provided written, informed consent for their de-identified data to be included in the database under supervision of the institutional review board.
Prostate specimens were submitted in their entirety and evaluated after fixation in formalin. Specimens were sectioned transversely in 3-mm intervals. Tumour volume was determined using a visual estimation. The area of tumour was measured in x and y diameters and multiplied by the depth, based on presence of tumour in subsequent sections and the thickness of sections. The sum total of all foci of tumour was the estimated tumour volume. This method of visual estimation was previously described and validated in other studies [7]. Primary and secondary Gleason grade was documented for each patient. The presence of extracapsular extension (ECE), seminal vesicle invasion (SVI), lymph node invasion (LNI) and SM status was noted.
Cox proportional hazards regression and Kaplan-Meier survival analysis were used to examine the relationship between pathological tumour volume and biochemical progression. For these analyses, tumour volume was compared as both a continuous and categorized ordinal (< 0.26, 0.26–0.50, 0.51–1.00, 1.01–2.00, 2.01–4.00, > 4.00 mL) variable. This categorization yielded approximate quintiles of volume, except that the smallest quintile was further divided in half. Kaplan-Meier survival curves were also generated for the subset of organ-confined tumours with negative SMs. A multivariable analysis was conducted controlling for PSA level, Gleason score, ECE, SVI, LNI and SM status. Biochemical recurrence was defined as a PSA level of > 0.2 ng/mL on two occasions after RP, or the initiation of secondary treatment ≥□6 months after surgery [8,9].
Results
The patient characteristics are shown in Table 1; most patients had a preoperative PSA level of < 10 ng/mL (80%) and pathology negative for ECE (74%), SVI (94%), LNI (98%) and SM (82%); most also had a Gleason score of ≤□7 (90%). The follow-up after RP was 0.5–14 years; there was a biochemical recurrence in 95 men (10%) during this period, with a median time to recurrence of 14 months, and a median follow-up of 24 months for those who did not have a recurrence.
Table 1.
Patient characteristics and pathological results
| Variable | Value |
|---|---|
| Mean (sd, range): | |
| age, years | 59 (7, 37–80) |
| PSA level, ng/mL | 7.5 (5.3, 1.2–100) |
| n (%): | |
| < 6 | 458 (50) |
| 6–10 | 274 (30) |
| 10.01–20 | 136 (15) |
| 20.01–30 | 17 (2) |
| > 30 | 8 (1) |
| Pathological Gleason score | |
| 2–6 | 337 (37) |
| 3 + 4 | 377 (41) |
| 4 + 3 | 106 (12) |
| 8–10 | 78 (9) |
| ECE | |
| Negative | 678 (74) |
| Positive | 236 (26) |
| SVI | |
| Negative | 857 (94) |
| Positive | 57 (6) |
| LNI | |
| Negative | 892 (98) |
| Positive | 22 (2) |
| SM | |
| Negative | 754 (82) |
| Positive | 160 (18) |
| Median (range) | |
| tumour volume, mL | 1.5 (0.1–87) |
| n (%) | |
| < 0.26 | 95 (10) |
| 0.26–0.50 | 97 (11) |
| 0.51–1.00 | 175 (19) |
| 1.01–2.00 | 187 (21) |
| 2.01–4.00 | 183 (20) |
| > 4.00 | 177 (19) |
Tumour volume did not correlate with biochemical survival on univariate analysis as a continuous variable (hazard ratio 1.00, P = 0.09, 95% CI 0.999–1.008). When volume was instead analysed as a categorized variable, hazard ratios for biochemical progression tended to increase with increasing tumour volume. However, the only statistically significant difference was for tumour volumes of > 4 mL (P < 0.05; Table 2).
Table 2.
Univariate analysis of categorized tumour volume as a predictor of biochemical failure, and multivariate analysis of predictors of biochemical failure
| Variable | Hazard ratio (95% CI) | P |
|---|---|---|
| Tumour volume | ||
| < 0.26 | reference | |
| 0.26–0.50 | 1.83 (0.45–7.32) | 0.393 |
| 0.51–1.00 | 1.44 (0.39–5.32) | 0.584 |
| 1.01–2.00 | 2.61 (0.76–8.97) | 0.127 |
| 2.01–4.00 | 2.52 (0.74–8.63) | 0.139 |
| > 4.00 | 4.3 (1.31–14.09) | 0.016 |
| Multivariate PSA, ng/mL | ||
| ≤□6 | reference | |
| 6.01–10 | 1.55 (0.90–2.50) | 0.113 |
| 10.01–20 | 2.06 (1.12–3.77) | 0.020 |
| 20.01–30 | 4.09 (1.49–11.2) | 0.006 |
| > 30 | 6.34 (2.15–18.7) | 0.001 |
| Gleason score | ||
| 2–6 | reference | |
| 3 + 4 | 2.25 (1.10–4.61) | 0.026 |
| 4 + 3 | 3.87 (1.70–8.75) | 0.001 |
| 8–10 | 4.34 (1.89–9.96) | 0.001 |
| SVI | 1.92 (1.05–3.50) | 0.034 |
| ECE | 2.01 (1.25–3.25) | 0.004 |
| SM | 1.61 (1.02–2.55) | 0.042 |
| LNI | 0.99 (0.62–1.68) | 0.963 |
| Tumour volume, mL | ||
| ≤ 0.25 | reference | |
| 0.26–0.50 | 1.26 (0.31–5.18) | 0.748 |
| 0.51–1.00 | 0.80 (0.21–3.09) | 0.749 |
| 1.01–2.00 | 1.27 (0.36–4.56) | 0.710 |
| 2.01–4.00 | 0.79 (0.22–2.87) | 0.721 |
| > 4.00 | 1.11 (0.31–3.96) | 0.869 |
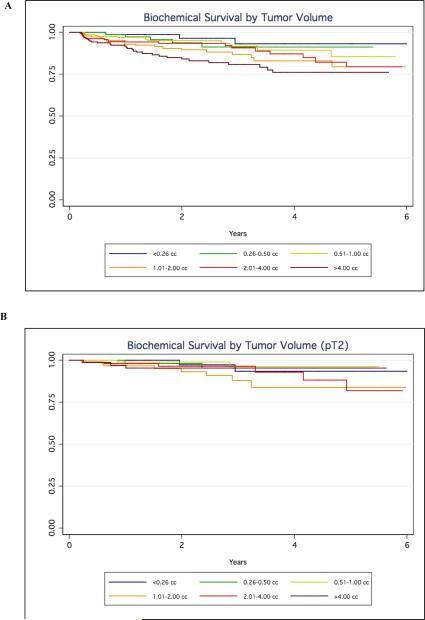
After controlling for known independent predictors of prostate cancer recurrence, no tumour volume was an independent predictor of outcome. As expected, PSA level, Gleason score, ECE, SVI and positive SMs were independent prognosticators of biochemical recurrence (P < 0.05; Table 2). Also, Kaplan-Meier analysis showed a higher risk of recurrence for tumours of > 4 mL (Fig. 1A), but this association was not apparent among organ-confined tumours with negative SMs (Fig. 1B).
Fig. 1.
Biochemical-free survival by tumour volume for a, all patients, and b, those with pT2 disease.
Subgroups were also analysed to examine the relationship between tumour volume and biochemical survival in low-risk patients (PSA level < 10 ng/mL, Gleason score ≤□6 and clinical stage T1c or T2a) [10]. In the cohort, 354 men met these criteria and 21 (5.9%) had cancer recurrence. Tumour volume did not correlate with biochemical recurrence-free survival on univariate or multivariate analysis. Of the 21 patients who had a biochemical recurrence, eight (38%) had ECE, three (14%) had SVI and 10 (48%) had a positive SM.
Discussion
With the increase in PSA screening, most men found to have prostate cancer currently present with clinically localized disease [5,6]. Numerous studies, mostly done before the widespread use of PSA testing, have shown that preoperative PSA level, Gleason score, LNI, SM status and pathological stage are independent predictors of cancer recurrence after treatment with RP [10]. However, there has been an ongoing search for additional variables, such as tumour volume, to help predict which patients are at high risk of recurrence and might therefore benefit from adjuvant treatment [2,4]. Nevertheless, the importance of tumour volume as an independent predictive variable remains controversial, especially given the recent stage migration that has occurred. In the present study of 856 patients, we found that tumour volume associated weakly with outcomes on univariate analysis, and that there was no evidence for tumour volume as a predictor of biological outcomes independent of prostate cancer stage, grade and PSA level. This conclusion also held true for patients who are considered at low risk of biochemical recurrence.
Studies in the PSA era analysing the effect of tumour volume on biochemical recurrence have yielded mixed results. In 2006, Nelson et al. [11] concluded that tumour volume was an independent predictor of PSA recurrence in 431 men treated by RP from 2000 to 2001. In that study, mean tumour volume associated with pathological stage and was significantly different between patients with and without recurrence (6.8 and 2.6 mL, respectively). On multivariate analysis, tumour volume as a continuous variable predicted biochemical recurrence. The results were not presented using tumour volume as a categorical variable or stratified by grade. In the present study with a larger cohort and longer follow-up, there was a greater risk of biochemical recurrence on univariate analysis for tumour volumes of > 4 mL. However, after adjusting for stage or grade, tumour volume was not significantly associated with progression as either a continuous or categorized variable.
Similarly, studies by Solomon et al. [12] and Kikuchi et al. [13] assessing the significance of tumour volume in clinically localized prostate cancer found that it added no independent prognostic information on multivariate analysis. Both studies had comparable mean and median tumour volumes to the present cohort, although the follow-up time was somewhat less. As in the present study, both groups showed that tumour volume (low or high) was not a predictor of PSA recurrence in a subset of low-risk patients.
Recent findings by Merrill et al. [14] in a large population undergoing RP for localized prostate cancer again confirmed this observation, finding that regardless of tumour volume, low-risk patients had a low rate of cancer progression. Notably, they found that tumour volume was a significant independent predictor of recurrence in patients with Gleason scores of ≥□7, although the sample size for this subgroup was small. Cheng et al. [15] reported that the maximum tumour diameter predicted biochemical recurrence and correlated with tumour volume in 364 patients, although the follow-up in that study was short (1.5 months to 2 years). By contrast, Van Oort et al. [16] reported no significant relationship between tumour volume and maximum tumour diameter with biochemical recurrence in high-risk localized prostate cancer, which is also consistent with our data.
A tumour volume of < 0.5 mL is often cited as a criterion, along with low Gleason score and stage, for defining insignificant prostate cancers [17]. In the present analysis we found no suggestion that tumours of < 0.5 mL but otherwise low-risk features (organ-confined, negative SMs, Gleason = 3 + 3) had any higher risk of recurrence than smaller tumours. Conversely, small tumours with higher grade or stage can show aggressive behaviour despite being small [13]. Based on our findings, tumour volume has limited value in predicting the clinical behaviour of localized prostate cancer, whether in the overall cohort or among those with otherwise low-risk features.
We recognize certain limitations of this study, most importantly that it was retrospective, and that tumour volume measurements were not available for all patients in the database. More than one pathologist reviewed the prostate specimens, which might have affected the reporting of tumour volume by visual estimation. In addition, the follow-up was limited in some men who have not recurred, particularly those diagnosed in the earlier years of the cohort. Lastly, all men in the current study had a RP and therefore were pre-selected based on certain risk features. Tumour volume could be a significant predictor in those men with more advanced disease, as reported previously, who were not well represented in this cohort [18,19].
In conclusion, in a contemporary cohort of men treated with RP for clinically localized disease, the risk of biochemical recurrence did not increase consistently with increasing tumour volume. There is no evidence that tumour volume predicts biological outcomes, independent of stage, Gleason grade and PSA level. Tumour volume should not be considered an independent marker of disease risk or prognosis in men with localized disease.
Acknowledgements
Supported by National Institutes of Health/National Cancer Institute, University of California- San Francisco SPORE Special Program of Research Excellence P50CA89520
Abbreviations
- RP
radical prostatectomy
- SM
surgical margin
- ECE
extracapsular extension
- SVI
seminal vesicle invasion
- LNI
lymph node invasion
References
- 1.Nicholson B, Theodorescu D. Angiogenesis and prostate cancer tumor growth. J Cell Biochem. 2004;91:125–50. doi: 10.1002/jcb.10772. [DOI] [PubMed] [Google Scholar]
- 2.Stamey TA, McNeal JE, Yemoto CM, et al. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999;281:1395–400. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- 3.Stamey TA, Freiha FS, McNeal JE, et al. Localized prostate cancer: relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer. 1993;71:933–8. doi: 10.1002/1097-0142(19930201)71:3+<933::aid-cncr2820711408>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Epstein JI, Carmichael M, Partin AW, et al. Is tumor volume an independent predictor of progression following radical prostatectomy? A multivariate analysis of 185 clinical stage B adenocarcinomas of the prostate with 5 years follow up. J Urol. 1993;149:1478–81. doi: 10.1016/s0022-5347(17)36421-2. [DOI] [PubMed] [Google Scholar]
- 5.Soh S, Kattan MW, Berkman S, et al. Has there been a recent shift in the pathological features and prognosis of patients treated with radical prostatectomy? J Urol. 1997;157:2212–8. [PubMed] [Google Scholar]
- 6.Cooperberg MR, Lubock DP, Mehta SS, Carroll PR. Time trend in clinical risk stratification of prostate cancer: Implications for outcomes (data from CaPSURE) J Urol. 2003;170:S21–S27. doi: 10.1097/01.ju.0000095025.03331.c6. [DOI] [PubMed] [Google Scholar]
- 7.Renshaw AA, Chang H, D'Amico AV. Estimation of tumor volume in radial prostatectomy specimens in routine clinical practice. Am J Clin Pathol. 1997;107:704–8. doi: 10.1093/ajcp/107.6.704. [DOI] [PubMed] [Google Scholar]
- 8.Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate specific antigen after anatomic radical retropubic prostatectomy. Urol Clin North Am. 1997;24:395–406. doi: 10.1016/s0094-0143(05)70386-4. [DOI] [PubMed] [Google Scholar]
- 9.D'Amico AV, Whittington R, Malkowicz SB, et al. The combination of preoperative prostate specific antigen and postoperative pathological findings to predict prostate specific antigen outcome in clinically localized prostate cancer. J Urol. 1998;160:2096–101. doi: 10.1097/00005392-199812010-00041. [DOI] [PubMed] [Google Scholar]
- 10.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999;17:1499–505. doi: 10.1200/JCO.1999.17.5.1499. [DOI] [PubMed] [Google Scholar]
- 11.Nelson BA, Shappell SB, Change SS, et al. Tumour volume is an independent predictor of prostate-specific antigen recurrence in patients undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int. 2006;97:1169–72. doi: 10.1111/j.1464-410X.2006.06148.x. [DOI] [PubMed] [Google Scholar]
- 12.Solomon K, Levrel O, Anastasiadis AG, et al. Prognostic significance of tumor volume after radical prostatectomy: a multivariate analysis of pathological prognostic factors. Eur Urol. 2003;43:39–44. doi: 10.1016/s0302-2838(02)00493-1. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi E, Scardino PT, Wheeler TM, et al. Is tumor volume an independent prognostic factor in clinically localized prostate cancer? J Urol. 2004;172:508–11. doi: 10.1097/01.ju.0000130481.04082.1a. [DOI] [PubMed] [Google Scholar]
- 14.Merrill MM, Lane BR, Reuther AM, et al. Tumor volume does not predict for biochemical recurrence after radical prostatectomy in patients with surgical Gleason score 6 or less prostate cancer. Urology. 2007;70:294–8. doi: 10.1016/j.urology.2007.03.062. [DOI] [PubMed] [Google Scholar]
- 15.Eichelberger LE, Koch MO, Ebel JN, et al. Maximum tumor diameter is an independent predictor of prostate-specific antigen recurrence in prostate cancer. Modern Pathol. 2005;18:886–90. doi: 10.1038/modpathol.3800405. [DOI] [PubMed] [Google Scholar]
- 16.Van Oort IM, Witjes JA, Kok DEG, et al. Maximum tumor diameter is not an independent prognostic factor in high risk localized prostate cancer. World J Urol. 2008;26:237–41. doi: 10.1007/s00345-008-0242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kattan MW, Eastham JA, Wheeler TM, et al. Counseling men with prostate cancer. A nomogram for predicting the presence of small, moderately differentiated, confined tumors. J Urol. 2003;170:1792–7. doi: 10.1097/01.ju.0000091806.70171.41. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi M, Kikuchi H, Ishibashi M, et al. Percentage of the positive area of bone metastasis is an independent predictor of disease death in advance prostate cancer. Br J Cancer. 2003;88:195–201. doi: 10.1038/sj.bjc.6600715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuya Y, Akakura K, Akimoto S, et al. Pattern of progression and survival in hormonally treated metastatic prostate cancer. Int J Urol. 1999;6:240–4. doi: 10.1046/j.1442-2042.1999.00060.x. [DOI] [PubMed] [Google Scholar]