Abstract
Fractures require adequate stability and blood supply to heal. The vascular supply to long bones is compromised in a fracture, and the ability to heal hinges on the ability of new blood vessels to proliferate from surrounding vessels in a process known as angiogenesis. This process is largely driven by the growth factor, vascular endothelial growth factor (VEGF), whose levels are increased locally and systemically during fracture healing. VEGF is involved in many steps throughout the fracture healing cascade, from initially being concentrated in fracture hematoma, to the promotion of bone turnover during the final remodeling phase. This article reviews the current literature surrounding the role of VEGF and other growth factors in reestablishing vascular supply to fractured bone, as well as medications and surgical techniques that may inhibit this process.
Keywords: VEGF, growth factor, fracture, angiogenesis, non-union, blood supply, vascular endothelial growth factor, fracture healing
Introduction
Fractures are one of the most common ailments that orthopaedic surgeons, regardless of specialty, are faced with [1]. Understanding the complex processes of fracture healing, down to the molecular level, is critical so that the surgeon can preserve and possibly augment these endogenous mechanisms. Osseous repair requires the coordination of many growth factors in a temporospatial fashion during the healing cascade. One such factor that has been extensively studied for its role in angiogenesis and fracture healing is vascular endothelial growth factor (VEGF). Bone is a highly vascular tissue and fractures disrupt this vasculature [2], depriving osteoblasts of oxygen, nutrients, growth factors, calcium, phosphate, and many other materials these cells use to form new bone. In addition to providing bone forming substrates, the vasculature around fractures also provides a source of stem cells which are capable of differentiating into osteoblasts [3]. The purpose of this paper is to review the role of VEGF in angiogenesis and fracture healing, as well as to highlight therapeutic measures that may compromise these processes.
Growth factors promoting angiogenesis during fracture healing
Vascular endothelial growth factor
VEGF has many isoforms, but VEGF-A is considered the prototype member in the family. Other isoforms within this family include VEGF-B, -C, and -D. The role of VEGF-B is currently unknown, and VEGF-C and -D isoforms are chiefly involved in lymphangiogenesis [4]. Of the isoforms, VEGF-A is the predominant factor in the regulation of angiogenesis and endothelial cell growth (referred to as VEGF from here forward). The human VEGF gene undergoes alternative splicing to produce the four major cleavage products with the 165 amino acid peptide being the isoforms with the greatest biological activity [5]. VEGF is produced by endothelial cells, macrophages, fibroblasts, smooth muscle cells, osteoblasts, and hypertrophic chondrocytes [6–8]. Similar to most peptide growth factors, VEGF binds to receptors (VEGFR-1 and 2) on the cell surface of its targets (Fig. 1) [5].
Fig. 1.
Vascular Endothelial Growth Factor receptor binding and downstream signaling pathway. The various pathways that VEGF stimulate within endothelial cells lead to their proliferation, migration, and survival, all of which are necessary for angiogenesis. PI3K, phosphoinositide 3-kinase; Akt/PKB, protein kinase B; p38MAPK, p38 mitogen-activated protein kinase; MEK, mitogen and extracellular kinase; Erk, extracellular regulated kinase. (Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved. Rini, B et al: Journal of Clinical Oncology 23 (5), 2005: 1028–1043)
Primary human osteoblasts express high levels of VEGFR-1 and signaling through VEGFR-1 on osteoblasts induces a strong chemotactic response [9]. VEGF also indirectly induces proliferation and differentiation of osteoblast precursor cells. This is achieved by the secretion of osteoanabolic factors, such as endothelin-I and insulin-like growth factor -I, by VEGF stimulated endothelial cells [10]. VEGF has also been shown to promote mesenchymal stem cell (MSC) chemotaxis [11]. The periosteum, muscle, adipose tissue, and bone marrow serve as rich sources of MSCs capable of differentiating into osteoblasts, while also providing endothelial progenitor cells that support postnatal vasculogenesis [12]. Vasculogenesis and angiogenesis, driven largely by locally and systemically elevated VEGF levels [13], allow for the delivery of osteogenic substrates, pericyte stem cells, and mesenchymal stem cells that are capable of differentiating into additional osteoblasts [3]. This establishes a positive feedback loop between osteoblasts and endothelium through the trophic factors secreted by these cells.
The bone morphogenetic proteins, known for their ability to promote mesenchymal stem cell (MSC) differentiation that leads to bone formation, also promote angiogenesis. Deckers, et al. found that BMP-2, BMP-4, and BMP-6 were capable of stimulating murine derived osteoblasts to secrete VEGF in culture and stimulated angiogenesis in fetal mice bone explants [8]. The authors used BMP antagonists to observe a significant decrease in VEGF production by osteoblasts, confirming that BMPs were responsible for the increased VEGF detected in culture. Additionally, the authors used anti-murine VEGF antibodies and noticed a blockade of BMP-induced angiogenesis [8]. The action of BMPs on osteoblasts establishes a positive feedback loop where the BMP-induced VEGF release causes vessel ingrowth, leading to the delivery of osteogenic precursor cells on which BMPs will act to further increase VEGF concentrations at the fracture site. These examples illustrate the synergistic role of BMP and VEGF in promoting angiogenesis and bone formation during fracture healing.
Other growth factors contributing to angiogenesis
There are many other factors that play a role in the reestablishment of vascular supply to bones during the healing process, but the precise mechanisms have not been as clearly elucidated as those for VEGF. The fibroblastic growth factors (FGF) were studied by Kawaguchi et al. who showed that a single dose of recombinant basic FGF (bFGF) could increase the structural integrity of fibula fractures in rats [14]. While the mechanism was not elucidated, this was hypothesized to have been mediated by bFGF’s ability to induce angiogenesis by promoting expression of VEGF, promote differentiation of MSCs into osteoblasts, and down-regulate the BMP antagonist, noggin [15, 16].
Thrombin, found in fracture hematoma, is inherently angiogenic through its action on PAR (protease activated receptors) type receptors [17]. The known angiogenic capacity of thrombin led to the production of thrombin peptide 508 (TP508), a twenty-three amino acid peptide (Chrysalin, OrthoLogic, Tempe, AZ). This peptide represents the receptor-binding domain of the thrombin protein. Because TP508 represents only a portion of the intact thrombin protein, it is able to mimic many aspects of the thrombin response without activating blood clotting. Thrombin normally activates PARs, but since TP508 is not proteolytic the peptide must interact with receptors distinct from the PARs, or with PARs at a non-proteolytic site. TP508 is interesting in regards to fracture repair because it was shown to have a dose-dependent chemotactic effect on human microvascular endothelial cells and osteoblasts. In a rat closed femur fracture model, a single injection on TP508 increased the mechanical strength of the femora as early as three weeks post fracture. These results were attributed to the effects that TP508 had on angiogenesis at the fracture site. TP508 treated femora showed a significant increase in vessel number and size in fracture callus as compared to controls [17]. TP508 is synthetically produced, but whether or not this peptide is a cleavage product of thrombin in vivo remains unknown.
Many other growth factors, enzymes, and cytokines play a role in angiogenesis and fracture repair. Refer to Table 1 for a more comprehensive listing.
Table 1.
Factors that promote angiogenesis and/or bone formation
| Factor | Angiogenesis Promoter | Bone Formation Promoter | Reference |
|---|---|---|---|
| Vitamin D3 [10] | + | + | Wang, 1997 |
| IGF-1 [10] | + | + | Wang, 1997 |
| Endothelin-1 [10] | + | Wang, 1997 | |
| PDGF-A [18] | + | Gerber, 2000 | |
| bFGF [15] | + | + | Carano, 2003 |
| PGE 1 and 2 [19] | + | + | Harada, 1994 |
| TGF-β [15] | + | + | Carano, 2003 |
| MMP1 [20] | + | + | Weiss, 2002 |
| MMP9 [21] | + | + | Weiss, 2005 |
| PTH(1–34) [22] | + | + | Manabe, 2007 |
| EPO [23] | + | + | Holstein, 2007 |
| Statins [24] | + | + | Simpson, 2006 |
| GDF-5 [25] | + | + | Sena, 2007 |
| IL-8 [26] | + | Reher, 1999 | |
| ANG [16] | + | Weiss, 2008 | |
| Ang-2 [16] | + | Weiss, 2008 | |
| PTN [16] | + | Weiss, 2008 |
Abbreviations: IGF- insulin growth factor, PDGF- platelet derived growth factor, FGF- fibroblastic growth factor, PGE- prostaglandin E, TGF- transforming growth factor, MMP- matrix metalloproteinase, PTH- parathyroid hormone, EPO- erythropoietin, GDF- growth differentiation factor, IL- interleukin, ANG- angiogenin, Ang- angiopoietin, PTN- pleiotrophin
The role of angiogenesis in fracture healing
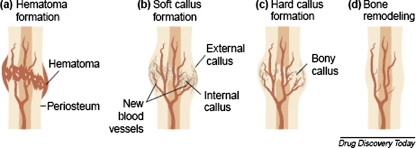
Fracture healing is a complex process that requires the coordinated response from four major types of tissue: bone marrow, cortical bone, periosteum, and undifferentiated fascia surrounding the fracture. Apposed fracture fragments require two essential factors for healing: blood supply and stability [27]. If rigid internal fixation is provided and the two fracture fragments are apposed with no micromotion between fragments, primary cortical, or osteonal healing will occur. This is mediated directly by osteoclasts and osteoblasts, with little participation from the surrounding tissues [28, 29]. More commonly, fractures are treated with external fixation, intramedullary fixation, or plating which allows micromotion to occur at the fracture site, leading to secondary healing via intramembranous and endochondral bone formation. The process of endochondral bone formation mimics the events which occur at the epiphyseal plate of growing long bones, with chondrogenesis occurring first, followed by matrix calcification, vascular invasion, and finally remodeling of this vascular calcified matrix into bone. In general, the five temporal phases of fracture healing are: hematoma formation, inflammation, endochondral bone formation coupled to angiogenesis, followed by cartilage removal with woven bone formation and bone remodeling (Fig. 2) [29]. VEGF, and the resulting angiogenesis, plays a role in many of the events that occur during each of these five temporal phases.
Fig. 2.
The stages of fracture repair. (a) Hematoma formation: following injury, fracture disrupts bony blood supply leading to hematoma formation in and around the bony defect; (b) Soft callus formation: fracture hematoma is rich in VEGF, which promotes blood vessel ingrowth from surrounding vessels (angiogenesis) along with the formation of a cartilage intermediate by endochondral ossification (internal callus) and the external callus (intramembranous ossification) (c) Hard callus formation: the callus is mineralized as hypertrophic chondrocytes undergo apoptosis (partially regulated by VEGF) and woven bone is formed and eventually replaced by lamellar bone (d) Bone remodeling: the fracture callus composed of primary lamellar bone is remodeled to secondary lamellar bone, and the vascular supply returns to normal. (Reprinted from Drug Discovery Today, Volume 8, Carano R and Filvaroff E, Angiogenesis and Bone Repair, pages 980–989, Copyright 2003, with permission from Elsevier.)
Hematoma formation phase
Fracture of a long bone severs the vessels that run longitudinally throughout the Haversian canals of the cortical bone, as well as periosteal and medullary canal vessels. There may also be damage to vessels in the surrounding soft tissues. The body responds to fractures both on the local and systemic levels, and with this principle in mind, Street et al. evaluated the difference between the circulating plasma levels of VEGF and the local VEGF concentrations within fracture hematoma [30]. Human fracture subjects had significantly elevated circulating VEGF levels compared to healthy controls, and the fracture hematoma contained 15-fold higher VEGF levels than did subject plasma. They also showed that interfragmentary hematoma, essentially a fibrin matrix, acts as a VEGF reservoir [30]. The high concentration of VEGF observed in fracture hematoma and the known roles that VEGF plays in endothelial cell proliferation, endothelial cell migration, osteoblast migration, and osteoblast activation, illustrates the fact that the formation of hematoma in fracture is an integral part of the repair process.
Fracture hematoma is extremely hypoxic and has been shown in healing rabbit fractures to have a mean oxygen tension as low as 6.25 mm Hg [31]. In 1992, researchers at Johns Hopkins discovered the link between hypoxia and the upregulation of proangiogenic factors, such as VEGF [32]. The transcription factor responsible is named hypoxia inducible factor-1α (HIF-1α), which activates genes involved in anaerobic metabolism, angiogenesis, and erythropoiesis. HIF expression in a rat femur fracture model was shown to steadily increase and peak to the point where expression was 5.9 fold higher than the intact contralateral femur. As expected, VEGF expression mirrored HIF expression, peaking at a 2.2 fold increase relative to the intact contralateral femur. VEGF expression rose steadily to its peak concentration then steadily declined thereafter, possibly due to blood vessel ingrowth and consequent rise in oxygen tension [33]. VEGF has been used to successfully promote healing in non-union and critical sized defects in rabbit fracture models [34–36], but given the potentially prohibitive costs associated with recombinant growth factor production, Shen et al. sought to inhibit the degradation of HIF with small molecules, thereby promoting endogenous VEGF production [37]. HIF is degraded under normoxic conditions following its hydroxylation by HIF prolyl hydroxylase (PHD) enzymes. Three small molecules, deferoxamine, L-mimosine, and Dimethyloxalylglycine that inhibit PHD were found to increase HIF signaling leading to increased VEGF production in vitro, increased capillary sprouting in a functional angiogenesis assay, and increased vascularity and callus size in a mouse femur fracture model [37].
Thrombin activity is necessary for hematoma formation. Studies have shown that low molecular weight heparin, which accelerates the breakdown of thrombin and decreases blood clot formation, had a detrimental effect on fracture healing in a rabbit model [38]. Thrombin has been shown in vivo and in vitro to act as a chemotactic factor for endothelial cells and to increase the production of αVβ3 integrin on the surface of these cells. This integrin facilitates migration, attachment, and survival of endothelial cells [39]. It is well known that thrombin binds to fibrinogen, catalytically cleaving it to fibrin, and forming a blood clot. Thrombin is also able to bind fibrin in a non-substrate fashion [40], as well as to subendothelial extracellular matrix (ECM). This binding is achieved through anchorage domains separate from the molecule’s catalytic cleavage site [41]. When thrombin is bound to fibrin and dermatan sulfate of the hematoma and ECM, respectively, it is protected from the inactivation by antithrombin-III, and can be liberated in active form upon hematoma lysis and ECM remodeling [41]. This serves as another example of how fracture hematoma serves as to sequester the necessary growth factors that initiate the revascularization of a fracture site.
Inflammatory phase
In humans, the inflammatory response to a fracture occurs in two general phases: early response, which occurs during the first days following trauma, and the later prolonged response which lasts from days to weeks. Mouse models have shown that during the early inflammatory response, cytokines TNF-α, IL-1α, and IL-1β are strongly expressed and localized primarily in macrophages and inflammatory cells in the marrow space and the periosteum nearest to the fracture [42]. Inflammatory cells and the cytokines they release promote extracellular matrix synthesis and recruit local fibrogenic cells to the site of fracture. During the inflammatory phase, these cytokines act to increase the expression of cell adhesion molecules on blood vessels allowing for neutrophils and monocytes to enter the extracellular matrix where they secrete matrix metalloproteinases that promote tissue remodeling. IL-1 and TNF-α, important mediators of osteoclast formation, stimulate monocyte precursors to fuse and form a multinucleated osteoclast. Also, IL-1 and TNF-α can act directly on mature osteoclasts to increase bone turnover [42]. In a mouse fracture model, IL-1 and TNF-α levels rose sharply in the acute phase of inflammation after which the levels fell back to baseline. IL-1 expression remained near baseline until approximately 21 days post fracture at which point the levels began to rise again. A similar trend was observed for TNF-α. This correlated with the resorption of calcified cartilage and the formation and remodeling of new woven bone. IL-1α also showed a biphasic pattern of expression, and at later time points was shown to be expressed along with TNF within cells lining the interface of newly formed trabecular bone [42]. From these results it can be concluded that the acute phase inflammatory cytokines, IL-1 and TNF-α, help regulate osteoclastogenesis early in the process of fracture repair, followed by trabecular bone formation and later by bone remodeling. Accordingly, mice null for TNF-α receptors show a delay in the initial healing phase, delayed chondrocyte apoptosis during endochondral bone formation, and delayed resorption of mineralized cartilage [43].
The inflammatory response during fracture healing occurs both on a local and systemic level. Increased cytokine and growth factor levels can be detected within the regenerating tissue surrounding fracture, as well as in the peripheral blood of these patients [7, 16, 30]. Various studies have evaluated the circulating levels of VEGF in patients with fractures of long bones and have uniformly demonstrated that peripheral blood VEGF levels in fracture patients are significantly increased as compared to healthy controls [13, 16, 30]. Weiss et al. also evaluated the serum levels of angiogenin (ANG), angiopoietin-2 (Ang-2), bFGF, and PDGF-AB in patients with successful union of long bone fractures, versus patients with non-union. The authors found that fracture patients had significantly elevated serum VEGF, bFGF, and PDGF-AB as compared to healthy controls, and patients achieving successful union had significantly increased serum bFGF and PDGF-AB as compared to patients with non-union [16]. Interestingly, two studies have shown that patients with non-union have elevated serum VEGF levels at all time points compared to patients with fracture union, though neither study found this increase to be statistically significant [13, 16]. Few studies of tissue samples harvested from patients with non-union exist, making correlation of local and systemic growth factor response difficult, therefore studies in sheep with delayed fracture healing have been performed [12]. While human peripheral blood studies found increased levels of circulating VEGF in non-union, sheep models of non-union have shown lower expression within callus of various factors necessary for healing, including VEGF, Ang-1 and -2, and bFGF as compared to successfully healed subjects [12]. By histology, the sheep model of non-union showed prolonged hematoma formation and less staining for blood vessels within the fracture defect for a longer time period than controls, suggesting that the sustained elevation in VEGF may be a consequence of prolonged hypoxia within the non-union [12].
Cartilage intermediate phase
Many studies on endochondral bone formation have focused on the epiphyseal plate as the model, because the growth of long bones mimics the process of endochondral bone formation. Fracture repair differs from long bone growth in that there is callus formation around the site of injury. The callus has two different tissue consistencies based on its geographical location around the fracture. Within the medullary canal and intercortical areas of fracture there is formation of “soft callus,” which will undergo endochondral ossification. In the subperiosteal regions, the soft tissue surrounding the fracture forms “hard callus,” which will form bone via intramembranous ossification [29]. The callus is composed mostly of calcified cartilage on which monocyte-derived chondroclasts act to remodel the tissue for bone deposition. In the calcified callus remodeling process chondroclasts liberate proangiogenic factors, including BMPs and VEGF from the extra cellular matrix, which signal for the invasion of the soft callus by periosteal and cortical blood vessels [28]. The vascular invasion allows for the delivery of mesenchymal stem cells, specifically the pericyte, which share a common basement membrane with the endothelial cells of the capillaries that penetrate the soft callus. It has been known that pericytes proliferate during the process of vascular repair, but Brighton et al. showed that the pericyte is able to express alkaline phosphatase, collagen, glycosaminoglycans, and osteocalcin, and thus were able to form calcified colonies in vitro [3]. These findings suggest that vessel invasion of fracture not only delivers the oxygen and nutrients needed for native cells to repair the defect, but also provides an additional source of mesenchymal stem cells (MSCs) that develop into osteoblasts, and cells from the macrophage/monocyte lineage that develop into osteoclasts.
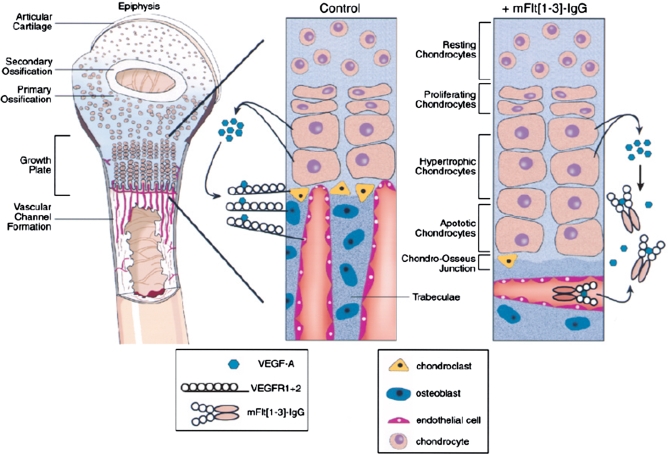
During endochondral bone formation, vascular invasion of cartilage occurs in the zone of hypertrophic chondrocytes. Normally, cartilage is an avascular tissue but various authors have shown that hypertrophic chondrocytes express and secrete VEGF as well as various VEGF receptor isoforms [44]. This suggests autocrine and paracrine roles for VEGF in vascular invasion of the cartilage at the zone of hypertrophy, as well as regulation of chondrocyte apoptosis [7, 18, 44, 45]. Supernatants from hypertrophic chondrocytes act powerfully to promote endothelial cell migration and invasion, showing that chondrocyte derived VEGF is viable in promoting angiogenesis in animal models [45]. Prior to assuming a hypertrophic phenotype during endochondral bone formation, the chondrocytes are actively dividing and assume a typical chondrocytic phenotype. The expression of VEGF by these cells coincides with terminal differentiation, progression to the hypertrophic phenotype, and a characteristic gene expression profile. The expression profile of hypertrophic chondrocytes includes an increase in alkaline phosphatase and collagen type-X expression, with decreased expression of collagen types II and IX as compared to proliferating chondrocytes [7]. Because chondrocytes express VEGFR-2, it suggests that VEGF acts in an autocrine fashion to promote the switch from a chondrocytic gene expression program to a more osteogenic program, and may regulate the apoptosis necessary for vascular invasion [45]. In support of this idea, the inhibition of angiogenesis at the growth plate with antibodies directed against VEGF delays chondrocyte death leading to increased numbers of hypertrophic chondrocytes and thus expansion of the growth plate [7]. Additionally, Matsumoto et al. used muscle derived stem cells that either over-expressed VEGF, or sFlt-1 (VEGF antagonist) to analyze apoptosis of chondrocytes and progression of osteoarthritis in a mouse model [46]. The authors found that inhibition of VEGF decreased articular chondrocyte apoptosis by TUNEL assay while improving macroscopic and cellular repair processes [46]. VEGF is known to support chondrocyte survival during development [47], but it is inhibition of VEGF in the epiphysis of the postnatal period that leads to increased chondrocyte survival. Inactivation of HIF-1α, leads to massive chondrocyte death, therefore apoptosis of hypertrophic chondrocytes is indirectly promoted by VEGF through its effect on vascular invasion, subsequent increased oxygen tension, and resultant decreased levels of active HIF-1α [47]. It should be noted that VEGF and the VEGFR are not normally found quiescent or physiologically proliferating chondrocytes, but rather limited to the epiphysis and sites of cartilage repair, such and endochondral bone formation [45, 48]. In summary, VEGF expression by chondrocytes and binding of the VEGFR during endochondral bone formation is essential for rudimentary bone matrix formation, hypertrophic chondrocyte apoptosis, and vascular penetration with pre-osteoblast and pre-osteoclast cell delivery to the site of bone formation (Fig. 3).
Fig. 3.
VEGF expression by hypertrophic chondrocytes in the epiphyseal plate, a model for endochondral bone formation. Hypertrophic chondrocytes release VEGF in an autocrine and paracrine fashion, acting on endothelial cells, chondroclasts, and osteoblasts to promote apoptosis of hypertrophic chondrocytes, blood vessel ingrowth, and woven bone formation. mFlt(1–3)-IgG is the murine derived soluble VEGF receptor used to sequester VEGF in the studies performed by Harper and Kalgsbrun (Nature Med 1999) leading to delayed apoptosis of hypertrophic chondrocytes, decreased vessel ingrowth, and decreased trabecular bone formation. (Reprinted from Trends in Cardiovascular Medicine, Volume 10(5), Gerber HP and Ferrara N, Angiogenesis and Bone Growth, pages 223–228, Copyright 2000, with permission from Elsevier.)
Remodeling phase
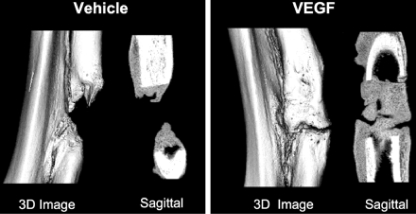
Surrounding the site of endochondral ossification an extensive callus has formed supporting the structurally weak cartilage intermediate, while also delivering periosteal blood vessels and MSCs to the fracture site. BMPs are expressed in fracture callus and are bound to the underlying extracellular matrix along with VEGF [49]. Both VEGF and BMPs increase the differentiation and metabolism of preosteoblasts, and BMP-4 is known to work synergistically with VEGF in promoting bone formation [15]. Bone formation must be paired with bone resorption and remodeling in order to convert woven bone into strong lamellar bone. VEGF increases the recruitment, survival and activity of osteoclasts, which are responsible for bone resorption and remodeling [35]. In 2002, Street et al. studied the effects of VEGF on bone repair and turnover in a mouse tibia fracture model. They found that when using a soluble VEGFR to sequester VEGF, calcified callus volumes were significantly reduced at all time points. Also, the amount of remodeling in the VEGF inhibited mice was decreased compared to controls. Finally, the authors found that VEGF inhibition decreases callus vascularity compared to untreated controls. As expected, treatment with excess VEGF increased mineral density in calcified callus and vascularity compared to controls (Fig. 4) [35]. When VEGF is inhibited in mouse femur fracture sites, there is also a lack of lamellar bone remodeling and osteoclastic cutting cones in the hard callus, highlighting the importance of VEGF during bone remodeling for both endochrondral and intramembranous ossification [15]. Recombinant human VEGF (rhVEGF) has been used in studies of experimental non-union animal models to evaluate whether local administration of the growth factor would promote union as compared to animals injected with the vehicle solution alone. Eckardt et al. showed that treating rabbit tibial non-union with rhVEGF increased the values for tibial failure torque, failure angle, stiffness, and cross-sectional area of callus as compared to controls [34]. Additionally, all of the rhVEGF treated tibiae went on to union as compared to only half of the control tibiae.
Fig. 4.
Local application of VEGF promotes healing of critical defects created in rabbit radii. Three-dimensional rendering μCT images of critical defects created in rabbit radii treated with either polylactic acid depot (PLAD) vehicle alone or PLAD+250 μg VEGF continuously released for 7 d after surgery, resulting in 91% increase (P = 0.02) in total callus volume and a 95% increase (P = 0.02) in calcified callus volume in the VEGF treated group. (Reproduced with permission from Endocrine Reviews, Vascular Endothelial Growth Factor: Basic Science and Clinical Progress, 25 (4) 2004: 581–611; Copyright 2004, The Endocrine Society.)
Therapeutic manipulations that inhibit angiogenesis
While some mechanical and pharmacologic interventions my enhance angiogenesis and promote fracture healing, others can inhibit these processes and lead to delayed or incomplete fracture healing. Impaired angiogenesis during fracture healing may lead to higher rates of infection, decreased healing, and eventual non-union [50].
Non-steroidal anti-inflammatory drugs
One of the most widely used class of drugs, especially in the orthopaedic setting, are the non-steroidal anti-inflammatory drugs (NSAIDs). While these drugs decrease pain and inflammation, they have also been implicated in delayed fracture healing and development of drug-induced non-union. NSAIDs inhibit cyclooxygenase (COX) mediated prostaglandin formation, specifically PGE2, which is produced by osteoblasts and is known to promote bone formation [51]. Murnaghan et al. evaluated whether or not NSAID-induced non-union was due to decreased vascular supply to the healing bone. The authors found that the mice treated with the selective COX2 inhibitor rofecoxib had decreased callus, increased cartilage, and increased fibrous tissue around the mid femoral diaphyseal fracture site [51]. Laser Doppler flowmetry was employed to assess the blood flow across the fracture gap in the mice and the authors showed that the NSAID treated group had significantly decreased blood flow at the fracture site at all time points. This suggested that the process of endochondral bone formation, which requires vessel ingrowth, was inhibited. Recently, van der Heide et al. reported that indomethacin (non-specific NSAID) and meloxicam (COX2 specific) did not result in a statistically significant difference in bony growth into a bone conduction chamber when placed into the tibia of a goat model [52]. Consequently, these authors suggested that NSAIDs may not adversely affect bone formation. While this may be true for bone ingrowth into a bone conduction chamber, these results should be considered with caution when generalized to all settings of bone formation. The bone conduction chamber is a model for membranous bone formation, but as stated previously, most fractures heal via endochondral bone formation. Additionally, numerous authors have found that NSAIDs, both in vivo and in vitro decrease new bone formation, delay fracture healing, and inhibit spinal fusion.(Table 2) [53–56].
Table 2.
Some drugs known to impair fracture healing
| Chemical | Inhibitor of Bone Formation | Inhibitor of Angiogenesis | Mechanism of Impaired Fracture Healing | Reference |
|---|---|---|---|---|
| Alendronate [57, 58] | + | + | Inhibits endothelial cell migration and capillary formation. Also known to inhibit spine fusion. | Hashimoto, 2007; Huang, 2005 |
| Dexamethasone [59, 60] | + | + | Inhibition of VEGF expression ingrowth plate chondrocytes. Also known to decrease bone graft incorporation in lumbar spine fusion. | Koedam, 2002; Sawin, 2001 |
| Nicotine [61] | + | 100% non-union rate in rabbits treated with systemic nicotine undergoing single level lumbar fusion. | Silcox, 1995 | |
| Thiazolidinediones [62] | + | PPAR-gamma agonists used in the treatment of diabetes causes decreased bone formation and decreased bone mass in rodent models. | Grey, 2008 | |
| Cytostatic Drugs [63] | + | + | These drugs, (e.g. Cyclophosphamide) kill cells that proliferate quickly such as the endothelial cells, fibroblasts, osteoblasts, and chondrocytes that are mitotically active during the fracture healing process. | Aspenberg, 2005 |
| Fluoroquinolones [63] | + | Adverse affects on growing cartilage, inclulding disruption of the cartilage callus. | Aspenberg, 2005 | |
| Enoxaparin (low molecular weight heparin) [38] | + | Dose-dependent decrease in osteoid area and alkaline phosphatase activity in osteoblasts. | Street, 2000 |
Soft tissue stripping during surgery
Certain interventions during orthopedic surgery have also been implicated in incomplete fracture healing and decreasing angiogenesis at the fracture site. Large, open, comminuted fracture such as the Gustilo-Anderson type IIIb tibial fractures are associated with intense soft tissue injury along with periosteal stripping [64]. As mentioned previously, the periosteum is an important source of osteoprogenitor cells and periosteal blood vessels that grow into the fracture defect and help to restore circulation. When the periosteum is stripped either surgically or traumatically, undoubtedly there is removal of the positive trophic factors this tissue provides. The effect of periosteal stripping on segmental fracture healing has been evaluated in a rat model [65]. Compared to rats that retained the periosteum around the osteotomy site, the stripped rats showed delayed fracture healing, reduced mechanical properties of the bone at 12 weeks post fracture, and delayed normalization of blood flow around the fracture defect. Upon biomechanical testing, the femora of the intact periosteum group had significantly larger bending moment and bending rigidity as compared to the periosteal stripping group [65]. The surgeon should aim to protect the periosteum and debride bony injuries judiciously as to preserve this source of stem cells and positive trophic factors for fracture healing.
High pressure pulsatile lavage
High-pressure pulsatile lavage (HPPL) irrigation, often used to debride open fractures, has also been shown to have a detrimental effect on fracture healing, particularly in the early stages [66]. Dirschl et al. found that in a rabbit medial femoral condyle osteotomy model, HPPL caused a significant decrease in new bone formation during the first two weeks of fracture healing as compared to a bulb syringe irrigated group and a non-irrigated control group. This effect was diminished after two weeks of healing as the rate of new bone formation returned to baseline for all groups, but twice as many of the HPPL treated animals developed non-union at the osteotomy site as compared to the two control groups [66]. These results should be interpreted with caution though because this was a closed intra-articular sterile fracture model and clinically HPPL is used as an effective way to debride large, open, comminuted, septic fractures. The clinician should be wary of using HPPL in a patient whose peripheral vasculature is already compromised, especially in the absence of gross wound contamination.
Summary
As early as 1763, the importance of blood vessels to bone health and repair was noted [15]. A key regulator in re-establishing blood supply to a site of fracture is the growth factor VEGF. VEGF plays a role in every step of the fracture repair cascade from being concentrated in fracture hematoma, to the final remodeling stages of fracture repair. VEGF accumulation within a fracture defect attracts osteoprogenitor cells and endothelial cells alike, creating an environment where these distinct cell types secrete trophic factors that mutually promote their proliferation and survival. This establishes a zone rich in growth factors and cells necessary for the repair process. VEGF is only one of an unknown number of factors that play an integral role in the process of angiogenesis and bone repair, but at this time the literature suggest that this is the most essential for angiogenesis and subsequent bone repair by replenishing the blood supply and population of bone forming cells.
Many studies surrounding factors that are known to increase angiogenesis and bone healing have been carried out in small animal models but only BMP-2 and -7 have made it to randomized controlled trials in humans. More clinical trials need to be conducted to evaluate the efficacy and safety of factors beyond the BMPs, especially with VEGF which has been shown many times over to be a necessary cytokine in the fracture healing process. Furthermore, because of the known positive feedback interactions between BMP and VEGF, using the two in conjunction could increase the efficacy as compared to their use in isolation.
Currently, our lack of knowledge surrounding the temporospatial relationship of growth factor expression in healing fractures makes it difficult clinically to decide when and where growth factors should be applied. There needs to be further studies, expanding on our preliminary knowledge of what factors are involved, in order to determine the order and location of expression of angiogenic and osteogenic growth factors so that these endogenous mechanisms can be mimicked and augmented. Accordingly, there needs to be further work in the development carrier vehicles so that the appropriate timing and dose of growth factor can be applied to the healing tissue.
Orthopaedic trauma will continue to be a major part of the practice of most orthopaedic surgeons. As our knowledge expands about the process of fracture healing and how to enhance it, the incidence of complications and the morbidity from mal-, non- and delayed union should be expected to decrease.
Footnotes
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Level of Evidence: N/A (Review Article).
References
- 1.Boden SD, Einhorn TA, Morgan TS, et al. An AOA critical issue. The future of the orthopaedic surgeon-proceduralist or keeper of the musculoskeletal system? J. Bone Joint Surg. Am. 2005;87(12):2812–2821. doi: 10.2106/JBJS.E.00791. [DOI] [PubMed] [Google Scholar]
- 2.Glowacki J, Angiogenesis in fracture repair. Clin. Orthop. Relat. Res. 1998; (355 Suppl): S82–S89 [DOI] [PubMed]
- 3.Brighton CT, Lorich DG, Kupcha R, et al. The pericyte as a possible osteoblast progenitor cell. Clin. Orthop. Relat. Res. 1992;275:287–299. [PubMed] [Google Scholar]
- 4.Testa U, Pannitteri G, Condorelli GL. Vascular endothelial growth factors in cardiovascular medicine. J. Cardiovasc. Med. (Hagerstown) 2008;9(12):1190–1221. doi: 10.2459/JCM.0b013e3283117d37. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 6.Josko J, Gwozdz B, Jedrzejowska-Szypulka H, et al. Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med. Sci. Monit. 2000;6(5):1047–1052. [PubMed] [Google Scholar]
- 7.Bluteau G, Julien M, Magne D, et al. VEGF and VEGF receptors are differentially expressed in chondrocytes. Bone. 2007;40(3):568–576. doi: 10.1016/j.bone.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Deckers MM, Bezooijen RL, Horst G, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143(4):1545–1553. doi: 10.1210/en.143.4.1545. [DOI] [PubMed] [Google Scholar]
- 9.Mayr-Wohlfart U, Waltenberger J, Hausser H, et al. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30(3):472–477. doi: 10.1016/S8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- 10.Wang DS, Miura M, Demura H, et al. Anabolic effects of 1,25-dihydroxyvitamin D3 on osteoblasts are enhanced by vascular endothelial growth factor produced by osteoblasts and by growth factors produced by endothelial cells. Endocrinology. 1997;138(7):2953–2962. doi: 10.1210/en.138.7.2953. [DOI] [PubMed] [Google Scholar]
- 11.Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J. Orthop. Res. 2008;26(10):1407–1412. doi: 10.1002/jor.20668. [DOI] [PubMed] [Google Scholar]
- 12.Lienau J, Schmidt-Bleek K, Peters A, et al., Differential regulation of blood vessel formation between standard and delayed bone healing. J. Orthop. Res. 2009 [DOI] [PubMed]
- 13.Sarahrudi K, Thomas A, Braunsteiner T, et al., VEGF serum concentrations in patients with long bone fractures: A comparison between impaired and normal fracture healing. J. Orthop. Res. 2009 [DOI] [PubMed]
- 14.Kawaguchi H, Kurokawa T, Hanada K, et al. Stimulation of fracture repair by recombinant human basic fibroblast growth factor in normal and streptozotocin-diabetic rats. Endocrinology. 1994;135(2):774–781. doi: 10.1210/en.135.2.774. [DOI] [PubMed] [Google Scholar]
- 15.Carano RA, Filvaroff EH. Angiogenesis and bone repair. Drug Discov. Today. 2003;8(21):980–989. doi: 10.1016/S1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- 16.Weiss S, Zimmermann G, Pufe T, et al., The systemic angiogenic response during bone healing. Arch. Orthop. Trauma Surg. 2008; 129: 989–997 [DOI] [PubMed]
- 17.Ryaby JT, Sheller MR, Levine BP, et al. Thrombin peptide TP508 stimulates cellular events leading to angiogenesis, revascularization, and repair of dermal and musculoskeletal tissues. J. Bone Joint Surg. Am. 2006;88(Suppl 3):132–139. doi: 10.2106/JBJS.F.00892. [DOI] [PubMed] [Google Scholar]
- 18.Gerber HP, Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc. Med. 2000;10(5):223–228. doi: 10.1016/S1050-1738(00)00074-8. [DOI] [PubMed] [Google Scholar]
- 19.Harada S, Nagy JA, Sullivan KA, et al. Induction of vascular endothelial growth factor expression by prostaglandin E2 and E1 in osteoblasts. J. Clin. Invest. 1994;93(6):2490–2496. doi: 10.1172/JCI117258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss S, Baumgart R, Jochum M, et al. Systemic regulation of distraction osteogenesis: a cascade of biochemical factors. J. Bone Miner. Res. 2002;17(7):1280–1289. doi: 10.1359/jbmr.2002.17.7.1280. [DOI] [PubMed] [Google Scholar]
- 21.Weiss S, Zimmermann G, Baumgart R, et al. Systemic regulation of angiogenesis and matrix degradation in bone regeneration–distraction osteogenesis compared to rigid fracture healing. Bone. 2005;37(6):781–790. doi: 10.1016/j.bone.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Manabe T, Mori S, Mashiba T, et al. Human parathyroid hormone (1–34) accelerates natural fracture healing process in the femoral osteotomy model of cynomolgus monkeys. Bone. 2007;40(6):1475–1482. doi: 10.1016/j.bone.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Holstein JH, Menger MD, Scheuer C, et al. Erythropoietin (EPO): EPO-receptor signaling improves early endochondral ossification and mechanical strength in fracture healing. Life Sci. 2007;80(10):893–900. doi: 10.1016/j.lfs.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Simpson AH, Mills L, Noble B. The role of growth factors and related agents in accelerating fracture healing. J. Bone Joint Surg. Br. 2006;88(6):701–705. doi: 10.1302/0301-620X.88B6.17524. [DOI] [PubMed] [Google Scholar]
- 25.Sena K, Sumner DR, Virdi AS. Modulation of VEGF expression in rat bone marrow stromal cells by GDF-5. Connect. Tissue Res. 2007;48(6):324–331. doi: 10.1080/03008200701692743. [DOI] [PubMed] [Google Scholar]
- 26.Reher P, Doan N, Bradnock B, et al. Effect of ultrasound on the production of IL-8, basic FGF and VEGF. Cytokine. 1999;11(6):416–423. doi: 10.1006/cyto.1998.0444. [DOI] [PubMed] [Google Scholar]
- 27.Reed AA, Joyner CJ, Brownlow HC, et al. Human atrophic fracture non-unions are not avascular. J. Orthop. Res. 2002;20(3):593–599. doi: 10.1016/S0736-0266(01)00142-5. [DOI] [PubMed] [Google Scholar]
- 28.Einhorn TA. The science of fracture healing. J. Orthop. Trauma. 2005;19(10 Suppl):S4–S6. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- 29.Phillips AM. Overview of the fracture healing cascade. Injury. 2005;36(Suppl 3):S5–S7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 30.Street J, Winter D, Wang JH, et al. Is human fracture hematoma inherently angiogenic? Clin. Orthop. Relat. Res. 2000;378:224–237. doi: 10.1097/00003086-200009000-00033. [DOI] [PubMed] [Google Scholar]
- 31.Brighton CT, Krebs AG. Oxygen tension of nonunion of fractured femurs in the rabbit. Surg. Gynecol. Obstet. 1972;135(3):379–385. [PubMed] [Google Scholar]
- 32.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992;12(12):5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komatsu DE, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34(4):680–688. doi: 10.1016/j.bone.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Eckardt H, Ding M, Lind M, et al. Recombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion model. J. Bone Joint. Surg. Br. 2005;87(10):1434–1438. doi: 10.1302/0301-620X.87B10.16226. [DOI] [PubMed] [Google Scholar]
- 35.Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. U. S. A. 2002;99(15):9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li R, Stewart DJ, Schroeder HP, et al. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. J. Orthop. Res. 2009;27(1):8–14. doi: 10.1002/jor.20658. [DOI] [PubMed] [Google Scholar]
- 37.Shen X, Wan C, Ramaswamy G, et al., Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J. Orthop. Res. 2009 [DOI] [PMC free article] [PubMed]
- 38.Street JT, McGrath M, O’Regan K, et al. Thromboprophylaxis using a low molecular weight heparin delays fracture repair. Clin. Orthop. Relat. Res. 2000;381:278–289. doi: 10.1097/00003086-200012000-00032. [DOI] [PubMed] [Google Scholar]
- 39.Tsopanoglou NE, Andriopoulou P, Maragoudakis ME. On the mechanism of thrombin-induced angiogenesis: involvement of alphavbeta3-integrin. Am. J. Physiol. Cell Physiol. 2002;283(5):C1501–C1510. doi: 10.1152/ajpcell.00162.2002. [DOI] [PubMed] [Google Scholar]
- 40.Mosesson MW. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005;3(8):1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 41.Bar-Shavit R, Eldor A, Vlodavsky I. Binding of thrombin to subendothelial extracellular matrix. Protection and expression of functional properties. J. Clin. Invest. 1989;84(4):1096–1104. doi: 10.1172/JCI114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kon T, Cho TJ, Aizawa T, et al. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner. Res. 2001;16(6):1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 43.Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J. Bone Miner. Res. 2003;18(9):1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 44.Gerber HP, Vu TH, Ryan AM, et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 45.Carlevaro MF, Cermelli S, Cancedda R, et al. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J. Cell Sci. 2000;113(Pt 1):59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto T, Cooper GM, Gharaibeh B, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60(5):1390–1405. doi: 10.1002/art.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zelzer E, Mamluk R, Ferrara N, et al. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131(9):2161–2171. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- 48.Murata M, Yudoh K, Masuko K. The potential role of vascular endothelial growth factor (VEGF) in cartilage: how the angiogenic factor could be involved in the pathogenesis of osteoarthritis? Osteoarthr. Cartil. 2008;16(3):279–286. doi: 10.1016/j.joca.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Kloen P, Doty SB, Gordon E, et al. Expression and activation of the BMP-signaling components in human fracture nonunions. J. Bone Joint Surg. Am. 2002;84-A(11):1909–1918. doi: 10.2106/00004623-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Swiontkowski MF, Aro HT, Donell S, et al. Recombinant human bone morphogenetic protein-2 in open tibial fractures. A subgroup analysis of data combined from two prospective randomized studies. J. Bone Joint Surg. Am. 2006;88(6):1258–1265. doi: 10.2106/JBJS.E.00499. [DOI] [PubMed] [Google Scholar]
- 51.Murnaghan M, Li G, Marsh DR. Nonsteroidal anti-inflammatory drug-induced fracture nonunion: an inhibition of angiogenesis? J. Bone Joint Surg. Am. 2006;88(Suppl 3):140–147. doi: 10.2106/JBJS.F.00454. [DOI] [PubMed] [Google Scholar]
- 52.Heide HJ, Hannink G, Buma P, et al. No effect of ketoprofen and meloxicam on bone graft ingrowth: a bone chamber study in goats. Acta Orthop. 2008;79(4):548–554. doi: 10.1080/17453670710015562. [DOI] [PubMed] [Google Scholar]
- 53.Goodman S, Ma T, Trindade M, et al. COX-2 selective NSAID decreases bone ingrowth in vivo. J. Orthop. Res. 2002;20(6):1164–1169. doi: 10.1016/S0736-0266(02)00079-7. [DOI] [PubMed] [Google Scholar]
- 54.Simon AM, O’Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J. Bone Joint Surg. Am. 2007;89(3):500–511. doi: 10.2106/JBJS.F.00127. [DOI] [PubMed] [Google Scholar]
- 55.Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J. Bone Joint Surg. Br. 2003;85(5):700–705. [PubMed] [Google Scholar]
- 56.Glassman SD, Rose SM, Dimar JR, et al. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1998;23(7):834–838. doi: 10.1097/00007632-199804010-00020. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto K, Morishige K, Sawada K, et al. Alendronate suppresses tumor angiogenesis by inhibiting Rho activation of endothelial cells. Biochem. Biophys. Res. Commun. 2007;354(2):478–484. doi: 10.1016/j.bbrc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 58.Huang RC, Khan SN, Sandhu HS, et al. Alendronate inhibits spine fusion in a rat model. Spine. 2005;30(22):2516–2522. doi: 10.1097/01.brs.0000186470.28070.7b. [DOI] [PubMed] [Google Scholar]
- 59.Koedam JA, Smink JJ, Buul-Offers SC. Glucocorticoids inhibit vascular endothelial growth factor expression in growth plate chondrocytes. Mol. Cell Endocrinol. 2002;197(1–2):35–44. doi: 10.1016/S0303-7207(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 60.Sawin PD, Dickman CA, Crawford NR, et al. The effects of dexamethasone on bone fusion in an experimental model of posterolateral lumbar spinal arthrodesis. J. Neurosurg. 2001;94(1 Suppl):76–81. doi: 10.3171/spi.2001.94.1.0076. [DOI] [PubMed] [Google Scholar]
- 61.Silcox DH, 3rd, Daftari T, Boden SD, et al. The effect of nicotine on spinal fusion. Spine. 1995;20(14):1549–1553. doi: 10.1097/00007632-199507150-00001. [DOI] [PubMed] [Google Scholar]
- 62.Grey A. Skeletal consequences of thiazolidinedione therapy. Osteoporos. Int. 2008;19(2):129–137. doi: 10.1007/s00198-007-0477-y. [DOI] [PubMed] [Google Scholar]
- 63.Aspenberg P. Drugs and fracture repair. Acta Orthop. 2005;76(6):741–748. doi: 10.1080/17453670510045318. [DOI] [PubMed] [Google Scholar]
- 64.Bartlett CS, 3rd, Weiner LS, Yang EC. Treatment of type II and type III open tibia fractures in children. J. Orthop. Trauma. 1997;11(5):357–362. doi: 10.1097/00005131-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 65.Utvag SE, Grundnes O, Reikeraos O. Effects of periosteal stripping on healing of segmental fractures in rats. J. Orthop. Trauma. 1996;10(4):279–284. doi: 10.1097/00005131-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 66.Dirschl DR, Duff GP, Dahners LE, et al. High pressure pulsatile lavage irrigation of intraarticular fractures: effects on fracture healing. J. Orthop. Trauma. 1998;12(7):460–463. doi: 10.1097/00005131-199809000-00005. [DOI] [PubMed] [Google Scholar]