Abstract
Prior work in the CCN field, including our own, suggested to us that there might be co-regulatory activity and function as part of the actions of this family of cysteine rich cytokines. CCN2 is now regarded as a major pro-fibrotic molecule acting both down-stream and independent of TGF-β1, and appears causal in the disease afflicting multiple organs. Since diabetic renal fibrosis is a common complication of diabetes, and a major cause of end stage renal disease (ESRD), we examined the possibility that CCN3 (NOV), might act as an endogenous negative regulator of CCN2 with the capacity to limit the overproduction of extracellular matrix (ECM), and thus prevent, or ameliorate fibrosis. We demonstrate, using an in vitro model of diabetic renal fibrosis, that both exogenous treatment with CCN3 and transfection with the over-expression of the CCN3 gene in mesangial cells markedly down-regulates CCN2 activity and blocks ECM over-accumulation stimulated by TGF-β1. Conversely, TGF-β1 treatment reduces endogenous CCN3 expression and increases CCN2 activity and matrix accumulation, indicating an important, novel yin/yang effect. Using the db/db mouse model of diabetic nephropathy, we confirm the expression of CCN3 in the kidney, with temporal localization that supports these in vitro findings. In summary, the results corroborate our hypothesis that one function of CCN3 is to regulate CCN2 activity and at the concentrations and conditions used down-regulates the effects of TGF-β1, acting to limit ECM turnover and fibrosis in vivo. The findings suggest opportunities for novel endogenous-based therapy either by the administration, or the upregulation of CCN3.
Keywords: Fibrosis, CCN regulation, Diabetic nephropathy, Anti-fibrotic therapy
Introduction
Glomerulosclerosis and interstitial fibrosis are common endpoints of chronic injury in the kidney, and are fundamental defects occurring in diabetic renal disease, largely accounting for the recent and dramatic elevation of end stage renal disease (ESRD). Angiotensin II inhibitors are the therapy of choice for this condition and have been shown to slow progression of renal failure in many patients. However, a need for more effective treatments capable of blocking or reversing progression remains. Over almost two decades our laboratory has been focused on identifying the factors responsible for the initiation and progression of renal fibrosis in diabetes. We and others have shown how an environment of hyperglycemia and intraglomerular hypertension interact to drive this fibrosis, both acting to increase the production, activation, and receptor binding of TGF-β (Riser et al. 1999, 2001). These collective findings have led to a search for novel, and common downstream targets. CCN2 is a cytokine/matricellular protein recently identified as playing a critical role in fibrosis, including that in diabetic nephropathy. First named connective tissue growth factor (CTGF), CCN2 is now recognized as a member of the CCN family. All of the six members demonstrate similarities in their multimodular structure, but differences in function (Brigstock et al. 2003; Perbal 2004). Three of the four constitutive modules show partial identity with insulin-like growth factor (IGF) binding proteins, Von Willebrand factor (VWF), and thrombospondin one (TSP1), whereas the C-terminal module contains a cysteine knot structure that appears to be critical to the heterodimerization of several matrix proteins and growth factors (Perbal 2001; Rachfal and Brigstock 2005).
Kidney mesangial cells (MC) are a cell type important in maintaining the normal structure and function of the glomerulus, producing various cytokine and growth factors and regulating the turnover of ECM in the structure. In diabetic nephropathy the MC is thought to be the primary cell type responsible for the accumulation of ECM that characterizes the lesion, particularly in early disease (Riser et al. 2000b). Our laboratory and others have shown that expression of CCN2 and its activity, are upregulated by those very factors known to be responsible for driving fibrosis in renal disease, including a high glucose environment, hypertensive force, and TGF-β (Riser et al. 2000b; Bollineni and Reddi 1993; Ziyadeh 2004). Although a downstream factor, mechanical strain and other recently identified elements appear able to stimulate CCN2 expression, independent of TGF-β. This supports our supposition that CCN2 will provide a more downstream and essential target for regulation of matrix metabolism in fibrosis (Hahn et al. 2000; Riser et al. 2000b). This is also supported by recent reports in several animal models of renal fibrosis, including diabetic nephropathy. Antisense oligonucleotides (AS-ODN) were used to specifically knock down CCN2 activity and produce a blockade of progressive disease (Guha et al. 2007; Okada et al. 2005; Yokoi et al. 2004).
We hypothesized existence of an endogenous regulatory molecule(s) that might be active in shutting down the augmented ECM turnover, for example in the process of normal wound healing, that therefore could be utilized to down-regulate this process in conditions of chronic insult and fibrosis. We suspected that one possibility for negative regulation of CCN2 might be CCN3 (formerly known as nephroblastoma overexpressed gene [NOV]). We had noted that some cell types tested that expressed high levels of CCN2 tended to express low levels of CCN3 (Li, CL, Perbal, B and B. Riser unpublished observations). Thus, we conducted studies to test our hypothesis both in vitro and in vivo.
Methods
Reagents
TGF-β1 was from R&D Systems (Minneapolis, MN). Purified rat collagen type I was from Upstate Biotechnology (Lake Placid, NY) and polyclonal anti-rat collagen type I from Chemicon International (Temecula, CA). The production of full-length recombinant human CCN2 protein in a baculovirus expression system has been described previously (Riser et al. 2000b). Recombinant human and mouse CCN3 (rhCCN3 and rmCCN3) were from R&D Systems and where generated from a DNA sequence encoding a mature CCN3 protein expressed in a mouse myeloma cell line. Anti-CCN3 antibodies included a rabbit polyclonal produced by us (Kyurkchiev et al. 2004) and monoclonals from R&D Systems. RPMI from Invitrogen (Grand Island, NY) and fetal bovine serum (FBS) from Gemini (Woodland, CA) were used for the growth medium.
Cell culture
The MC used were from a cloned line (16KC2) derived from Fischer rat glomeruli as previously described (Riser et al. 1998). MC were grown long term in RPMI 1,640 medium (RPMI) containing antibiotics and 5 mM glucose, plus 10% FBS. For many experiments, cells were seeded in normal growth medium in 24-well tissue culture plates, and grown for three days. On the fourth day, cells were washed with RPMI containing 1% FBS (RPMI-1), then incubated in fresh RPMI-1. Twenty-four hours later, the cells were washed and exposed to RPMI-1 with added cytokines, as indicated in the figures. After 48 h, all cells were again washed and incubated in RPMI-1 plus cytokines for one or two additional days before harvest. In all cases, heparin (50 μg/ml) was added to all wells prior to harvest to induce the release of cell- or matrix-bound CCN2, as previously described (Riser et al. 2000b). For determination of cellular proliferation and standardization of ELISA data, results were expressed per cell, based on the amount of DNA determined using the CyQuant Cell Proliferation Assay Kit, catalog # C-7026, from Molecular Probes (Eugene, Oregon). By running samples against a standard curve generated using increasing numbers of MC, the cell number in each test well could be determined.
Generation of a stable cell line over-expressing human CCN3
To generate a stable cell line expressing hrCCN3, MC were seeded into culture plates, and grown for 24 h. The transfecting mixture containing 20 μg CCN3 expression vector bCB6+ (Chevalier et al. 1998) was incubated with 15 μl of transfection reagent, Lipofectamin 2000 (Invitrogen, Carlsbad, CA), in 1 ml serum free RPMI for 30 min at room temperature. This preparation was then added to cells with fresh medium and incubated for 4 days. On the fourth day the medium was replaced, and after two additional days cells were dispersed and re-plated with 1 mg/ml G418 antibiotic, to select for stable antibiotic resistant colonies. These G418-resistant cell lines were then analyzed by RT-PCR using human specific CCN3 primers to assess expression of hCCN3.
Transfection of the col1 promoter construct and promoter analysis by luciferase measurement
Cells were seeded on 24-well plates and transfected 18 h later with col1a2 constructs kindly provided by Dr. William Schnaper (Poncelet et al. 1999). Renilla-luciferase pRL-SV40 was used as a control to normalize for transfection efficiency. Transfection was performed with the Invitrogen reagent Lipofectamin 2000. Briefly, 0.8 μg of collagen promoter constructs or 0.01 μg pRL-SV40 control constructs were mixed with 1 μl Lipofectamin 2000 in 100 μl serum free medium. The mixtures were incubated for 30 min at room temperature and added to the cells with 1 ml of fresh medium. After 18 h the medium was replaced with one containing 2% FBS, and cells were incubated for additional 18 h. Either TGF-β1 (2 ng/ml) or control vehicle was added to the cells. In some experiments, the transfected cells were pretreated for 4 h with 0–300 ng/ml CCN3 before adding TGF-β. Then, 24 h later, the cells were washed with PBS, and extracts were prepared using 150 µl of reporter lysis buffer (Promega Inc, Madison, WI). Luciferase activities of the promoter construct and the internal control were measured by adding 15 μl of extract with 50 μl luciferase substrate and 50 μl stop-and-go reagent. The luciferase activity determined in the assay was normalized utilizing the transfection efficiency. The experiments were performed with triplicate samples.
RNA extraction, reverse transcription and PCR
RNA extraction was carried out using Trizol reagent under methods provided by Invitrogen (Chomczynski and Sacchi 1987). Synthesis of cDNA was carried out using random hexamers and Moloney murine leukemia virus reverse transcriptase at 42°C for 1 h starting with 5 µg of total RNA. Two µl of cDNA was then used for PCR. The sequences of primers used in PCR amplification are shown in Table 1. PCR analyses were done using an Applied Biosystem thermocycler (Applied Biosystems, Foster, CA). Electrophoresis of the amplification products was in 1 and 2% agarose gels. Bands were visualized by ethidium bromide staining, and intensities determined by densitometer scanning with subsequent analysis using the NIH Image program.
Table 1.
Sequences of primers used in PCR amplification
| Gene | Strand | PCR primer sequence (5′–3′) |
|---|---|---|
| hCCN3 | Sense | ATGCAGAGTGTGCAGAGCAC |
| Anti-sense | TTACATTTTCCCTCTGGTAGTCTTCA | |
| rCcn3 | Sense | TCTGTGGGATCTGCAGTGAC |
| Anti-sense | ATTGTTCTGAGGGCAGTTGG | |
| mCcn3 | Sense | GCACCAAGAAATCCCTGAAA |
| Anti-sense | GAGGGCAGTTGGAGTAGCAG | |
| rCcn2 | Sense | AGTCTCTTCTGCGACTTCGG |
| Anti-sense | GCAACTGCTTTGGAAGGACT | |
| mCcn2 | Sense | AGCAGCTGGGAGAACTGTGT |
| Anti-sense | TGGTATTTGCAGCTGCTTTG | |
| rCol1 | Sense | TGCTGCCTTTTCTGTTCCTT |
| Anti-sense | AAGGTGCTGGGTAGGGAAGT |
ELISA
An ELISA was used to quantify levels of cytokines and collagen. An indirect ELISA was used for CCN2 protein measurements for the conditioned media, as we have previously described (Riser et al. 2000b). For CCN3, a direct ELISA was used for tissue-culture samples from rat cells, and an indirect ELISA was used for rat cells transfected to express hCCN3. In brief, for the direct ELISA, the samples and recombinant standards (diluted in the same medium as the samples) were incubated at room temperature to allow binding to the 96-well plate. The unbound sites were then blocked with 1% BSA + 0.05% Tween 20. After washing, a primary antibody was added (MAB1640 from R&D Systems, Inc., Minneapolis, MN) or K19-immunized rabbit serum (Kyurkchiev et al. 2004). For the indirect ELISA, the plate was first coated with MAB1640, then blocked, then incubated with sample and standard before giving K19-immunized rabbit serum. After further washing, horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA, anti-rabbit 111-035-003, or anti-mouse 115-035-003) was added, followed by more washing and HRP-substrate (Enhanced K-βlue TMB Substrate, 308175, Neogen Corp., Lexington, KY). The color intensity was allowed to develop before being read at 650 nm using a microplate reader (Thermo Max, Molecular Devices Corp., Sunnydale, CA).
The measurement of COL1 was done by direct ELISA, as previously described (Cooker et al. 2007) and similar to the method described here for CCN3, except that incubations were carried out at 4°C to minimize aggregation of the molecule, and the samples and standards were incubated in the plate overnight. The blocking solution and secondary antibody dilution buffer contained 5% nonfat dry milk + 0.05% Tween 20. The BSA blocking solution above was used to dilute the primary antibody (AB755P, Chemicon). The substrate color was developed at room temperature, and read as above.
Western blotting
Biological samples were prepared by mixing one volume of a sample with one volume of the loading buffer with 10% 2-mercaptoethanol and 2% SDS. Twenty μl of each prepared sample were then subjected to SDS–PAGE on a 4–15% Tris-HCL gradient gel (Bio-Rad) and transferred to a PVDF membrane (Millipore, Bedford, MA). The membrane was blocked with 5% nonfat dried milk in TBS + 0.1% Tween 20 for 1 h at room temperature and then incubated with a specific polyclonal anti-CCN3 antibody (K19 at 1:1,000 dilution) produced by us, against a 19 amino acid sequence in C-terminal module. To measure phosphho-Smad3 activity, a specific rabbit monoclonal antibody (Cell Signaling, Danvers, MA) was used. A horseradish peroxidase-conjugated secondary antibody (1:15,000 dilution, Amersham, Piscataway, NJ) and horseradish substrates (Pierce, Rockford, IL) were used to label the bands, which were enhanced with the chemiluminescence system (Pierce) and were developed using Amersham X-ray film.
Immunohistochemistry
MC grown on chamber slides were fixed in methanol. Immunoperoxidase labeling was performed at ambient temperature in a humidified chamber. Endogenous peroxidase was first blocked in 0.3% hydrogen peroxide/methanol. Cells were then rinsed in TBS, and non-specific binding was blocked in 1% BSA/TBS. Primary antibody, anti-CCN3 (K19, 1:250 dilution in 1% BSA/TBS) was applied, then cells were washed 5× in TBS/0.025% Brij35. Secondary antibody conjugate (1:10 in BSA/TBS; HRP Polymer Conjugate, Broad Spectrum; Zymed Laboratories, San Francisco, CA) was applied, then washed in TBS/Brij and one in TBS only. Stable DAB (Invitrogen, Burlington, ON) was applied while monitoring color development. Cells were counterstained in hematoxylin and dehydrated before mounting.
Animal experiments
All animal studies were approved by the local institutional review board. Male diabetic db/db mice and their nondiabetic db/m littermates were from Jackson Laboratories (Bar Harbor, ME).
Statistics
The procedure MIXED (SAS software) was used to perform the analyses. Differences of least squares means estimates were performed for the pair-wise comparisons of groups. P-values less than 0.05 were considered statistically significant. For analyses in which there were only two groups, a Student’s t test was performed, and P-values less than 0.05 were considered statistically significant.
Results
Effect of CCN3 treatment on TGF-β1 stimulated CCN2 and COL1 production
We used our previously reported in vitro model of diabetic nephropathy (Riser et al. 2000a) in which cultured MC are incubated in a 2% fetal bovine serum (FBS)-containing medium causing low replication, after which the cells are exposed for 1–4 days to a medium with TGF-β1 (2 ng/ml), or a control medium only. This treatment replicates the in situ diabetic condition characterized by elevated TGF-β1 due to hyperglycemia and hypertensive forces. As occurs in vivo, cultured MC under these conditions produce an increase in CCN2 mRNA and protein, followed by an increased production of type I collagen (COL1) mRNA and protein (Riser and Cortes 2001; Riser et al. 2000a). In this model, TGF-β-stimulated collagen synthesis is blocked by CCN2 antisense oligonucleotides (ODN) (Abdel-Wahab et al. 2002; Riser et al. 2006), as has been shown in vivo (Guha et al. 2007; Okada et al. 2005; Yokoi et al. 2004).
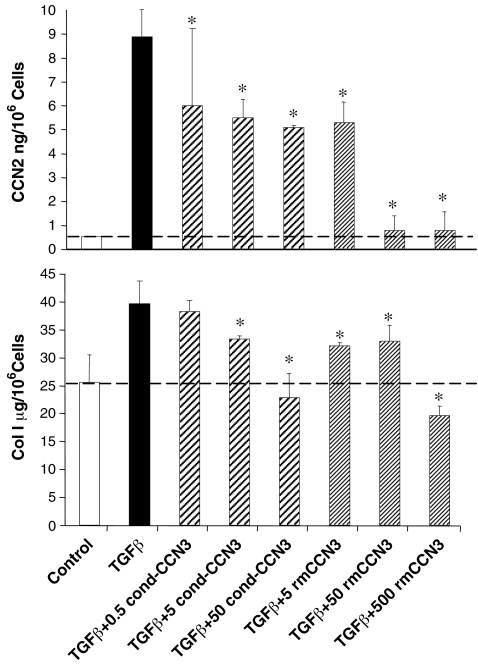
In the present experiments MC were exposed for 1 h to a conditioned medium (NCI-H295R human cell line) containing a high levels of CCN3 (0.5–50 ng/ml) (Thomopoulos et al. 2001), immediately prior to TGF-β1 treatment (2 ng/ml) for 96 h. Alternatively, in the same manner, purified recombinant mouse CCN3 (5–500 ng/ml), rmCCN3) was used. Our results showed that there was a low CCN2 baseline level, but this level was greatly increased by treatment with TGF-β1, as expected (Fig. 1). Exogenously added CCN3, at increasing concentrations, produced a clear dose-dependent reduction of CCN2 levels that began at the lowest concentration tested. The purified rmCCN3 was able to induce a total blockade of CCN2. Baseline secretion of COL1 was also increased in response to TGF-β1 exposure as expected (Fig. 1), and this stimulation was totally abrogated by pre-treatment with either the rmCCN3 or the conditioned media with CCN3. Again this occurred in a dose-dependent manner. There was a slight, but statistically significant, reduction of COL1 production, below the constitutive (non- TGF-β simulated) level in response to the highest concentrations of CCN3.
Fig. 1.
CCN3 reduces TGFβ1-stimulated CCN2 and COL1 production in cultured rat renal mesangial cells (MC). CCN2 secretion is significantly increased by addition of TGFβ1 (2 ng/ml) alone to the culture medium for 96 h, and this stimulated production is reduced in a dose-dependent fashion by adding either a conditioned medium (0.5–50 ng/ml, cond-CCN3) from NCI-H295R cells enriched in CCN3, or direct addition of recombinant mouse CCN3 (5–500 ng/ml, rmCCN3) for 1 h prior to treatment with TGFβ1. A similar pattern is observed with TGFβ1-stimulated COL1 production in MC. The error bars represent the mean ± the standard deviation for three measurements. * indicates a significant difference (P < 0.05) from TGF-β1 stimulated conditions due to CCN3 treatment
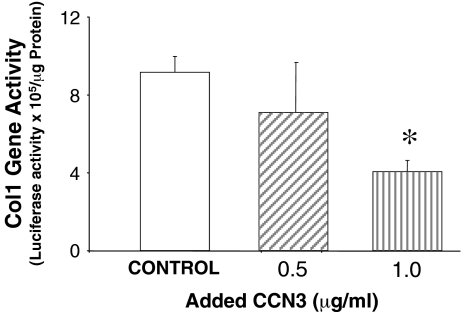
Next, we examined the effect of CCN3 on collagen gene regulation. The human col1 promoter linked to luciferase was expressed in this MC line using transient transfection. The cells were then treated to 0.5 or 1.0 μg/ml of rmCCN3 at 24 h after transfection, and luciferase activity was measured (Fig. 2). Collagen gene activation was reduced by CCN3 in a dose-dependent manner.
Fig. 2.
CCN3 inhibits COL1 promoter activity in cultured MC. Human col1 promoter was linked to luciferase in MC, and the COL1 promoter activity was measured as luciferase activation. Renilla-luciferase pRL-SV40 was used as a control to normalize for transfection efficiency (data not shown). CCN3 (0.5 or 1.0 μg/ml) was administered 24 h after transfection of the promoter construct, and significantly inhibited COL1 promoter activity in a dose dependent manner. The error bars represent the mean ± the standard deviation for three separate transfections. * indicates a significant difference of P = 0.001
CCN3 expression in MC and its regulation by TGF-β1
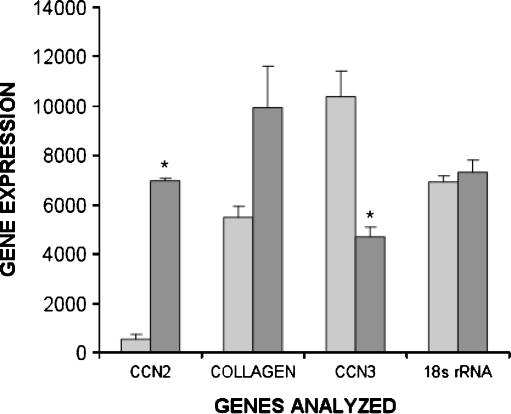
Since CCN3 had effects on MC that were opposing those of CCN2, and the latter is strongly stimulated by TGF-β1, we examined whether TGF-β1 might act to repress CCN3 activity. Indeed, PCR analysis demonstrated CCN3 mRNA expression in our unstimulated MC in culture (Fig. 3). Whereas, exposure to TGF-β1 (2 ng/ml) resulted in a highly significant increase in CCN2 mRNA expression (P = 0.00001) and an apparent elevation of COL1 mRNA (P = 0.06) as expected, there was an opposite down-regulatory effect on CCN3 expression in response to TGF-β1 (P = 0.01), as the baseline, untreated CCN3 expression level was greatly reduced (Fig. 3). The low CCN2 and COL1 mRNA levels, with high CCN3 transcript amounts in non-TGF-β1 stimulated cultures were reflected in a marked level of CCN3 protein secretion, as determined by ELISA (control, 1,011 ± 59 ng/106 cells, mean ± SEM). Likewise, the exposure to TGF-β1 that had increased CCN2 and COL1 mRNA, and down-regulated CCN3 mRNA, also significantly (P < 0.05) reduced the level of secreted CCN3 protein (TGF-β1 treated, 529 ± 45 ng/106 cells, mean ± SEM).
Fig. 3.
TGFβ1 exposure induces opposite effects on mRNA levels for CCN2 and COL1 versus CCN3 in cultured MC. TGFβ1 (2 ng/ml) significantly increased expression of CCN2 (* is P = 0.00001) and COL1 (P = 0.06) when added to the culture medium, but reduced CCN3 expression (* is P = 0.01) as determined by semiquantitative RT-PCR measured in the linear range. This semi-quantitative data were obtained by scanning PCR DNA fragments using densitometry (arbitrary units). The values were normalized utilizing 18s rRNA as an internal control. TGFβ1 did not affect expression of 18s RNA. The error bars represent the mean ± the standard deviation among three replicates. The experiement was repeated with similar results
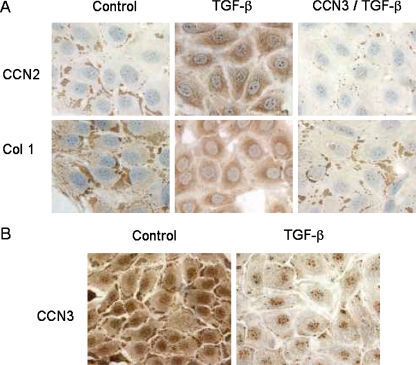
We also performed immunohistochemical staining of MC to gain insight into the cellular localization and distribution of these molecules, and the effect of TGF-β1 on this distribution. Results showed that in untreated MC, both CCN2 and COL1 produced similar staining patterns, that is, they were both primarily localized to discrete, intensely staining areas at, or near, the cell membrane with limited diffuse staining (Fig. 4A). Treatment with TGF-β1 (5 ng/ml) for 48 h resulted in a dramatic change from this focal, peripheral localization to a very diffuse but strong cytoplasmic distribution of both CCN2 and COL1, suggesting new (and quite coordinated) synthesis. A 1 h pre-treatment with CCN3 (500 ng/ml) effectively blocked this transition (Fig. 4A). These studies also demonstrated that, as opposed to CCN2 (and COL1), unstimulated MC exhibited heavy but diffuse staining of the cytoplasm and nucleus for CCN3, with a much smaller fraction localized to the cell membrane (Fig. 4B). Also, quite opposite to that observed for CCN2, cytoplasmic CCN3 staining was largely lost in response to TGF-β1 exposure (Fig. 4B).
Fig. 4.
TGFβ1 causes converse effects on CCN2 and COL1 localization in cultured MC (48 h) compared to CCN3, and pre-treatment with CCN3 for 1 h reverses the localization pattern for CCN2 and COL1 in response to TGFβ1. Using immunocytochemistry, CCN2 and COL1 localize as dense staining in the peripheral regions of the cells under control conditions (a), but this localization is lost when TGFβ1 (5 ng/ml) is added to the incubation medium, and a new pattern of diffuse but intense, staining throughout the cytoplasm is observed (a). In each case, addition of CCN3 (500 ng/ml) to the incubation medium reverses the effects of TGFβ1 (a). Conversely, CCN3 is homogenously distributed throughout the cytoplasm in the absence of TGFβ1 (b), but becomes more diffusely distributed and accumulates as dense bodies at the cell periphery with addition of TGFβ1 (b)
Effect of transfection with over-expression of the human CCN3
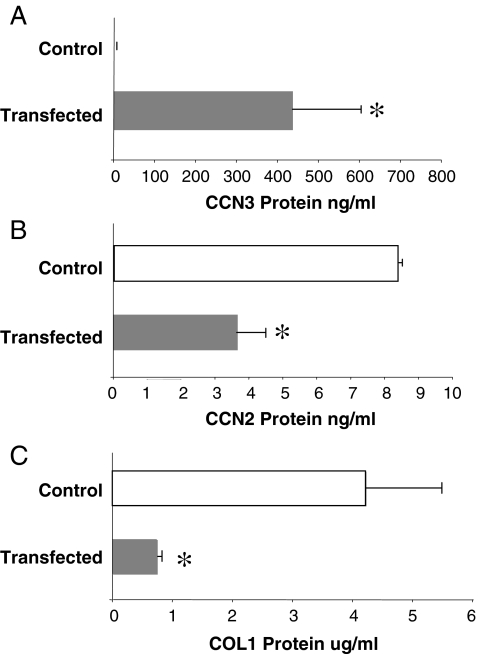
In addition to determining the effect of exogenous treatment with CCN3, we tested the effect of the upregulation of endogenous CCN3 gene expression. We transfected cultured MC with the human CCN3 gene, producing a stable cell line with high expression of hCCN3. This was first confirmed by RT-PCR at the mRNA level (not shown) and at the protein level by quantitative ELISA. As was the case for mRNA, hCCN3 protein secretion was not present in the control, empty vector transfected cells, but a high level of human CCN3 protein was produced in cultures of the CCN3-transfected cells (Fig. 5A). This marked level of hCCN3 induced a greater than 50% reduction in CCN2 (Fig. 5B) secretion and a near total blockade of the COL1 produced (Fig. 5C).
Fig. 5.
Transfection of cultured MC with human recombinant CCN3 (hCCN3) causes a reduction in CCN2 and COL1 production. Panel A confirms that transfection of MC with hrCCN3 produces a significant increase in CCN3 protein, as determined by indirect ELISA (anti-human antibody). Specificity is indicated by a zero control value (a). This increased production of endogenous CCN3 causes a resultant reduction in CCN2 (b) and COL1 (c) protein in the cells. The error bars represent the mean ± the standard deviation for three measurements. * indicates a significant difference at P < 0.05
In these experiments, cell growth was largely arrested prior to and during exposure to cytokines by reducing the FBS concentration to 2%. However in order to verify this, cell numbers were determined by measurement of total DNA at the time of assay and showed no significant effect of any treatment on replication (not shown). This lack of an effect of the cytokines on replication was verified by cell cycle analysis using fluorescent-assisted cell sorting, thus demonstrating that the observed effects of CCN3 on CCN2 and COL1 as well as the effects of TGF-β were not due to an alteration in cell growth.
Smad signaling and CCN3
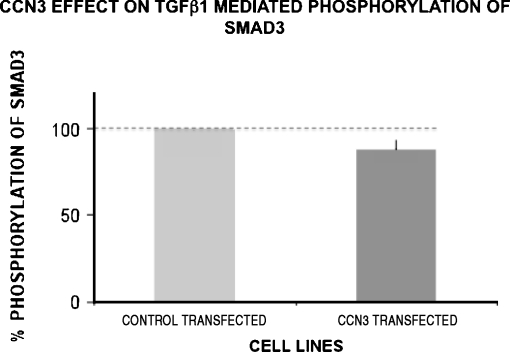
To determine if the observed effects of CCN3 might partially be mediated through an effect on the upstream molecule TGF-β1, we examined phosphorylated Smad3 (pSmad3) signaling under conditions of reduced and elevated CCN3. Control-transfected MC showed low or absent Smad3 activity, as evidenced by no visible band on Western blot analysis, but as expected a strong band resulted from exposure to TGF-β1. When tested using the CCN3 over-expressing cells (Fig. 6), there was a small, but non-statistically significant, effect of endogenous CCN3 on TGF-β1 (3 ng/ml) stimulation of pSmad3 activity. In our previous studies we likewise observed that exogenous treatment of MC with CCN3 (300 ng/ml) produced little, or no, reduction in pSmad2 activity (Riser et al. 2009).
Fig. 6.
Transfection of cultured MC with CCN3 does not significantly reduce TGF-β1 stimulated pSmad3 activity. CCN3 over-expressing cells were stimulated with TGF-β1 (3 ng/ml). The transfected cells had 88.1 ± 5.7 (mean±SD) percent of control pSmad3 activity (P = 0.1) for two separate experiments
CCN3 expression in vivo in diabetic renal fibrosis
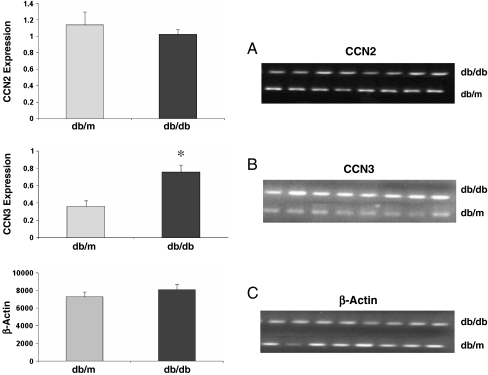
To begin elucidating the possible in vivo relevance of these in vitro findings, we conducted studies using db/db diabetic mice and their healthy counterparts (db/m). We have previously shown that increased renal CCN2 mRNA expression is part of an early response to diabetes and a marker of the glomerulosclerosis that characterizes the diabetic nephropathy lesion in this model (Riser et al. 2000b). In the later stage of diabetic nephropathy using the STZ rat model, and in patients, we also reported that expression as measured by CCN2 protein in urine decreases toward the non-diabetic base line amount (Riser et al. 2003). In the present experiments, db/db mice were sacrificed late in the course of disease (6 months of age, 5 months of diabetes). Kidney cortex was analyzed for specific mRNA expression by RT-PCR. Results confirmed the expression of renal CCN2 mRNA. At this late stage of progression, the expression CCN2 levels were not significantly different in the diabetic mice (db/db) compared to the healthy (db/m) controls, suggesting a late stage return to pre-disease baseline levels, as described below (Fig. 7A). Analysis of the same samples for CCN3 demonstrated the presence of mRNA in the control, non-diabetic animals (Fig. 7B). Interestingly however, in contrast to CCN2, there was a marked increase (approximately 2.1 fold average) in CCN3 expression in all of the diabetic (db/db) animals as compared to the healthy non-diabetic animals.
Fig. 7.
CCN3 expression is increased at a late stage in a model of type two diabetic renal fibrosis, and CCN2 expression is not significantly different from control. Graphs show CCN2 (a) and CCN3 (b) expression in kidney cortex of diabetic (db/db) and control (db/m) mice, using semi-quantitative RT-PCR, after 5 months of disease. Data is expressed as the intensity of RT-PCR bands (arbritrary densitometry units). Values are normalized by using β-actin as a housekeeping gene (c). There is a significant large increase (* is P < 0.01) in CCN3 mRNA, and no significant change in CCN2 mRNA. Error bars represent the mean ± the standard error for eight measurements
Since CCN3 mRNA expression was dramatically increased in the kidney of the late stage diabetic mice, we also examined urine for CCN3 protein. Samples analyzed by Western blotting demonstrated that whereas healthy (db/m) mice had little or no measurable urinary CCN3, all of the diabetic mice with fibrosis showed multiple immunoreactive bands (Fig. 8). The strong band at approximately 55 kDa likely represents the full-length CCN3 protein, whereas the less prominent and lower molecular weight band at approximately 27 kDa corresponds to the truncated half-length CCN3 isoform (Perbal et al. 1999). A larger immunoreactive band also observed may represent CCN3 bound to other urinary proteins or complexed with smaller isoforms of CCN2, and was absent in the healthy animals.
Fig. 8.
CCN3 is excreted in the urine of diabetic mice with late stage fibrosis, but not in non-diabetic control animals. Western blot analyses of the urines from diabetic (db/db,) and control (db/m) mice show multiple immunoreactive forms of CCN3 in the diabetic animals, but virtually none in healthy control mice. This includes a strong band in the diabetic mice at approximately 55 kDa (likely representing full-length CCN3), and a band at approximately 27 kDa (likely representing a half-fragment of CCN3)
Discussion
Fibrosis is often depicted as wound healing gone awry. Wound healing allows for the removal of damaged cells and altered tissues in a carefully regulated manner, and this restructuring is followed by resumed normal function. In a similar fashion, fibrosis also appears to be an attempt at healing in response to a marked insult, but normal completion of the process is prevented due to altered regulation. The process of wound healing frequently occurs in response to tissue damage accompanying inflammation, followed by cell migration, production of regulatory cytokines, and increased formation and turnover of ECM. This generally involves fibroblasts or fibroblast-like cells, which may be derived from mesenchymal cells or myofibroblasts. Upon completion of this process and resumption of normal structure and function, the inflammation disappears along with a down-regulation of the cytokines driving the response. A fundamental difference with fibrosis, which may be largely responsible for driving the over-accumulation of ECM and scar formation is, the presence of a chronic stimulus or injury. In diabetic nephropathy this is thought to be largely due to a combination of renal cell exposure to hyperglycemia, as well as increased intraglomerular hypertension. We recently hypothesized that in addition to the elimination of the injurious insult, there is a molecule that acts to down-regulate ECM metabolism in the late stages of normal wound healing, and its regulation may be altered in fibrosis (Riser et al. 2009). Thus, such a molecule could be utilized therapeutically to shut down, or reverse the accelerated ECM production and turnover that occurs in fibrosis. We had previously observed that CCN2 and CCN3 are often reported to have opposite effects on cellular replication. We therefore speculated that CCN3 might be a regulatory molecule for CCN2 that when repressed would allow the upregulation of CCN2 for wound healing and fibrosis, and when increased would down-regulate CCN2 and serve to terminate the wound healing process or to prevents fibrosis.
In testing the hypothesis, we showed here that cultured MC exposed to exogenous CCN3, or transfected to express high levels of endogenous CCN3, strongly blocks TGF-β1-stimulated CCN2 activity (measured by mRNA, protein secretion, and cell localization). We also documented that CCN3 is able to reduce the activation of the collagen promoter, and produce a near total blockade of TGF-β1 stimulated synthesis, secretion, and cellular redistribution of intracellular COLI. In support of the latter finding, our immunolocalization studies revealed in unstimulated MC that both CCN2 and COL1 were primarily abundant as focal areas of dense deposit at, or near, the cell-cell interface, and limited as diffuse cytoplasmic molecules. However, following TGF-β1 exposure, the membrane-associated focal areas disappear and are replaced by diffuse, dense staining of the entire cytoplasm, likely representing new synthesis of the molecules (supported by our ELISA secretion data). The pre-exposure to CCN3 virtually blocked this redistribution and synthesis of CCN2 and COL1. In contrast to CCN2, in the absence of TGF-β1, MC produced endogenous CCN3 primarily as abundant, diffuse cytoplasmic staining with some apparent nuclear staining. Upon exposure to TGF-β1, this heavy diffuse localization was greatly reduced, and dense deposits at the cell periphery now appeared. This reverse effect of TGF-β1 on CCN3, as compared to CCN2, was supported by both examination of associated mRNA and secreted protein.
In a second set of experiments designed to begin examining the relevance of this in vitro regulatory role of CCN3 in fibrosis, we examined its expression in the db/db mouse model of human diabetic nephropathy. In this model, animals become hyperglycemic by 1 month of age, and by 5 months show dramatic increases in the normally low glomerular CCN2 expression (Riser et al. 2000b). Also by 5 months of age, the db/db mice develop clinical signs of diabetic nephropathy, mainly mesangial lesions characterized by expansion of the mesangial ECM, and albuminuria. This resembles early stage diabetic nephropathy. With extended time, full proteinuria develops, and limited interstitial disease may occur. However, unlike human disease, there is a lack of progression to extensive glomerular sclerosis with collapsing of glomerular capillaries and consistent interstitial fibrosis. Similarly in the streptozotocin (STZ)-induced rodent model of type I diabetes and renal fibrosis, like virtually all animal models of diabetic nephropathy, there is an early progression, but a lack of advancement to renal failure (Breyer et al. 2005). The reason for this limited progression is unclear. We have shown in the STZ rat model that the level of urinary CCN2 is reflective of progression (Riser et al. 2003). The excreted level is dramatically upregulated, from a low baseline, by 2 weeks following the onset of hyperglycemia. Excreted CCN2 peaks around 3 months of disease, and then starts to fall. However, this urinary CCN2 never fully returns to the level of the healthy control animal. We suspected that this unexplained blockade of progression to organ failure in these rodent models, as compared to most humans, might be due to the upregulation of CCN3, which acts to block the CCN2 activity and ECM metabolism late in disease.
Our finding here, in control mice, of constitutive renal CCN3 expression that was greatly increased during late, non-progressing disease supports this idea, and could explain the reduction of CCN2 to pre-disease levels observed at this same period. The upregulation in renal CCN3 mRNA levels could also have resulted in increased expression and action of the protein in the kidney, since our Western blot analysis of urine indicated substantial levels of CCN3 protein in urine of diabetic mice, but little or no detectable CCN3 in the urine of healthy, non-diabetic mice during the same period. The apparent elevation of CCN3-related protein in the urine is therefore consistent with the upregulation of CCN3 mRNA in the kidney of diabetic mice. The finding on Western analysis of full-length CCN3, as well as half-length, and larger immunoreactive isoforms is similar to what we have reported for urinary CCN2 in patients with chronic kidney disease (Riser et al. 2003). The truncated form could result from pH exposure and/or proteolytic cleavage, and based on the CCN3-specific antibody used suggests that this truncated form contains the C-terminal module of CCN3. The inability to detect sharp, discreet protein bands perhaps also suggests such degradation. However, similar truncated forms have been observed in other body fluids and in culture, suggesting differential post-translational modification and perhaps unique biological function, rather than simply representing breakdown products of the full-length form (Brigstock et al. 1997; Perbal et al. 1999; Riser et al. 2003, 2000b). The large molecular weight form (substantially greater than 55 kDA) observed in the db/db mice could represent CCN3 bound to either another isoform of CCN or an unrelated protein.
Collectively the data support our hypothesis that in renal injury, including diabetic nephropathy, TGF-β1 activity is quite quickly increased, and it and other factors including increased mechanical strain drive CCN2 activity upward, while at the same time down-regulating CCN3 (Fig. 9). This cytokine environment then is conducive to increased metabolism and remodeling of COLI and other ECM proteins. A reduced CCN3 level is necessary for a significant CCN2 response and in turn the increased synthesis of ECM. In acute injury as the wound is repaired, the suppression of CCN3 may be removed, and its increase will have the effect of lowering CCN2 and ECM metabolism. Exactly how CCN3 is regulated is not clear at this time, but one may speculate that in chronic injury with fibrosis such as occurs in diabetic nephropathy, either CCN3 is continually maintained at low levels, or perhaps increases late in disease progression (as observed in the db/db mouse), but is not in sufficient elevated amounts to completely halt progression.
Fig. 9.
A working hypothesis for the regulation of CCN2 and COL1 formation in wound healing and fibrosis. In response to stimuli favoring wound healing, TGFβ causes an increase in CCN2 production, which in turn stimulates COL1 formation and synthesis of the extracellular matrix (ECM). CCN3, an endogenous inhibitor of CCN2 production and COL1 formation, is transiently inhibited by TGFβ, thus allowing remodeling of the ECM. During the later stages of wound healing, however, inhibition of CCN3 is reversed by a mechanism not yet defined (?), thereby suppressing COL1 production and terminating the process of wound healing. During fibrosis, the normal up-regulation of CCN3 following the initial phase of COL1 production is prevented, resulting in an uncontrolled production of extracellular matrix leading to fibrotic changes
The mechanism for CCN3 down-regulation of CCN2 and collagen activity was not elucidated in our studies. However, the finding of a quite direct relationship between CCN3-induced reduction of CCN2 and the reduction in COLI, supports the idea that this is at least in part mediated by a direct effect on CCN2 expression and activity. Although in a recent study by van Roeyen and associates CCN3 expression was shown to be inversely associated with glomerular cell proliferation and was negatively regulated by platelet-derived growth factor (PDGF)-BB (van Roeyen et al. 2008), we do not think that this is the case in our study. We used an in vitro model of renal fibrosis that mimics the largely non-proliferating conditions found in chronic kidney disease, including that associated with diabetes (Hostetter 1995). Our cell cycle analysis confirmed that under the low FBS concentrations used, not only was there minimal replication of MC during the period of study, but also none of the treatments induced a significant change in cell cycling (data not shown). We also do not think that the CCN3 effect on CCN2 and COL1 can be explained by interference with TGF-β1 signaling. pSmad3 activity was strongly up-regulated following TGF-β1 exposure, but CCN3-transfection and overexpression had only a small, non-statistically significant, suppression of this response. Addition of exogenous CCN3 had a similar effect, showing little or no reduction in pSmad3 activity. These observations are consistent with the interpretation that the observed effects of CCN3 on CCN2 and COLI are not mediated through blocking TGF-β1 signaling.
It is clear from previous studies that CCN3 mRNA is present in multiple organs, including the kidney, but there is an absence of information about cell specific localization, regulatory factors, or the normal functon of CCN3 in any developed organ (Chevalier et al. 1998; Liu et al. 1999). In normal nephrogenesis, CCN3 protein is tightly associated with normal differentiation of the glomerulus and is expressed in Wilms tumors (Chevalier et al. 1998). We and others have been responsible for demonstrating a link between cancer cell growth and CCN3 expresson (Glukhova et al. 2001; McCallum et al. 2006; Niu et al. 2005), whereas recently a role for CCN3 as a regulator of human hematopoietic stem or progenitor cell growth has been shown (Gupta et al. 2007). Pleotrophic effects in development, normal physiology, and pathology are indicated. Our study has added a role for CCN3 in wound healing and fibrosis, with great significance indicated in the complications of diabetes. It additionally shows the ability of one CCN molecule to regulate the production and function of another member. Not only does this demonstrate a new paradigm for understanding the interaction of CCN molecules, but could provide a novel therapeutic approach for disease intervention, not only in diabetic nephropathy, but in the altered ECM metabolism and pathology that is the response to a variety of insults, and in multiple organ systems.
Acknowledgments
We thank Dr. William Snapper at Northwestern University for providing the col1a2 construct. This work was supported by a grant from the Juvenile Diabetes Foundation International (BLR).
Competing interests statement No competing interests to be disclosed by any of the authors. BLR is a full-time employee of Baxter Healthcare, but holds an adjunct appointment and supervises an academic laboratory at Rosalind Franklin University of Medicine and Science, where the work was carried out.
Footnotes
Grant number(s): Juvenile Diabetes Foundation International (to BLR) # 1-2001-380.
References
- Abdel-Wahab N, Weston BS, Roberts T, Mason RM. Connective tissue growth factor and regulation of the mesangial cell cycle: role in cellular hypertrophy. J Am Soc Nephrol. 2002;13(10):2437–2445. doi: 10.1097/01.ASN.0000031828.58276.02. [DOI] [PubMed] [Google Scholar]
- Bollineni JS, Reddi AS. Transforming growth factor-beta 1 enhances glomerular collagen synthesis in diabetic rats. Diabetes. 1993;42(11):1673–1677. doi: 10.2337/diabetes.42.11.1673. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Bottinger E, Brosius FC, 3rd, Coffman TM, Harris RC, Heilig CW, et al. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16(1):27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- Brigstock DR, Steffen CL, Kim GY, Vegunta RK, Diehl JR, Harding PA. Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids. Identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem. 1997;272(32):20275–20282. doi: 10.1074/jbc.272.32.20275. [DOI] [PubMed] [Google Scholar]
- Brigstock DR, Goldschmeding R, Katsube KI, Lam SC, Lau LF, Lyons K, et al. Proposal for a unified CCN nomenclature. Mol Pathol. 2003;56(2):127–128. doi: 10.1136/mp.56.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier G, Yeger H, Martinerie C, Laurent M, Alami J, Schofield PN, et al. novH: differential expression in developing kidney and Wilm’s tumors. Am J Pathol. 1998;152(6):1563–1575. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Cooker LA, Peterson D, Rambow J, Riser ML, Riser RE, Najmabadi F, et al. TNF-{alpha}, but not IFN-{gamma}, regulates CCN2 (CTGF), collagen type I, and proliferation in mesangial cells: possible roles in the progression of renal fibrosis. Am J Physiol Renal Physiol. 2007;293(1):F157–F165. doi: 10.1152/ajprenal.00508.2006. [DOI] [PubMed] [Google Scholar]
- Glukhova L, Angevin E, Lavialle C, Cadot B, Terrier-Lacombe M-J, Perbal B, et al. Patterns of specific genomic alterations associated with poor prognosis in high-grade renal cell carcinomas. Cancer Genet Cytogenet. 2001;130(2):105–110. doi: 10.1016/S0165-4608(01)00477-0. [DOI] [PubMed] [Google Scholar]
- Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. Faseb J. 2008;21:3355–3368. doi: 10.1096/fj.06-6713com. [DOI] [PubMed] [Google Scholar]
- Gupta R, Hong D, Iborra F, Sarno S, Enver T. NOV (CCN3) functions as a regulator of human hematopoietic stem or progenitor cells. Science. 2007;316(5824):590–593. doi: 10.1126/science.1136031. [DOI] [PubMed] [Google Scholar]
- Hahn A, Heusinger-Ribeiro J, Lanz T, Zenkel S, Goppelt-Struebe M. Induction of connective tissue growth factor by activation of heptahelical receptors. Modulation by Rho proteins and the actin cytoskeleton. J Biol Chem. 2000;275(48):37429–37435. doi: 10.1074/jbc.M000976200. [DOI] [PubMed] [Google Scholar]
- Hostetter TH. Progression of renal disease and renal hypertrophy. Annu Rev Physiol. 1995;57:263–278. doi: 10.1146/annurev.ph.57.030195.001403. [DOI] [PubMed] [Google Scholar]
- Kyurkchiev S, Yeger H, Bleau AM, Perbal B. Potential cellular conformations of the CCN3(NOV) protein. Cell Commun Signal. 2004;2(1):9. doi: 10.1186/1478-811X-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Liu X-J, Crowe PD, Kelner GS, Fan J, Barry G, et al. Nephroblastoma overexpressed gene (NOV) codes for a growth factor that induces protein tyrosine phosphorylation. Gene. 1999;238(2):471–478. doi: 10.1016/S0378-1119(99)00364-9. [DOI] [PubMed] [Google Scholar]
- McCallum L, Price S, Planque N, Perbal B, Pierce A, Whetton AD, et al. A novel mechanism for BCR-ABL action: stimulated secretion of CCN3 is involved in growth and differentiation regulation. Blood. 2006;108(5):1716–1723. doi: 10.1182/blood-2006-04-016113. [DOI] [PubMed] [Google Scholar]
- Niu Z, Ito M, Awakura Y, Takahashi T, Nakamura E, Ito N, et al. The expression of NOV and WT1 in renal cell carcinoma: a quantitative reverse transcriptase-polymerase chain reaction analysis. J Urol. 2005;174(4 Pt 1):1460–1462. doi: 10.1097/01.ju.0000173008.73741.80. [DOI] [PubMed] [Google Scholar]
- Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, et al. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol. 2005;16(1):133–143. doi: 10.1681/ASN.2004040339. [DOI] [PubMed] [Google Scholar]
- Perbal B. The CCN family of genes: a brief history. Mol Pathol. 2001;54(2):103–104. doi: 10.1136/mp.54.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363(9402):62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Perbal B, Martinerie C, Sainson R, Werner M, He B, Roizman B. The C-terminal domain of the regulatory protein NOVH is sufficient to promote interaction with fibulin 1C: a clue for a role of NOVH in cell-adhesion signaling. Proc Natl Acad Sci U S A. 1999;96(3):869–874. doi: 10.1073/pnas.96.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncelet AC, Caestecker MP, Schnaper HW. The transforming growth factor-beta/SMAD signaling pathway is present and functional in human mesangial cells. Kidney Int. 1999;56(4):1354–1365. doi: 10.1046/j.1523-1755.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- Rachfal AW, Brigstock DR. Structural and functional properties of CCN proteins. Vitam Horm. 2005;70:69–103. doi: 10.1016/S0083-6729(05)70003-0. [DOI] [PubMed] [Google Scholar]
- Riser BL, Cortes P. Connective tissue growth factor and its regulation: a new element in diabetic glomerulosclerosis. Ren Fail. 2001;23(3–4):459–470. doi: 10.1081/JDI-100104729. [DOI] [PubMed] [Google Scholar]
- Riser BL, Cortes P, Yee J, Sharba AK, Asano K, Rodriguez-Barbero A, et al. Mechanical strain- and high glucose-induced alterations in mesangial cell collagen metabolism: role of TGF-beta. J Am Soc Nephrol. 1998;9(5):827–836. doi: 10.1681/ASN.V95827. [DOI] [PubMed] [Google Scholar]
- Riser BL, Ladson-Wofford S, Sharba A, Cortes P, Drake K, Guerin CJ, et al. TGF-beta receptor expression and binding in rat mesangial cells: modulation by glucose and cyclic mechanical strain. Kidney Int. 1999;56(2):428–439. doi: 10.1046/j.1523-1755.1999.00600.x. [DOI] [PubMed] [Google Scholar]
- Riser BL, Cortes P, Yee J. Modelling the effects of vascular stress in mesangial cells. Curr Opin Nephrol Hypertens. 2000;9(1):43–47. doi: 10.1097/00041552-200001000-00008. [DOI] [PubMed] [Google Scholar]
- Riser BL, Denichilo M, Cortes P, Baker C, Grondin JM, Yee J, et al. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol. 2000;11(1):25–38. doi: 10.1681/ASN.V11125. [DOI] [PubMed] [Google Scholar]
- Riser BL, Varani J, Cortes P, Yee J, Dame M, Sharba AK. Cyclic stretching of mesangial cells up-regulates intercellular adhesion molecule-1 and leukocyte adherence: a possible new mechanism for glomerulosclerosis. Am J Pathol. 2001;158(1):11–17. doi: 10.1016/S0002-9440(10)63938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riser BL, Cortes P, DeNichilo M, Deshmukh PV, Chahal PS, Mohammed AK, et al. Urinary CCN2 (CTGF) as a possible predictor of diabetic nephropathy: preliminary report. Kidney Int. 2003;64(2):451–458. doi: 10.1046/j.1523-1755.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- Riser B, Foroni A, Karoor S. CCN2 (CTGF) in the pathogenesis of diabetic renal disease: a target for therapeutic intervention. In: Morgensen C, Cortes P, editors. The kidney and diabetes mellitus. 6. Boston: Kluwer; 2006. [Google Scholar]
- Riser BL, Najmabadi F, Perbal B, Peterson DR, Rambow J, Riser ML, Sukowski E, Yeger H, Riser SC. CCN3 (NOV) is a negative regulator of CCN2 (CTGF) and a novel endogenous inhibitor of the fibrotic pathway in an in vitro model of renal disease. Am J Pathol. 2009;174(5):1725–1734. doi: 10.2353/ajpath.2009.080241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos GN, Kyurkchiev S, Perbal B. Immunocytochemical localization of NOVH protein and ultrastructural characteristics of NCI-H295R cells. J Submicrosc Cytol Pathol. 2001;33(3):251–260. [PubMed] [Google Scholar]
- van Roeyen CR, Eitner F, Scholl T, Boor P, Kunter U, Planque N. CCN3 is a novel endogenous PDGF-regulated inhibitor of glomerular cell proliferation. Kidney Int. 2008;73:86–94. doi: 10.1038/sj.ki.5002584. [DOI] [PubMed] [Google Scholar]
- Yokoi H, Mukoyama M, Nagae T, Mori K, Suganami T, Sawai K, et al. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc Nephrol. 2004;15(6):1430–1440. doi: 10.1097/01.ASN.0000130565.69170.85. [DOI] [PubMed] [Google Scholar]
- Ziyadeh FN. Mediators of diabetic renal disease: the case for TGF-b as the major mediator. J Am Soc Nephrol. 2004;15(1, Suppl. 1):S55–S57. doi: 10.1097/01.ASN.0000093460.24823.5B. [DOI] [PubMed] [Google Scholar]