Abstract
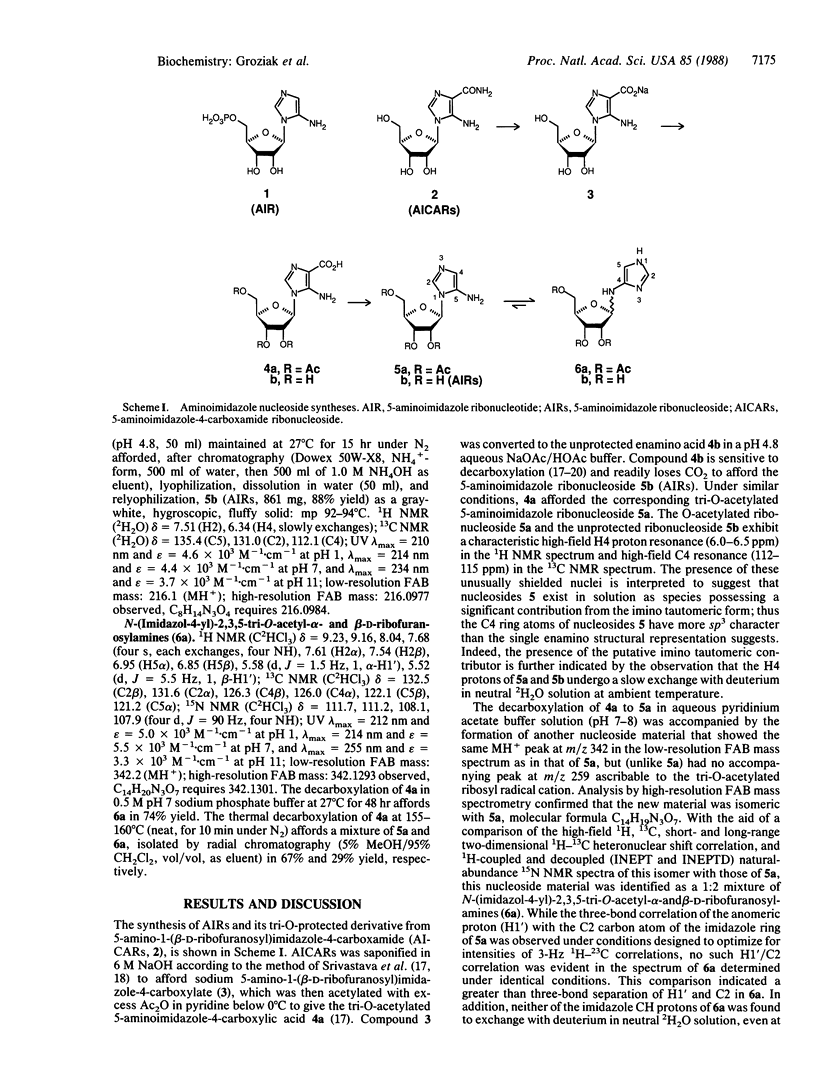
5-Amino-1-beta-D-ribofuranosylimidazole 5'-monophosphate (AIR, 1) is the ubiquitous precursor to the purine ribonucleotides in vivo, and it serves as the biochemical precursor to the pyrimidine portion of thiamin (vitamin B1) in certain prokaryotic organisms. The corresponding ribonucleoside (AIRs, 5b) was prepared via chemical (nonenzymatic) synthesis from 5-amino-1-beta-D-ribofuranosylimidazole-4-carboxamide. The tri-O-acetylated derivative of AIRs (5a) was also prepared, and it was shown to undergo a facile ring transformation in aqueous pH 7 buffer to afford N-(imidazol-4-yl)-2,3,5-tri-O-acetyl-D-ribofuranosylamine as a 1:2 mixture of alpha and beta anomers (6a). Under similar conditions, compound 5b affords the corresponding unprotected beta-ribonucleosides 6b. This Dimroth-type ring transformation reaction of 5 to 6, which occurs primarily in neutral aqueous solution, may be responsible for the previously reported lability of AIRs and its derivatives. It may also have relevance to the postulated early biotic pathway to the 9- and 3-substituted purine nucleotide components of an all-purine biopolymer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert A. The Dimroth rearrangement. XI. Catalysis by methylamine salts. Preparation of 7-methylamino- and 6,7-dihydro-7-imino-v-triazolo(4,5)pyrimidines via 4-ethoxymethyleneamino-1,2,3-triazoles. J Chem Soc Perkin 1. 1973;22:2659–2663. doi: 10.1039/p19730002659. [DOI] [PubMed] [Google Scholar]
- Albert A. The Dimroth rearrangement. XII. Transformation by alkali of 4-amino-3-benzyl-1,2,3-triazole and its 5-substituted derivatives into the corresponding 4-benzylamino isomers. Retrogression of this reaction in neutral solvents. J Chem Soc Perkin 1. 1970;2:230–235. doi: 10.1039/j39700000230. [DOI] [PubMed] [Google Scholar]
- Cusack N. J., Shaw G., Litchfield G. J. Purines, pyrimidines, and imidazoles. XXXVI. Carboxylation of some 5-aminoimidazoles and related compounds, including nucleosides and nucleotides, with potassium hydrogen carbonate in aqueous solution. J Chem Soc Perkin 1. 1971;8:1501–1507. doi: 10.1039/j39710001501. [DOI] [PubMed] [Google Scholar]
- Estramareix B., David S. Biosynthesis of thiamine: origin of the methyl carbon atom of the pyrimidine moiety in Salmonella typhimurium. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1136–1141. doi: 10.1016/0006-291x(86)90369-4. [DOI] [PubMed] [Google Scholar]
- Franks M., Green C. P., Shaw G., Litchfield G. J. Purines, pyrimidines, and imidazoles. XXV. Some chemical reactions and interconversions of intermediates in the sequence of biosynthesis de novo of purine nucleotides leading to imidazole nucleotides. J Chem Soc Perkin 1. 1966;24:2270–2274. doi: 10.1039/j39660002270. [DOI] [PubMed] [Google Scholar]
- Hill A. R., Jr, Kumar S., Leonard N. J., Orgel L. E. Template-directed oligomerization of 3-isoadenosine 5'-phosphate. J Mol Evol. 1988;27(2):91–95. doi: 10.1007/BF02138366. [DOI] [PubMed] [Google Scholar]
- Horton J. K., Stevens M. F. A new light on the photo-decomposition of the antitumour drug DTIC. J Pharm Pharmacol. 1981 Dec;33(12):808–811. doi: 10.1111/j.2042-7158.1981.tb13944.x. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Monny C., Laursen R. A., Leonard N. J. Polynucleotide analogues. VIII. Poly 3-isoadenylic acid. Biochim Biophys Acta. 1966 May 19;119(2):258–267. doi: 10.1016/0005-2787(66)90184-5. [DOI] [PubMed] [Google Scholar]
- Schrimsher J. L., Schendel F. J., Stubbe J. Isolation of a multifunctional protein with aminoimidazole ribonucleotide synthetase, glycinamide ribonucleotide synthetase, and glycinamide ribonucleotide transformylase activities: characterization of aminoimidazole ribonucleotide synthetase. Biochemistry. 1986 Jul 29;25(15):4356–4365. doi: 10.1021/bi00363a027. [DOI] [PubMed] [Google Scholar]
- Srivastava P. C., Ivanovics G. A., Rousseau R. J., Robins R. K. [Nucleosides of 4-substituted imidazoles]. J Org Chem. 1975 Oct 3;40(20):2920–2924. doi: 10.1021/jo00908a015. [DOI] [PubMed] [Google Scholar]
- Srivastava P. C., Mancuso R. W., Rousseau R. J., Robins R. K. Nucleoside peptides. 6. Synthesis of certain N-(5-amino-1-(beta-D-ribofuranosyl)imidazole-4-carbonyl)amino acids related to naturally occurring intermediates in the purine biosynthetic pathway. J Med Chem. 1974 Nov;17(11):1207–1211. doi: 10.1021/jm00257a600. [DOI] [PubMed] [Google Scholar]
- Thomas H. J., Avery L. N., Brockman R. W., Montgomery J. A. 5-Amino-4-(diazoacetyl)-1-beta-D-ribofuranosylimidazole, a new antileukemic agent. J Med Chem. 1987 Feb;30(2):431–434. doi: 10.1021/jm00385a030. [DOI] [PubMed] [Google Scholar]
- Townsend L. B. Imidazole nucleosides and nucleotides. Chem Rev. 1967 Oct;67(5):533–563. doi: 10.1021/cr60249a002. [DOI] [PubMed] [Google Scholar]
- Wächtershäuser G. An all-purine precursor of nucleic acids. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1134–1135. doi: 10.1073/pnas.85.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Kumaoka H. Precursor of carbon atom five and hydroxymethyl carbon atom of the pyrimidine moiety of thiamin in Escherichia coli. J Nutr Sci Vitaminol (Tokyo) 1983 Aug;29(4):389–398. doi: 10.3177/jnsv.29.389. [DOI] [PubMed] [Google Scholar]



