Abstract
The most important clinical strategy for management of patients with hemophilia is the avoidance of recurrent hemarthroses by means of continuous, intravenous hematological prophylaxis. When only intravenous on-demand hematological treatment is available, frequent evaluations are necessary for the early diagnosis and treatment of episodes of intra-articular bleeding. The natural history of the disease in patients with poorly controlled intra-articular bleeding is the development of chronic synovitis and, later, multi-articular hemophilic arthropathy. Once arthropathy develops, the functional prognosis is poor. Treatment of these patients should be conducted through a comprehensive program by a multidisciplinary hemophilia unit. Although continuous prophylaxis can avoid the development of the orthopedic complications of hemophilia still seen in the twenty-first century, such a goal has not, so far, been achieved even in developed countries. Therefore, many different surgical procedures such as arthrocentesis, radiosynoviorthesis (radiosynovectomy) (yttrium-90, rhenium-186), tendon lengthenings, alignment osteotomies, joint arthroplasties, removal of pseudotumours, and fixation of fractures are still frequently needed in the care of these patients.
Keywords: hemophilia, orthopedic surgery, musculo-skeletal complications
Introduction
Hemophilia is a rare inherited bleeding disorder characterized by the absence of one of the coagulation factors including factor VIII in hemophilia A and factor IX in hemophilia B. The type of inheritance is sex-linked (chromosome X) and recessive. That means that grandfathers transmit the disease to 50% of their grandchildren through their daughters (who are known as carriers and do not suffer the disease). However, occasionally, there are some spontaneous mutations.
The lack of these specific coagulation factors renders the patient prone to abnormal bleeding. Eighty percent of hemorrhages occur in the musculo-skeletal system, while 20% take place in the central nervous system and other organ systems. The high percentage of musculo-skeletal bleedings explains why hemophilia is an orthopedic condition of great interest to the orthopedic surgeon [1]. The purpose of this review is to describe the prevalence and grades of hemophilia, some basic hematological concepts, and what the orthopedic surgeon should know regarding the management of the musculo-skeletal manifestations of hemophilia.
Prevalence and grades of hemophilia
The average prevalence of hemophilia is approximately one per 10,000 of the population. If the world population is estimated at six billion, there are most likely about 600,000 patients afflicted with the disease. In Spain, the incidence is lower than this world average. There are approximately 3,000 known cases in a population of 46 million. We believe this lower incidence is the result the nationwide efforts in prenatal diagnosis primarily involving chorionic villus biopsy and, in positive cases, termination of pregnancy.
There are three grades of hemophilia according to the percentage of coagulation factor in the patient’s blood: severe (<1%), moderate (1% to 5%), and mild (>5%). Generally speaking, the less the percentage of coagulation factor, the greater the risk of hemorraghic complications.
Basic hematological concepts for the orthopedic surgeon
When I started to treat hemophiliacs 35 years ago, the hematological management used was called on-demand. In other words, the deficient coagulation factor was only intravenously injected when the patient suffered a hemorrhage. With this treatment, most of patients developed a severe destruction of the joints in the second or third decade of life (hemophilic arthropathy).
Later on, some authors observed that using coagulation factors in a prophylactic way could diminish the rate of bleeding and its consequences. It was the beginning of the so-called continuous prophylaxis, which can be primary (started when the children begin to walk or just after the first hemarthrosis) or secondary (started after several or many articular hemorrhages) [2]. It is important to remember that the coagulation factors can only be injected intravenously and that they are very expensive. In fact, in our hospital, 40% of the annual pharmaceutical budget is dedicated to factor concentrates related to hemophilia.
In developed countries, the ideal current hematological treatment is primary prophylaxis. In addition to the cost, this treatment regimen exposes the patient to the risks of chronic intravenous therapy including the risks associated with indwelling central venous catheters. Unfortunately, this treatment does not completely protect the patient from articular hemorrhages and subclinical bleeding. The intravenous injections of the deficient coagulation factor can be performed in a day hospital or at home (after teaching patients and their families the proper technique). Unfortunately, only 15–20% of the world population has access to this ideal treatment of hemophilia. That means that 80–85% of hemophiliacs in the world are currently treated on-demand or even untreated. As a result of ideal prophylactic therapy, hemophiliacs in Spain and other developed countries have achieved a similar survival as that of the normal population.
Another current problem in adult hemophiliacs is that the majority of them were infected with HIV and/or hepatitis C during the 1980s. This tragic consequence occurred as a result of the inability to detect common contamination of the blood supply by HIV and hepatitis C viruses during that decade. Today, most factor concentrates are safe regarding the aforementioned viruses, including plasma-derived concentrates and recombinant factors. Because HIV and hepatitis C infection can be transmitted by exposure to infected blood or blood components, orthopedists are at risk for HIV and hepatitis infection during surgical procedures.
For any surgical procedure, clotting factor replacement should be infused preoperatively until a minimum level of 100% of normal is achieved. The level should then be maintained at 60% of normal for 14 postoperative days. Thereafter, clotting factor replacement is infused to obtain a level of 30% of normal prior to rehabilitation sessions for 8–10 weeks; 10% to 20% of patients develop antibodies against coagulation factors (so-called inhibitors). In these patients, the control of hemostasis for surgery cannot be obtained by means of ordinary coagulation factors. In these circumstances, patients will need bypassing agents to control hemostasis, such as recombinant factor VII-activated and/or factor eight inhibitor bypassing agent. Both of them are tremendously expensive, and their efficacy is not as good as the efficacy of ordinary factors.
Musculo-skeletal problems of hemophilia
In hemophiliac patients, any intramuscular injections should be avoided due to the risk of producing hematomas at the injection site. Aspirin and NSAIDs should be avoided due to the risk of gastrointestinal bleeding. COX-2 agents may be prescribed and have been found to be very effective in the management of articular pain and swelling in affected joints.
It is well known that about 90% of people with severe hemophilia experience chronic degenerative changes (hemophilic arthropathy) in one to six major joints (ankles, elbows, knees) by the second or third decade of life. Such degenerative changes are mainly due to spontaneous recurrent intra-articular hemorrhages. A critical factor for avoiding hemophilic arthropathy is the prevention of articular hemorrhages by means of prophylactic treatment. However, despite regular infusions of antihemophilic concentrate at an early age (prophylactic treatment), recurrent hemarthrosis and the possibility of hemophilic arthropathy still persist in some patients (subclinical hemorrhages). The pathogenesis of the progression from recurrent hemarthrosis to hemophilic arthropathy, in particular in the early stages, is incompletely understood.
Hemophilic arthropathy is characterized by chronic proliferative synovitis and cartilage destruction. Both events are the consequence of recurrent intra-articular bleeding. However, the exact pathogenesis is still poorly understood. In vitro studies performed by Dutch authors have shown that a 4-day duration of blood exposure produces a blood concentration- and time-dependent inhibition of cartilage matrix formation and an increased release of matrix components, both events resulting in a loss of matrix [3]. All these adverse effects can be partially prevented by N-acetylcysteine, suggesting the involvement of oxygen metabolites. Short-term exposure of cartilage to blood results in a three-fold increase in chondrocyte apoptosis. The addition of either specific caspase-3 inhibitor or a pan-caspase inhibitor decreases the number of apoptotic chondrocytes and partially restores proteoglycan synthesis in the cartilage after exposure to blood.
In vivo experiments have shown that a single joint hemorrhage results in lasting adverse changes in chondrocyte activity and cartilage matrix integrity. The clinical relevance of these findings could be that possibly one episode of hemarthrosis in childhood before the initiation of prophylactic treatment may result in joint damage after up to a decade or so later. The role of iron seems to be paramount in the development of hemophilic arthropathy, notably in the induction of synovial changes (synovial proliferation). A murine model of has documented that after 14 days of a major joint hemorrhage, the massive swelling of the joint resolves but the tissues remain brown with hemosiderin and the joint cavity becomes filled with a dense inflammatory cell infiltrate [4]. Vascular hyperplasia is also evident. The articular surface is irregular with pannus formation and the underlying bone is dysmorphic. After 30 days, there is marked cartilage and subchondral bone erosion. This would appear to suggest that preventive interventions should be implemented with urgency because a relatively short exposure of cartilage to blood may result in long-lasting changes in chondrocyte metabolism that may eventually lead to chronic degenerative changes (hemophilic arthropathy).
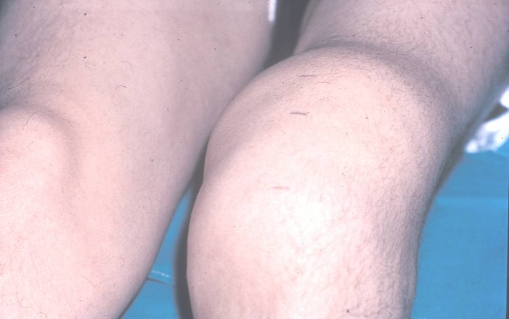
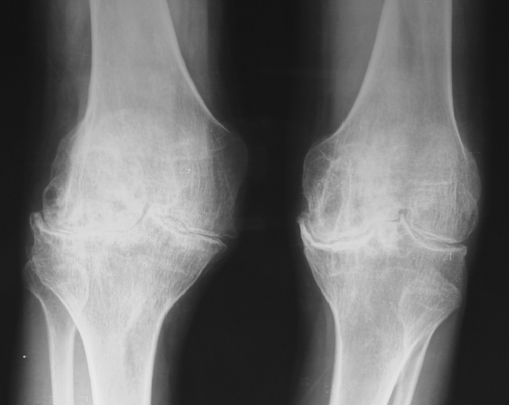
The pathogenesis of joint involvement in hemophilia begins with one or more hemarthroses when children start to walk. If the intra-articular blood is not evacuated early, it will affect the synthesis of proteogycans by chondrocytes, which eventually leads to the apoptosis of the chondrocytes. The synovium attempts to eliminate the excessive blood, resulting in a hypertrophic and fragile synovial lining which easily rebleeds, leading to a vicious cycle of hemarthrosis-synovitis-hemarthrosis. Finally, the intra-articular blood results in severe, acute pain, and flexion contracture of the involved joints. If untreated, they will eventually become fixed contractures. Unless these recurrent hemarthroses can be prevented, these patients will develop chronic synovitis (Fig. 1), hemarthrosis, pain, and eventually destruction of the joint (hemophilic arthropathy; Fig. 2). Hemophilic arthropathy is commonly multi-articular, affecting the knees, ankles, elbows, hips, and shoulders. When they occur, acute hemarthroses must be evacuated after the administration of factor to avoid the long-term consequences of intra-articular blood.
Fig. 1.
A photograph demonstrating chronic hemophilic synovitis of the knee
Fig. 2.
Radiographs of a 36-year-old patient with bilateral hemophilic arthropathy of the knee
Muscle hematomas
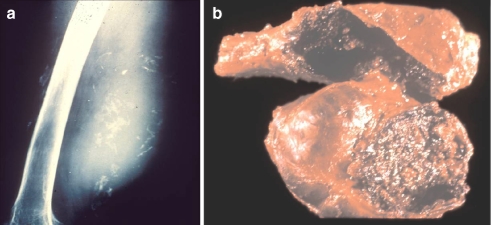
Another problem in hemophilia is the spontaneous development of muscle hematomas. Iliopsoas muscle hematoma is very common, although any other muscle in the anatomy can be involved. It is important to be aware of the risk of compartment syndromes and nerve compressions. Generally speaking, muscle hematomas require weeks or months of hematological treatment until their total disappearance (which can be proved by ultrasonography and/or CT scan). When a hematoma is not totally resolved, rebleeding will tend to occur, eventually resulting in a hemophilic pseudotumor (Fig. 3a). In the best-case scenario, a pseudotumor can be removed (Fig. 3b), although it can be very difficult if the pseudotumor is abdominal (pelvic pseudotumors) [5].
Fig. 3.
a A radiograph illustrating a posterior thigh pseudotumor which developed six years following a spontaneous thigh hematoma. b This photograph demonstrates the surgical specimen which resulted from the removal of the pseudotumor
It is very likely that with the advent of widespread maintenance therapy, pseudotumours will be less common in the future. It is important that they are diagnosed early, and prevention of muscular hematomas is the key to reducing their incidence. There are a number of therapeutic alternatives for this dangerous condition: surgical removal, percutaneous management, exeresis and filling of the dead cavity, irradiation, and embolization. The management of the patient with a hemophilic pseudotumor is complex and with a high rate of potential complications. Surgical excision is the treatment of choice but should only be carried out in major hemophilia centers by a multidisciplinary surgical team. The main postoperative complications are death infection, fistulization, and pathological fractures (requiring even amputation of the affected limb). Pelvic pseudotumors can even become complicated by fistulization to the large bowel and obstruction of the ureters. Untreated, proximal pseudotumors will ultimately destroy soft tissues, erode bone, and may produce neurovascular complications.
Anesthesia in the hemophiliac patient
The type of anesthesia to be used for surgery is another hot topic in hemophilia. Many anesthesiologists fear spinal anesthesia for the risk of neurological lesion in case of an intraneural hemorrhage. That is why general anesthesia is preferred in the majority of centers for hemophiliacs. General anesthesia is the obvious choice when, occasionally, a double or triple surgical procedure is planned for the same session [6]. However, this is very risky, and we do not use it routinely in our center.
Radiosynovectomy
Chronic hemophiliac synovitis can be treated medically by radiosynovectomy. The indications for radiosynovectomy include chronic hemophilic synovitis causing recurrent hemarthroses, unresponsive to hematological treatment. Radiosynovectomy involves the injection of Yttrium-90 for knees and Rhenium-186 for elbows and ankles (Table 1). These agents reduce the synovial and hypertrophy, decreasing the number and frequency of hemarthroses. On average, the efficacy of the procedure ranges from 76% to 80% and can be performed at any age. The procedure also slows cartilage destruction. Radiosynovectomy can be repeated up to three times with 3 month in between treatments. After 30 years of using radiation synovectomy worldwide, no damage has been reported in relation to the radioactive materials. Several joints can be injected in a single session, although no more than two joints to be injected at the same time is recommended.
Table 1.
Injection of Yttrium-90 for knees and Rhenium-186 for elbows and ankles
| Isotope | 90Y | 186Re |
|---|---|---|
| Radioactive half-life (days) | 2.8 | 3.8 |
| Radiation | Beta | Beta and gamma |
| TPP (mm) | 2.8 | 1 |
TPP Therapeutic penetration power
For the management of hemophilic synovitis, radiation synovectomy is a very easy, safe, and efficient procedure. In our center, we use it before arthroscopic synovectomy. The aim of both radiosynovectomy and arthroscopic synovectomy is to remove the inflamed and hypervascular synovium as soon as possible in order to prevent the onset of hemophilic arthropathy [7–15]. Ideally, however, these methods should be performed before the articular cartilage has eroded. Radioactive synovectomy is a relatively simple, virtually painless, and inexpensive technique for the treatment of chronic hemophilic synovitis, even in patients with inhibitors. Thus, radioactive synovectomy is the best choice for patients with persistent synovitis. Personal experience and the general recommendation among orthopedic surgeons and hematologists is that when three early consecutive radiosynovectomies (repeated every 3 months) fail to halt synovitis, an arthroscopic synovectomy should be immediately considered.
Hemophilic contracture is seen most commonly as an equinus deformity of the ankle or a flexion contracture of the knee or elbow. Treatment options are varied, and decision-making is based on the degree of the contracture, its chronicity, the presence of articular subluxation, the patient’s ability to participate in treatment, and the available medical facilities.
The available treatments fall into four categories: physiotherapy, orthotics, corrective devices, and surgical procedures. Treatment should be primarily by physiotherapy, splinting, and corrective devices. The late or severe case may require surgical correction in the form of soft-tissue procedures. Soft-tissue correction of muscle shortening may be performed such as lengthening of the Achilles tendon for equinus deformity of the ankle or hamstring release of the flexor muscles of the knee. Lower femoral osteotomy has been used for correction of flexion deformity at the knee joint. Mechanical distraction using external fixators for treatment of severe knee flexion contractures has been recently reported with satisfactory results. The main principle underlying the treatment of hemophilic contracture is the restoration of the patient’s lifestyle and mobility rather than anatomic or radiographic normality [16].
For flexion contractures, we recommend conservative treatment (physiotherapy). Only when they are fixed do we indicate surgical procedures: Z-lengthening of Achilles tendon for equinus deformity of the ankle or hamstring release in the knees. In severe contractures of the knee, we advocate external fixation for progressive extension of the joint.
Once the joint with hemophiliac arthropathy is destroyed (severe arthropathy), we follow principles which are similar to those applied to degenerative osteoarthritis (osteotomies, arthrodeses, or joint replacements). However, the risk of infection in hemophiliacs is higher than in normal population, especially in those who are HIV-positive.
Encouraging clinical experience has increased the indications for prosthetic knee arthroplasty in hemophiliacs [1, 17]. The medical status, physical disability, age, and projected activity levels are the major factors in determining treatment for the patient with unilateral or bilateral hemophilic arthropathy of the knee. In more severely involved knees of patients with hemophilia, flexion contracture is a common deformity. In addition, valgus, external rotation deformity, and posterior subluxation of the tibia may exist. The surgeon must have the expertise and experience to correct these deformities sufficiently when performing a total knee arthroplasty. In hemophiliacs, we routinely perform posterior stabilized prosthesis, using antibiotic cement and resurfacing of the patella. In extremely deformed knees, a constrained condylar knee or even a rotating-hinge prosthesis may be necessary. It is common in hemophilia for the tibia and distal femur to be quite small which may require small or even custom prosthetic components (preoperative planning as usual is very important).
At this point in time, TKA should be indicated in hemophilia patients suffering from severe knee pain and disability. However, the expected high risk of infection and other postoperative complications is a concern. The orthopedic surgeon should weigh the risks and benefits carefully. Clinical and immunological status should be considered before suggesting total knee replacement to a hemophilia patient.
The improvements in knowledge of arthroplasty fixation techniques and antibiotic prophylaxis are giving rise to a reduction in significant complications for hemophilia sufferers requiring total hip arthroplasty [1, 18, 19]. Following consideration of the risks and benefits, with age, functional demands, life expectancy, and concurrent immunological compromise, the patient with disabling hip arthropathy may very well benefit from hip replacement. As most of the patients are relatively young, it is our practice to use cementless prostheses, with a tapered femoral stem and an acetabular component that can be supplemented with screws.
Fractures
Fractures are more and more common in hemophiliacs because of their active lifestyles. For fractures, closed plaster casts are not recommended in hemophilia for the risk of compartment syndrome. We always prefer rigid internal fixation to external fixation, as usual under hematological control.
The goal of modern fracture treatment must be to obtain an optimal outcome with the patient’s return to full activity as soon as possible. Today, internal stabilization is indicated in most displaced fractures in the adult, whereas external fixation remains the best choice for initial stabilization with severe soft-tissue injuries. If a fracture is correctly treated in a hemophilic patient, it will progress to healing in a similar time frame to those occurring in the general population.
Conclusions
Any surgical procedure in hemophilia must be performed under factor cover; that is to say, under hematological control. Assistance by a consulting hematologist is encouraged even when perfoming arthrocentesis and radiosymonectomy. Factor supplementation is also recommended for periods when intensive physiotherapy is needed especially after surgical procedures (to avoid rebleeding during the rehabilitation program).
The close cooperation between hematologists, orthopedic surgeons, rehabilitation physicians, and physiotherapists is paramount to get satisfactory results after orthopedic procedures in these patients. Although continuous prophylaxis can slow the development of the orthopedic complications of hemophilia even in developed countries, these complications still arise. Therefore, orthopedic surgeons are still needed to carry out many different surgical procedures [1, 20–23]. Unfortunately, HIV infection as a consequence of repeated factor infusion raises risk of postoperative infection after any surgical procedure, especially joint arthroplasty [24, 25].
Footnotes
The author certifies that he has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Rodriguez-Merchan EC. Management of orthopedic complications of haemophilia. J. Bone Joint Surg. (Br) 1998;80B:191–196. [PubMed] [Google Scholar]
- 2.Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty-five years experience of prophylactic treatment in severe hemophilia A and B. J. Intern. Med. 1992;232:25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 3.Jansen NW, Roosendaal G, Bijlsma JW, Degroot J, Lafeber FP. Exposure of human cartilage tissue to low concentrations of blood for a short period of time leads to prolonged cartilage damage: an in vitro study. Arthritis Rheum. 2007;56:199–207. doi: 10.1002/art.22304. [DOI] [PubMed] [Google Scholar]
- 4.Valentino LA, Hakobyan N, Rodriguez N, Hoots WK. Pathogenesis of haemophilic synovitis: experimental studies on blood-induced joint damage. Haemophilia. 2007;13(Suppl. 3):10–13. doi: 10.1111/j.1365-2516.2007.01534.x. [DOI] [PubMed] [Google Scholar]
- 5.Sevilla J, Alvarez MT, Hernandez D, et al. Therapeutic embolization and surgical excision of hemophilic pseudotumour. Haemophilia. 1999;5:360–363. doi: 10.1046/j.1365-2516.1999.00330.x. [DOI] [PubMed] [Google Scholar]
- 6.Horoszowski H, Heim M, Schulman S, Varon D, Martinowitz U. Multiple joint procedures in a single operative session on hemophilic patients. Clin. Orthop. Relat. Res. 1996;328:60–64. doi: 10.1097/00003086-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Silva M, Luck JV, Jr, Siegel ME. 32P chromic phosphate radiosynovectomy for chronic haemophilic synovitis. Haemophilia. 2001;7(Suppl. 2):40–49. doi: 10.1046/j.1365-2516.2001.00109.x. [DOI] [PubMed] [Google Scholar]
- 8.Manco-Johnson MJ, Nuss R, Lear J, Wiedel J, Geraghty SJ, Hacker MR, Funk S, Kilcoyne RF, Murphy J. 32P radiosynoviorthesis in children with hemophilia. J. Pediatr. Hematol. Oncol. 2002;24:534–539. doi: 10.1097/00043426-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Chew EM, Tien SL, Sundram FX, Ho YK, Howe TS. Radionuclide synovectomy and chronic haemophilic synovitis in Asians: a retrospective study. Haemophilia. 2003;9:632–637. doi: 10.1046/j.1365-2516.2003.00799.x. [DOI] [PubMed] [Google Scholar]
- 10.Li P, Chen G, Zhang H, Shen Z. Radiation synovectomy by 188Re-Sulfide in haemophilic synovitis. Haemophilia. 2004;10:422–427. doi: 10.1111/j.1365-2516.2004.00913.x. [DOI] [PubMed] [Google Scholar]
- 11.Grmek M, Milcinski M, Fettich J, Benedik-Dolnicar M, Brecelj J. Radiosynoviorthesis for treatment of hemophilic hemarthrosis-Slovenian experience. Cancer Biother Radiopharm. 2005;20:338–343. doi: 10.1089/cbr.2005.20.338. [DOI] [PubMed] [Google Scholar]
- 12.Lofqvist T, Petersson C, Nilsson IM. Radioactive synoviorthesis in patients with hemophilia with factor inhibitor. Clin. Orthop. Relat. Res. 1997;343:37–41. [PubMed] [Google Scholar]
- 13.Wiedel JD. Arthroscopic synovectomy of the knee in hemophilia. 10- to 15-year followup. Clin. Orthop. Relat. Res. 1996;328:46–53. doi: 10.1097/00003086-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Greene WB. Synovectomy of the ankle for hemophilic arthropathy. J. Bone Joint Surg. (Br) 1994;76A:812–819. doi: 10.2106/00004623-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Merchan EC. Methods to treat chronic haemophilic synovitis. Haemophilia. 2001;7:1–5. doi: 10.1046/j.1365-2516.2001.00481.x. [DOI] [PubMed] [Google Scholar]
- 16.Heim M, Horoszowski H, Varon D, Schulman S, Martinowitz U. The fixed flexed and subluxed knee in the hemophilic child: what should be done? Haemophilia. 1996;1:47–50. doi: 10.1111/j.1365-2516.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 17.Cohen I, Heim M, Martinowitz V, Chechick A. Orthopedic outcome of total knee replacement in haemophilia A. Haemophilia. 2000;6:104–109. doi: 10.1046/j.1365-2516.2000.00375.x. [DOI] [PubMed] [Google Scholar]
- 18.Kelley SS, Lachiewicz PF, Gilbert MS, Bolander ME, Jankiewicz JJ. Hip arthroplasty in hemophilic arthropathy. J. Bone Joint Surg. (Am) 1995;77A:828–834. doi: 10.2106/00004623-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Lofqvist T, Sanzen L, Petersson C, Nilsson IM. Total hip replacement in patients with hemophilia. Acta. Orthop. Scand. 1996;67:321–324. doi: 10.3109/17453679609002323. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Merchan EC. Orthopedic surgery of haemophilia in the 21st century: an overview. Haemophilia. 2002;8:360–368. doi: 10.1046/j.1365-2516.2002.00562.x. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Merchan EC. Total knee arthroplasty in patients with haemophilia who are HIV-positive. J. Bone Joint Surg. (Br) 2002;84-B:170–172. doi: 10.1302/0301-620X.84B2.13015. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Merchan EC. Total knee replacement in haemophilic arthropathy. J. Bone Joint Surg. (Br) 2007;89B:186–188. doi: 10.1302/0301-620X.89B2.18682. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Merchan EC. Bone fractures in the haemophilic patient. Haemophilia. 2002;8:104–111. doi: 10.1046/j.1365-2516.2002.00628.x. [DOI] [PubMed] [Google Scholar]
- 24.Ragni MV, Crossett LS, Herndon JH. Postoperative infection following orthopedic surgery in human immunodeficiency virus-infected hemophiliacs with CD4 counts < or =200/mm3. J. Arthoplasty. 1995;10:716–721. doi: 10.1016/S0883-5403(05)80065-8. [DOI] [PubMed] [Google Scholar]
- 25.Phillips AM, Sabin CA, Ribbans WJ, Lee CA. Orthopedic surgery in hemophilic patients with human immunodeficiency virus. Clin. Orthop. Relat. Res. 1997;343:81–87. doi: 10.1097/00003086-199710000-00016. [DOI] [PubMed] [Google Scholar]