Abstract
Natural killer1 cells hold promise for cancer therapy. NK cytotoxicity can be enhanced by expression of chimeric antigen receptors that re-direct specificity toward target cells by engaging cell surface molecules expressed on target cells. We developed a regulatory-compliant, scalable non-viral approach to engineer NK cells to be target-specific based on transfection of mRNA encoding chimeric receptors. Transfection of eGFP mRNA into ex vivo expanded NK cells (N=5) or purified unstimulated NK cells from peripheral blood (N=4) resulted in good cell viability with eGFP expression in 85% ± 6% and 86% ± 4%, 24 hours after transfection, respectively. An mRNA encoding a receptor directed against CD19 (anti-CD19-BB-z) was also transfected into NK cells efficiently. Ex vivo expanded and purified unstimulated NK cells expressing anti-CD19-BB-z exhibited enhanced cytotoxicity against CD19+ target cells resulting in ≥80% lysis of acute lymphoblastic leukemia and B-lineage chronic lymphocytic leukemia cells at effector target ratios lower than 10:1. The target-specific cytotoxicity for anti-CD19-BB-z mRNA-transfected NK cells was observed as early as 3 hours after transfection and persisted for up to 3 days. The method described here should facilitate the clinical development of NK-based antigen-targeted immunotherapy for cancer.
Keywords: NK cells, chimeric antigen receptor, electroporation, non-viral, transfection, mRNA
Introduction
The capacity of natural killer1 cells to exert cytotoxicity against a variety of cancer cell types makes them an attractive tool for anti-cancer therapy2–7. Data gathered in the setting of allogeneic hematopoietic stem cell transplantation (HSCT) indicate that donor selection based on the degree of mismatch between expression of killer immunoglobulin-like receptors (KIRs) on donor NK cells and HLA Class I molecules expressed by the patient cells should maximize NK cell killing of target cells4,8–10, hence augmenting the efficacy of HSCT6,7,11. In addition, it was reported that the infusion of haploidentical NK cells in a non-myeloablative transplant setting could produce remissions in patients with acute myeloid leukemia5. Although NK cell cytotoxicity has a wide spectrum, some cancer cell types appear less susceptible or refractory to NK cell killing, because of failure to activate NK cells, induction of suppression or both. Among these relatively NK-resistant cell types are lymphoid malignancies such as acute lymphoblastic leukemia (ALL), B-cell chronic lymphocytic leukemia (B-CLL) and B-cell non-Hodgkin lymphoma. 3,12–16
Chimeric antigen receptor has been studied since late 80’s17–22. It generally contains a single chain variable fragment (scFv) as the extracellular antigen recognition unit and multiple lymphocyte activation domains as the intracellular activation part. Most work has been focused in arming T cells with this chimeric antigen receptor for anti-tumor effect21–23. NK cells transduced with chimeric antigen receptor have also been exploited for anti-tumor effect. Various combinations of intracellular activation domains were studied. The inclusion of both signal molecules of CD3ξ and 41BB was found not only to be optimal for mediating more activation signals, and producing higher IFN-γ and GM-CSF by NK cells after contacting to antigen-specific target cells18, but also to help NK cells to both direct their reactivity towards specific cell types and significantly augment their cytotoxicity19,20,22,24–26, Thus, expression of a receptor binding CD19, a surface molecule widely expressed in B cell malignancies, and delivering activation signals through CD3z and 4-1BB (anti-CD19-BB-z) could overcome KIR-mediated inhibition and render NK cell highly cytotoxic against ALL cells19. Therefore, the development of a practical and efficient method for large-scale expression of chimeric antigen receptors in NK cells should have important clinical applications.
Electroporation is an established and efficient non-viral method for loading molecules into a wide variety of cell types27–31. We here report a practical scalable method to efficiently transfect expanded and purified unstimulated NK cells through electroporation of mRNA encoding the anti-CD19-BB-z chimeric antigen receptor. With this method, we observed a high transfection efficiency and high cell killing of CD19-positive target cells. This method can be applied to chimeric receptors with different specificities, is suitable for large-scale transfection and it should be easily translatable to a clinical setting.
Materials and Methods
Cells
The CD19+ human B-lineage ALL cell line, OP-132, and the genetically engineered myeloid leukemia cell line, K562, co-expressing 4 1BB ligand and membrane bound IL-1518 were generated at St Jude Children’s Research Hospital (Memphis, TN) and were maintained in RPMI-1640 supplemented with 10% fetal bovine serum and antibiotics. Leukemic cells were obtained from the peripheral blood of patients with B-CLL with appropriate informed consent and approval (George Washington Hospital). Peripheral blood from healthy donor cells was obtained from BRT Laboratories, Inc (Baltimore, MD). Peripheral blood mononuclear cells (PBMC) were prepared with Ficoll density gradient centrifugation, washed twice with phosphate buffered saline (PBS), and frozen in liquid nitrogen until use.
Purified unstimulated NK cells were selected from PBMC according to the manufacture’s protocol using a Miltenyi NK cell isolation kit (Auburn, CA) and frozen in liquid nitrogen until use. NK cell expansion was achieved as previously described by Imai et al18. Briefly, peripheral blood mononuclear cells were cultured with irradiated and thawed K562 cells co-expressing 4-1BB ligand and membrane-bound IL-15. Cultures were performed in the presence of 10 IU/ml-100 U/ml IL-2 in the presence of 10% FBS and antibiotics.
Gene constructs used for electroporation
A transgene encoding anti-CD19 single chain variable region conjugated with the 4-1BB intracellular domain and the CD3z domain was cloned from the parental plasmid pMSCVanti-CD19BBZ19 into pVAX1 (Invitrogen, Carlsbad, CA). The cloning was performed by digesting the parental plasmid pMSCVanti-CD19BBZ and the pVAX1 vector with EcoR I and Xho I and ligating using T4 DNA ligase (NEW ENGLAND BioLab, Beverly, MA). The cloned pVAX1-αCD19BBZ was used as a template for in vitro mRNA transcription after linearization with XbaI. The mRNA encoding anti-CD19-BB-z (mRNA anti-CD19-BB-z) was transcribed in vitro with T7 RNA polymerase using the Ambion mMESSAGE mMACHINE T7 Ultra kit (Ambion, Austin, TX). The mRNA quality and quantity was analyzed by 1% agarose gel electrophoresis after 15 min denaturation at 70°C in mRNA denaturation buffer (Invitrogen, Carlsbad, CA) and quantitation by UV spectrophotometry (OD260/280). The mRNA encoding eGFP (mRNA-GFP) was transcribed in vitro using template of Cla I-linearized pCI-eGFP plasmid cloned into the pCI backbone (Promega, Madison, WI) and the same Ambion kit as mentioned above.
Electroporation
Purified unstimulated NK cells were thawed in a 37°C water bath and incubated for 0.5–1h at 37°C in prewarmed fresh complete medium (RPMI-1640+10% FBS+ antibiotics) before transfection. Expanded NK cells were harvested for transfection 6–12 days after expansion. After collection, both expanded and purified unstimulated NK cells were washed once with EP buffer (MaxCyte, Gaithersburg, MD), mixed with 100μg/ml mRNA (unless specified, at 1-3e8 cells/ml), transferred into MaxCyte processing chamber (Gaithersburg, MD), and transfected using the program “Expanded-NK#3” and “unstimulated-NK#1” for expanded and purified unstimulated NK cells, respectively, using a MaxCyte GT system (MaxCyte, Gaithersburg, MD). Immediately after electroporation, cells were recovered from the processing chamber and incubated for 20 min at 37°C, and then resuspended and cultured in complete medium. Expression was analyzed by flow cytometry using a FACSCalibur and CellQuest software (Becton Dickinson, San Jose, CA).
The MaxCyte GT system (MaxCyte, Gaithersburg, MD) has capabilities for both static small volume (as mentioned above) and computer-controlled flow large volume electroporation31. The flow electroporation is achieved by computer-controlled repeated cycles of electroporation. Each cycle consists of three steps: flowing in a fraction of cells into flow chamber, transfection and flowing out the transfected cells for collection. If mRNA transfection could maintain the similar transfection efficiency from fraction to fraction, mRNA transfection could be scaled up. About 10–20 transfection cycles with 4ml/cycle (holding capacity for MaxCyte current chamber) could achieve relevant volume for clinical applications. However, considering the cost in primary cell expansion and mRNA production, using not too high cell volume for transfection to obtain meaningful scale-up result is pursued in this study. Because of this, a mini-flow chamber with 0.8ml holding-volume capacity (manuscript in preparation) was designed, aiming at achieving 10–20 fractions in flow electroporation with about 10ml cell volume. NK cells expanded for 10 days were washed twice with 10 ml of EP buffer (MaxCyte, Gaithersburg, MD), resuspended with EP buffer to a diluted cell concentration (5–14ml at 4e7 cells/ml), added with 20μg/ml final concentration of mRNA encoding GFP, and flowed through the mini flow-chamber for transfection with the MaxCyte GT system using the program “Expanded-NK#1-Flow”. The transfected cells were collected by fractions with ≅ 0.8ml/fraction during the flow electroporation, incubated for 20min in the same EP buffer for each fraction, cultured and analyzed by FACS 6h-1d post transfection. Small volume of the mRNA-cell mixture was set aside for regular small volume static electroporation using “expanded-NK#3” program (mentioned above) to compare the scale-up efficiency.
Detection of chimeric antigen receptor expression and immunophenotyping
To detect anti-CD19-BB-z expression, NK cells were stained with goat anti-mouse (Fab)2 polyclonal antibody conjugated with biotin (Jackson Immuno Research Labs, West Grove, PA) followed by staining with peridinin chlorophyll protein-(PerCp; Becton Dickinson, San Jose, CA) labeled streptavidin. Cells stained with goat biotin-conjugated IgG followed by streptavidin-PerCp were used for negative gating.
The following antibodies were used for immunophenotypic characterization of NK cells: anti-CD3 conjugated with fluorescein isothiocyanate (FITC), anti-CD19 conjugated with phycoerythrin (PE), anti-CD16-PE, and anti-CD56-PE.
Cell killing assay
To facilitate a high throughput analysis of cell killing, we developed a simple non-radioactive cell killing assay that is based on FACS analysis combined with acetoxymethyl-calcein (calcein-AM, Molecular Probes, Eugene, OR)-labeled target cells (manuscript in preparation). Briefly, calcein-AM pre-labeled target cells (105 cells in 100μl) were co-cultured with 100 μl of either transfected, non-transfected NK cells or just fresh medium at various E:T ratios in each well of a 96-well U-bottom tissue culture plate in triplicates (Costar, Cambridge, MA). The 96-well plate was centrifuged at 400g for 5 min prior to cell culture in a 37°C, 5% CO2 incubator. The cells were resuspended and transferred to 4mL FACS tubes for FACS analysis at the indicated time points.
In some experiments, a previously reported antibody staining method was used for measuring cell killing19. Briefly, unstained target cells (105 cells in 100μl) were co-cultured with 100 μl of either transfected, non-transfected NK cells or just fresh medium at various E:T ratios in each well of a 96-well U-bottom tissue culture plate in triplicates (Costar, Cambridge, MA). After centrifugation at 400g for 5 min, the cells were cultured for the indicated cell-killing time. The cells were harvested and stained with anti-CD56-PE and/or anti-CD19-FITC antibodies for 20 min on ice. After washing in PBS, the cells were resuspended with 200 μl of PBS and analyzed by flow cytometry.
Cell killing assays were performed in the absence of exogenous IL-2. During acquisition, the FACS collection time was kept constant at 15 seconds. The specific cell lysis (%) was calculated using the following formula:
where NTarget is the number of viable target cells cocultured with NK cells and NControl is the number of viable target cells cultured alone, in the absence of NK cells. Cell killing was assessed at 4 h, unless specified otherwise.
Statistic analysis
Data was presented as mean ± standard deviation. Unpaired Student’s t-test (Microsoft Excel) with two tails was used to determine the significance of results. Statistical significance was determined as P < 0.05.
Results
eGFP transfection of human NK cells
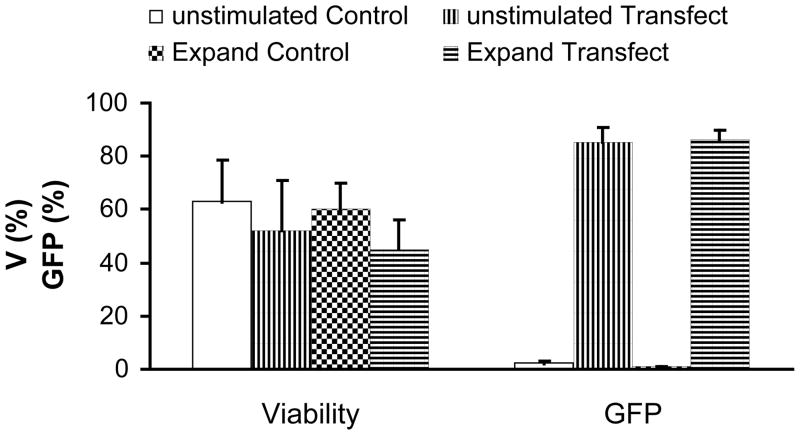
In our first set of experiments, we tested whether eGFP could be expressed in NK cells after transfection of the encoding mRNA mediated by electroporation. Percentage of GFP-positive NK cells after transfection was high, ranging from 81% to 92% (86% ± 5%) in experiments with purified unstimulated NK cells (n =3) and with NK cell expanded for 6–12 days by coculture with K562-mb15-41BBL cells (n = 3) (Fig. 1a and b). Transfection did not significantly affect cell viability (Fig. 1a and 1b). The mRNA-transfected cells had a decreased proliferation rate for the first day post transfection, but they typically recovered and exhibited similar proliferation rates as control cells later. The transfected NK cells expanded to 58% ± 7% (n = 4) of the cell numbers of control cells during the 4-day culturing period post transfection. The mRNA concentration in the range of 25μg/ml to 200μg/ml resulted in similar cell post-EP proliferation (data not shown).
Fig 1. Transfection of mRNA in expanded and purified unstimulated NK cells.
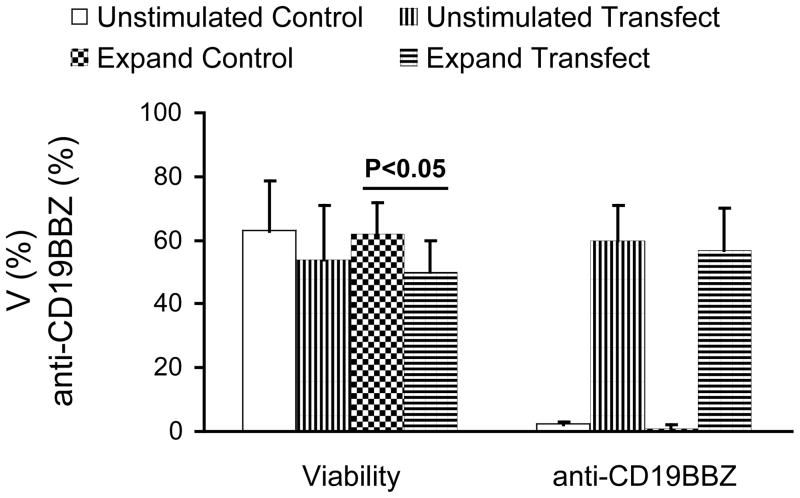
a. FACS analysis of NK cells transfected with mRNA-GFP. Expanded NK cells (left panels) and purified unstimulated (right panels) NK cells are depicted. b. Summary of post-electroporation viability and transfection efficiency of expanded and unstimulated NK cells transfected with mRNA-GFP. Results were obtained from two donors done in triplicates. c FACS analysis of transfection of mRNA-anti-CD19-BB-z into expanded (left panels) and unstimulated (right panels) NK cells. d. Summary of post-electroporation viability and anti-CD19-BB-z expression of expanded and unstimulated NK cells transfected with mRNA encoding anti-CD19-BB-z. Results of expanded NK cells were obtained from two donors, performed 10 times in total and unstimulated NK cells from four donors, performed 5 times in total. The expressions of transfected cells are significantly higher than that of control (p<0.01). The viabilities of transfected cells are insignificantly different from that of control cells (p>0.05), except one indicated in the figure.
Transfection of the anti-CD19-BB-z chimeric receptor in NK cells
The effect of NK cell transfection on viability and expression efficiency was further studied using mRNA encoding a functional transgene, anti-CD19-BB-z. As shown in Fig 1c, anti-CD19-BB-z mRNA was transfected efficiently into expanded and purified unstimulated NK cells. Expression was dose dependent, increasing with the increase of the used mRNA concentration from 25μg/ml to 200μg/ml (data not shown). Transfection at day 1 post transfection did not dramatically affect the percentage of viable cells as gated in the R1 region in this typical transfection result, with viabilities of control and transfected cells of 66% and 54% for expanded and 67% and 64% for unstimulated NK cells, respectively. As shown in Fig 1c, a clear anti-CD19-BB-z expression was achieved in both expanded and purified unstimulated NK cells (Fig 1d). Percentage of anti-CD19BB-z-positive NK cells after transfection was high, ranging from 33% to 81% (58% ± 12%) in experiments with unstimulated NK cells (n = 5) and with NK cell expanded for 6–12 days by coculture with K562-mb15-41BBL cells (n = 10) (Fig. 1c and d). Expression of anti-CD19-BB-z in expanded NK cells decreased with time but remained detectable up to day 4 post-transfection (data not shown). In unstimulated NK cells, anti-CD19-BB-z expression for the observed 2 day expression was detected with a 20%-30% decrease from day 1 to day 2. Similar cell proliferation characteristics were observed for anti-CD19-BB-z and eGFP transfected cells in the case of expanded NK cells (data not shown).
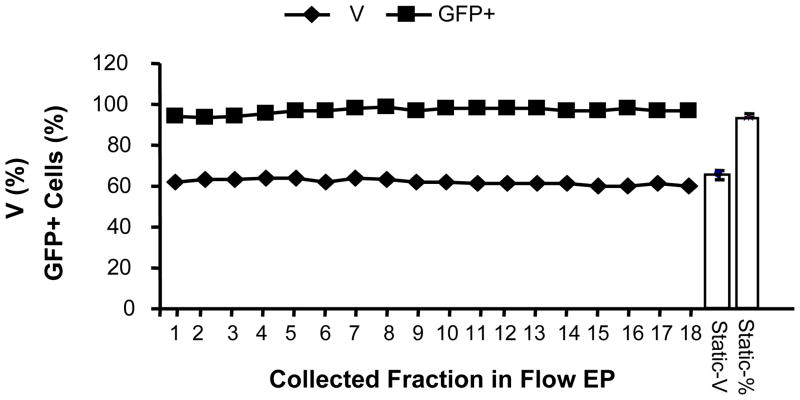
To demonstrate the feasibility of scalability of mRNA transfection, we report here a novel computer-controlled flow electroporation of mRNA, in which the expanded NK cells were electroporated when they were pumped to flow through the flow chamber. The result was shown in Fig 2. The transfection efficiency for collected fractions after flow electroporation and samples after static electroporation (30μl each, n=4) led to a range of 92%–98% and 94% ± 1% in GFP expression respectively. There is basically no decrease in transfection efficiency from fraction to fraction within 18 fractions. Also, the transfection efficiency of each fraction in flow electroporation is comparable to that of samples in 30μl static electroporation. It was a successful scale up transfection from 30ul to 14ml, a 400–500 folders increase. Our further study will need to overcome the cost restriction for mRNA production and the primary cell expansion so that we could exploit further scale up study with chamber of 3.5ml holding-volume capacity and routine cell concentration of 1-3e8 cells/ml, which could lead to ≥1×1010 cell processing capacity at the current 18 fraction flow electroporation level.
Fig 2. Scale-up flow electroporation of mRNA encoding eGFP.
The result of FACS analysis of 10-day expanded NK cells transfected with mRNA-GFP was depicted. NK cells were resuspended in 14 ml, flow electroporated, and collected with 18 fractions of 0.8ml each. The viability, GFP+ cell percentage (%) and mean fluorescence intensity (MFI) were represented for each fraction of the flow electroporated and samples (n=4) of the regularly electroporated (30μl for each electroporation). Results are representative of three independent experiments.
Leukemic cell killing by NK cells transfected with anti-CD19BB-z
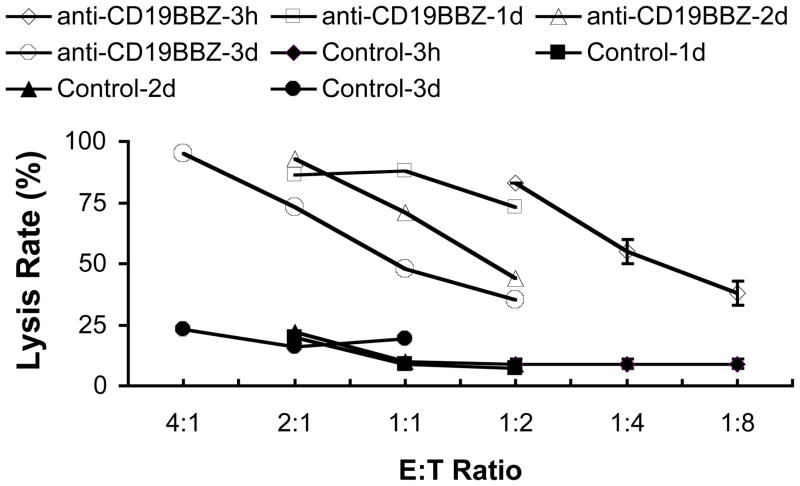
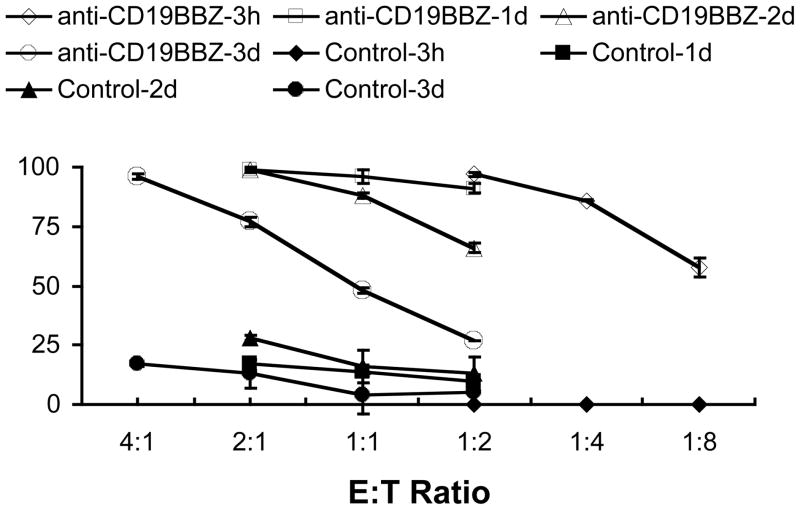
Expanded NK cells transfected with anti-CD19-BB-z effectively and specifically lysed CD19+ ALL cells. By contrast, NK cells transfected with GFP and NK cells electroporated without mRNA (EP alone) exerted no significant cytotoxicity over that exerted by non-electroporated NK cells (Fig 3a and b). The degree of ALL cell lysis by NK cells expressing anti-CD19-BB-z receptors depended on the concentration of mRNA used. As shown in Fig 4, cell killing was seen as early as 3h post transfection (Fig 4a-1 and 2). A 4-hour coculture resulted in approximately 80% lysis of the OP-1 cells at E: T ratio of 1: 2;16-hour coculture led to almost 100% lysis of the OP-1 cells at the same 1:2 ratio. NK cells transfected with anti-CD19-BB-z mRNA maintained their specific killing activity for up to 3 days post-transfection (Fig 4b). On day 3 post-transfection, anti-CD19-BB-z mRNA-transfected cells could still lyse 80% to 90% of the target OP-1 cells in a 4-hour assay at E: T ratio of 2: 1 and 4: 1, respectively (Fig 4b).
Fig 3. Specific killing of OP-1 cells by expanded NK cells.
a. Cell killing analyzed by Calcein-AM method. b. Cell killing analyzed by CD19-PE antibody staining method.
Fig 4. Duration of specific OP-1 killing by expanded NK cells transfected with anti-CD19-BB-z mRNA.
a-1. Four hour killing assay was performed by calcein-AM method at 3h post transfection. NK cells were transfected with 0, 50, 100 and 200μg/ml anti-CD19-BB-z mRNA. a-2. 1d killing assay was performed by calcein-AM method at 3h post transfection. NK cells were transfected with 0, 50, 100 and 200 μg/ml anti-CD19-BB-z mRNA. b-1. Four hour killing assay was performed by calcein-AM method at 1d, 2d and 3d post transfection. NK cells were transfected with 100 μg/ml anti-CD19-BB-z mRNA. b-2. 1d killing assay was performed by calcein-AM method at 1d, 2d and 3d post transfection. NK cells were transfected with 100 μg/ml anti-CD19-BB-z mRNA. Results are representative of three independent experiments.
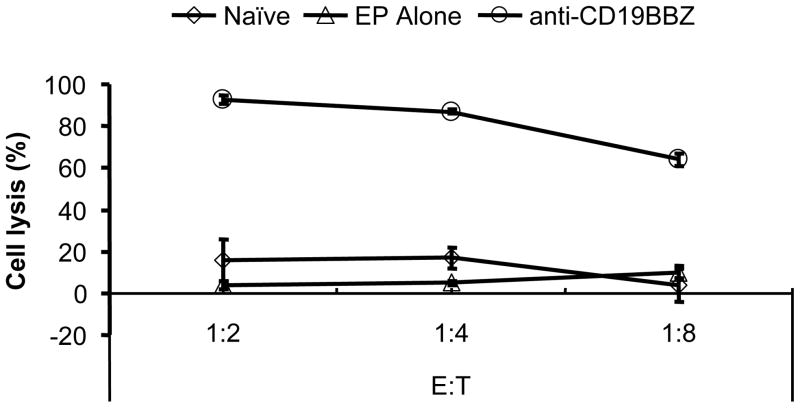
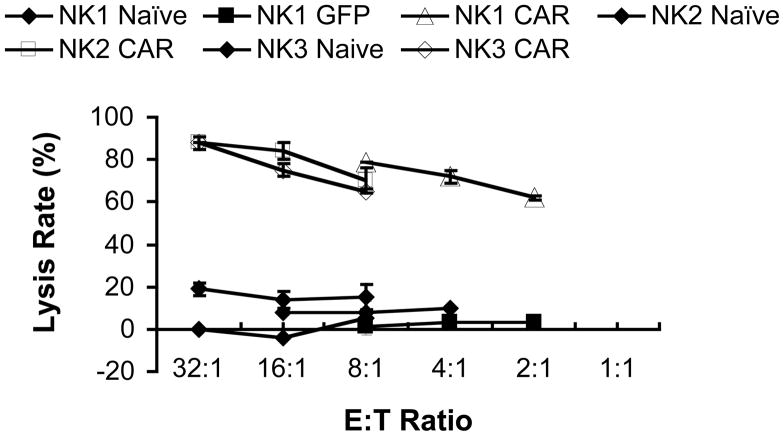
When purified unstimulated NK cells were transfected with anti-CD19-BB-z mRNA, they also became highly cytotoxic to ALL cells, although to a less extent than expanded activated NK cells (Fig. 5). About 80% of the target cells were lysed in the 4-hour assay at an E: T ratio of 8:1. The naive (no EP) and GFP mRNA-transfected unstimulated NK cells did not lyse target cells. Purified unstimulated NK cells from both donors demonstrated similar killing ability.
Fig 5. Specific OP-1 cell killing by unstimulated NK cells.
Transfected-NK cells (n=2) were tested for OP-1 cell killing 1d post transfection.
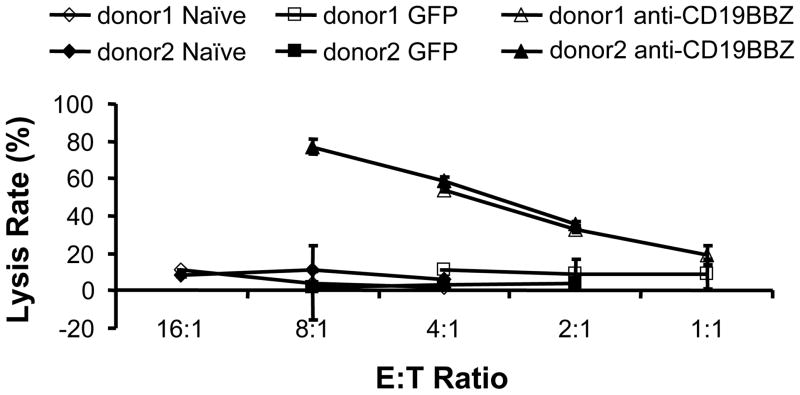
We next assessed whether anti-CD19-BB-z-transfected NK cells, either expanded or unstimulated, could kill primary B-lineage leukemia cells. Fig 6 shows the killing of CLL cells from two donors using either expanded NK cells from one donor (Fig 6a) or purified unstimulated NK cells from three donors (Fig 6b). The killing efficiency for expanded NK cells was greater than that for purified unstimulated NK cells. At E:T ratio of 2:1 and 1:1, anti-CD19-BB-z-transfected expanded NK cells could lyse about 80%–90% of target CLL cells; whereas, purified unstimulated NK cells could reach this killing level only at an 8:1 E:T ratio.
Fig 6. Specific allogeneic B-CLL killing by anti-CD19-BB-z-expressed NK cells.
a. Killing of B-CLL cells (2 donors) by expanded NK cells from one donor but by two independent expansions was depicted. NK cells were either untransfected (naive) or transfected with anti-CD19-BB-z. b. Killing of B-CLL cells (2 donors) by unstimulated NK cells (3 donors) that were either untransfected (naive), transfected with eGFP mRNA (GFP) or transfected with anti-CD19-BB-z. CAR represents anti-CD19-BB-z chimeric antigen receptor. The killings of B-CLL cells by anti-CD19BBz-transfected cells are all significantly higher than that by control or GFP-transfected cells (p<0.05).
Discussion
In this study, we developed a scalable method for efficient mRNA transfection in human NK cells. When an anti-CD19 chimeric antigen receptor was expressed using this method, NK cells acquired powerful and specific cytotoxicity against CD19+ leukemic cells. These results illustrate one of the potential applications of the technology, i.e. redirecting the specificity of NK cells towards specific tumor cell types and augmenting their specific cytotoxicity.
Even though plasmid DNA electroporation has been most frequently used for electroporation, DNA uptake mediated by electroporation results in toxicity to many cells, including unstimulated and expanded hematopoietic cells33,34, which hinders its application in primary cells35–42. A great deal of attention has been dedicated to develop a method that could not only achieve high transfection efficiency but also preserve cell viability. The utility of mRNA transfection has recently been recognized in the development of cell-based therapeutic agents43 since it helps maintaining a high cell viability. The successful mRNA transfection of NK cells in the current report added to the mRNA transfection of dendritic cells41,44, primary resting leukemia cells45, CD34 hematopoeitic stem cells46 and primary T and B cells47 and demonstrated the power of this nonviral method41,42,45,47,48. Unlike DNA, mRNA is unable to integrate into the host cell genome. Retroviral vectors can mediate effective transduction of chimeric antigen receptor in NK and T cells18,23–25. Although effective, this strategy is laborious. With the method described here, high levels of receptor expression can be achieved within hours in a single-step procedure. While mRNA transfection avoids integration into the host cell genome, this advantage is balanced by the possible limitation associated with transient expression of non-integrated genetic material. Whether transient expression and short-term (up to one week) target cell killing are sufficient to have significant impact on in vivo tumor cell killing and tumor burden is an important consideration.
The simplicity, safety, cost effectiveness and efficiency of the method described here to genetically modify NK cells is very well suited for clinical applications. An immediate application that we envisage is the redirection of NK cells from donors towards molecules expressed in the tumor cells of the recipient. Thus, purified NK cells from apheresis products would be transfected with mRNA encoding a chimeric antigen receptor specific for the target molecule (e.g., CD19 in case of B-cell malignancies). The modified NK cells would then be ready for infusion after a few hours. If larger numbers of NK cells are required and/or if multiple infusions are planned, expanded NK cells could be used instead.
Acknowledgments
Special thanks are due to Nick Chopas for his help in equipment preparation for the study. The authors want to express their gratitude to Dr. Gary Moroff and Ms. Amy Neuschaferand at AMERICAN RED CROSS HOLLAND LAB (15601 Crabbs Branch Way, ROCKVILLE, MD) for training and usage of a Gammacell 1000 irradiator.
Footnotes
No interest conflict with MaxCyte: Hiroyuki Fujisaki and Dario Campana.
References
- 1.Schroers R, Hildebrandt Y, Hasenkamp J, et al. Gene transfer into human T lymphocytes and natural killer cells by Ad5/F35 chimeric adenoviral vectors. Exp Hematol. 2004;32:536–546. doi: 10.1016/j.exphem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri L, Mancusi A, Burchielli E, Aversa F, Martelli MF, Velardi A. Natural killer cell alloreactivity in allogeneic hematopoietic transplantation. Curr Opin Oncol. 2007;19:142–147. doi: 10.1097/CCO.0b013e3280148a1a. [DOI] [PubMed] [Google Scholar]
- 4.McKenna DH, Jr, Sumstad D, Bostrom N, et al. Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007;47:520–528. doi: 10.1111/j.1537-2995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 5.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 6.Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002;100:1935–1947. doi: 10.1182/blood-2002-02-0350. [DOI] [PubMed] [Google Scholar]
- 7.Chiorean EG, Miller JS. The biology of natural killer cells and implications for therapy of human disease. J Hematother Stem Cell Res. 2001;10:451–463. doi: 10.1089/15258160152509073. [DOI] [PubMed] [Google Scholar]
- 8.Lanier LL. Activating and inhibitory NK cell receptors. Adv Exp Med Biol. 1998;452:13–18. doi: 10.1007/978-1-4615-5355-7_2. [DOI] [PubMed] [Google Scholar]
- 9.Passweg JR, Tichelli A, Meyer-Monard S, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–1838. doi: 10.1038/sj.leu.2403524. [DOI] [PubMed] [Google Scholar]
- 10.Schellekens J, Rozemuller EH, Petersen EJ, van den Tweel JG, Verdonck LF, Tilanus MG. Activating KIRs exert a crucial role on relapse and overall survival after HLA-identical sibling transplantation. Mol Immunol. 2008;45:2255–2261. doi: 10.1016/j.molimm.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Klingemann HG. Natural killer cell-based immunotherapeutic strategies. Cytotherapy. 2005;7:16–22. doi: 10.1080/14653240510018000. [DOI] [PubMed] [Google Scholar]
- 12.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 13.Verdonck LF, Petersen EJ, Lokhorst HM, et al. Donor leukocyte infusions for recurrent hematologic malignancies after allogeneic bone marrow transplantation: impact of infused and residual donor T cells. Bone Marrow Transplant. 1998;22:1057–1063. doi: 10.1038/sj.bmt.1701496. [DOI] [PubMed] [Google Scholar]
- 14.Collins RH, Jr, Goldstein S, Giralt S, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. 2000;26:511–516. doi: 10.1038/sj.bmt.1702555. [DOI] [PubMed] [Google Scholar]
- 15.Gratwohl A, Brand R, Apperley J, et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2006;91:513–521. [PubMed] [Google Scholar]
- 16.Abbott BL. Recent advances in chronic lymphocytic leukemia. Cancer Invest. 2006;24:302–309. doi: 10.1080/07357900600620525. [DOI] [PubMed] [Google Scholar]
- 17.Eshhar Z. The T-body approach: redirecting T cells with antibody specificity. Handb Exp Pharmacol. 2008:329–342. doi: 10.1007/978-3-540-73259-4_14. [DOI] [PubMed] [Google Scholar]
- 18.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 20.Biagi E, Marin V, Giordano Attianese GM, Dander E, D’Amico G, Biondi A. Chimeric T-cell receptors: new challenges for targeted immunotherapy in hematologic malignancies. Haematologica. 2007;92:381–388. doi: 10.3324/haematol.10873. [DOI] [PubMed] [Google Scholar]
- 21.Cooper LJ, Ausubel L, Gutierrez M, et al. Manufacturing of gene-modified cytotoxic T lymphocytes for autologous cellular therapy for lymphoma. Cytotherapy. 2006;8:105–117. doi: 10.1080/14653240600620176. [DOI] [PubMed] [Google Scholar]
- 22.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172:104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 23.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 24.Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 25.Brentjens RJ. Novel approaches to immunotherapy for B-cell malignancies. Curr Hematol Rep. 2005;4:64–72. [PubMed] [Google Scholar]
- 26.Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–1828. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann U. Electrical breakdown, electropermeabilization and electrofusion. Rev Physiol Biochem Pharmacol. 1986;105:176–256. [PubMed] [Google Scholar]
- 28.Golzio M, Rols MP, Teissie J. In vitro and in vivo electric field-mediated permeabilization, gene transfer, and expression. Methods. 2004;33:126–135. doi: 10.1016/j.ymeth.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Hui S, Li L. In vitro and ex vivo delivery of genes to cells by electroporation. In: Jaroszeski MJ, Gilbert R, Heller R, editors. Electrically mediated delivery of molecules to cells: electrochemotherapy, electrogene therapy and transdermal delivery by electroporation. Humana Press; Totowa, NJ: 2000. pp. 157–172. [Google Scholar]
- 30.Xie TD, Sun L, Tsong TY. Study of mechanisms of electric field-induced DNA transfection. I. DNA entry by surface binding and diffusion through membrane pores. Biophys J. 1990;58:13–19. doi: 10.1016/S0006-3495(90)82349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li LH, Shivakumar R, Feller S, et al. Highly efficient, large volume flow electroporation. Technol Cancer Res Treat. 2002;1:341–350. doi: 10.1177/153303460200100504. [DOI] [PubMed] [Google Scholar]
- 32.Manabe A, Coustan-Smith E, Kumagai M, et al. Interleukin-4 induces programmed cell death (apoptosis) in cases of high-risk acute lymphoblastic leukemia. Blood. 1994;83:1731–1737. [PubMed] [Google Scholar]
- 33.Li LH, Sen A, Murphy SP, Jahreis GP, Fuji H, Hui SW. Apoptosis induced by DNA uptake limits transfection efficiency. Exp Cell Res. 1999;253:541–550. doi: 10.1006/excr.1999.4666. [DOI] [PubMed] [Google Scholar]
- 34.Li LH, McCarthy P, Hui SW. High-efficiency electrotransfection of human primary hematopoietic stem cells. Faseb J. 2001;15:586–588. doi: 10.1096/fj.00-0447fje. [DOI] [PubMed] [Google Scholar]
- 35.Bell MP, Huntoon CJ, Graham D, McKean DJ. The analysis of costimulatory receptor signaling cascades in normal T lymphocytes using in vitro gene transfer and reporter gene analysis. Nat Med. 2001;7:1155–1158. doi: 10.1038/nm1001-1155. [DOI] [PubMed] [Google Scholar]
- 36.Van Tendeloo VF, Willems R, Ponsaerts P, et al. High-level transgene expression in primary human T lymphocytes and adult bone marrow CD34+ cells via electroporation-mediated gene delivery. Gene Ther. 2000;7:1431–1437. doi: 10.1038/sj.gt.3301252. [DOI] [PubMed] [Google Scholar]
- 37.Lai W, Chang CH, Farber DL. Gene transfection and expression in resting and activated murine CD4 T cell subsets. J Immunol Methods. 2003;282:93–102. doi: 10.1016/j.jim.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Trompeter HI, Weinhold S, Thiel C, Wernet P, Uhrberg M. Rapid and highly efficient gene transfer into natural killer cells by nucleofection. J Immunol Methods. 2003;274:245–256. doi: 10.1016/s0022-1759(02)00431-3. [DOI] [PubMed] [Google Scholar]
- 39.Maasho K, Marusina A, Reynolds NM, Coligan JE, Borrego F. Efficient gene transfer into the human natural killer cell line, NKL, using the Amaxa nucleofection system. J Immunol Methods. 2004;284:133–140. doi: 10.1016/j.jim.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Li LH, Biagi E, Allen C, et al. Rapid and efficient nonviral gene delivery of CD154 to primary chronic lymphocytic leukemia cells. Cancer Gene Ther. 2006;13:215–224. doi: 10.1038/sj.cgt.7700883. [DOI] [PubMed] [Google Scholar]
- 41.Landi A, Babiuk LA, van Drunen Littel-van den Hurk S. High transfection efficiency, gene expression, and viability of monocyte-derived human dendritic cells after nonviral gene transfer. J Leukoc Biol. 2007 doi: 10.1189/jlb.0906561. [DOI] [PubMed] [Google Scholar]
- 42.Van De Parre TJ, Martinet W, Schrijvers DM, Herman AG, De Meyer GR. mRNA but not plasmid DNA is efficiently transfected in murine J774A.1 macrophages. Biochem Biophys Res Commun. 2005;327:356–360. doi: 10.1016/j.bbrc.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 43.Sullenger BA, Gilboa E. Emerging clinical applications of RNA. Nature. 2002;418:252–258. doi: 10.1038/418252a. [DOI] [PubMed] [Google Scholar]
- 44.Holtkamp S, Kreiter S, Selmi A, et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108:4009–4017. doi: 10.1182/blood-2006-04-015024. [DOI] [PubMed] [Google Scholar]
- 45.Li Linhong CA, Shivakumar Rama, Jonathan M. Weiss, Joseph C. Fratantoni, Linda N. Liu. Efficient Non-Viral Gene Delivery to Primary, Leukemia B Cells by mRNA Transfection. Molecular Therapy. 2004;9:220. [Google Scholar]
- 46.Li LH, Shivakumar R, Allen C, et al. Engineering stem cell products for enhanced potency and biological function. Annual Conference of International Society of Cell Therapy; 2006; 2006. [Google Scholar]
- 47.Zhao Y, Zheng Z, Cohen CJ, et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13:151–159. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabinovich PM, Komarovskaya ME, Ye ZJ, et al. Synthetic messenger RNA as a tool for gene therapy. Hum Gene Ther. 2006;17:1027–1035. doi: 10.1089/hum.2006.17.1027. [DOI] [PubMed] [Google Scholar]