The 50th anniversary of the publication of Hans-Christoph Lüttgau and Rolf Niedergerke's seminal 1958 paper recently passed. This ‘citation classic’ (cited 551 times to date), is generally acknowledged when the story of cellular Na–Ca exchange is related. Anniversaries can prompt a re-evaluation of original papers, but their context is often neglected; this celebration of the work mainly focuses there.
Lüttgau and Niedergerke's declared aim (p. 486) was to ‘obtain information on [the] antagonism between Na and Ca, in the hope of finding thereby some clue to the mode of activation of the contractile system’ (i.e. the process now termed excitation–contraction (EC) coupling). They analysed a perplexing property of cardiac muscle known since Sydney Ringer's observations: extracellular Ca ions (Cao) promote contractility but the effect is enhanced if [Nao] is reduced. The phenomenon had first been quantified ten years earlier by Wilbrandt & Koller (1948) who showed the contractile equivalence of various conditions of [Ca]o and [Na]o, provided the quotient [Ca]o/[Na]o2 remained constant.
Wilbrandt and Koller's approach was dominated by the then still novel membrane potential paradigm for understanding cell function. Ions were assumed to act at the cell membrane, considered Ca impermeant, with binding and local accumulation taking place according to Donnan equilibria. The ionic milieu thus modified the surface potential which, in turn, influenced the transmembrane potential (Em), seen as the factor controlling muscle activity (in some unspecified way). By 1958, ideas had moved considerably. The Hodgkin–Huxley–Katz model for Em and excitability was established, but radioactive tracer studies had begun revealing non-negligible fluxes of several cations, including Ca. Furthermore, Niedergerke, himself then recently a co-discoverer of the sliding filament mechanism, had extended earlier work showing that direct injection of Ca ions into (skeletal) muscle leads to activation. However, another dominant concept was A. V. Hill's diffusion-time analysis with an interpretation militating against a ‘surface originating’ activator in muscle (for large skeletal fibres at least). Against this, in 1958 several planks of our present understanding of EC coupling were still absent: (1) the regulatory role of Ca on the myofilaments, (2) Ca influx during the cardiac action potential, and (3) the sarcoplasmic reticulum as an intracellular Ca store. Furthermore, knowledge of the ATP-driven, plasmalemmal Na–K exchange pump was in its infancy.
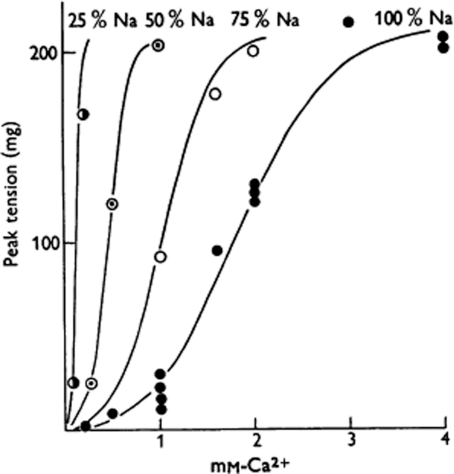
German-born Hans-Christoph Lüttgau arrived in 1956 at University College London (where Ringer had worked) from Bern, Switzerland (where Wilbrandt and Koller had worked). Bernard Katz suggested research with Niedergerke, whom Lüttgau knew from studying in Göttingen. The Journal of Physiology paper appeared after a prior summary in Nature (1957). The latter was published in the same issue as one by Niedergerke & Harris (1957) that reported Ca fluxes in frog heart under similar [Na]o and [Ca]o variation, noting that reduced [Na]o leads to Ca accumulation resulting from both reduced Ca loss and increased uptake. Lüttgau and Niedergerke made a detailed study of isometric twitch (Fig. 1) and contracture tension in frog ventricle strips. The antagonistic specificity for Ca and Na over other ions was revealed. By quantifying the influence of membrane potential (controlled by varying [K]o), they extended the scope for understanding Ca–Na antagonism. Results were successfully modelled by assuming a competition between Ca and Na for ‘a negatively charged substance’, accessible to the external medium, only the Ca-bound form of which activates contraction.
Figure 1. Effect of diminished NaCl on twitch tension.
Maximum twitch tension of a heart strip equilibrated with various compositions of Ringer's fluid plotted against Ca2+ concentrations at four different NaCl concentrations (NaCl of Ringer's fluid is replaced by osmotically equivalent sucrose). ‘Reference curve’ at 100% NaCl is drawn empirically; curves at 75, 50 and 25% NaCl have been constructed from the ‘reference curve’, assuming peak tensions to be constant at given values of the [Ca2+]:[Na+]2 ratio. Frequency of stimulation 2/min; diameter of strip 0.35 mm. (Figure and legend from Lüttgau & Niedergerke, 1958.)
Alan Hodgkin is amongst those acknowledged in the Journal of Physiology paper. In fact, he had refereed it, but broke conventional anonymity to exchange letters with the authors. Hodgkin developed a descriptive algebraic model for the findings that linked membrane potential and the [Ca]o/[Na]o2 quotient (i.e. the results of Figs 8 and 9). But he also suggested a mechanism: ‘assume a store of CaR (an activator) in a ‘third space’ which is at the same potential as the external solution; assume CaR has one negative charge’ (A. L. Hodgkin, unpublished letter of April 1958 kindly provided by H.-Ch. Lüttgau). The model is then developed along lines described in Lüttgau & Niedergerke (1958) but confers the observed voltage sensitivity. Hodgkin's modelling and the ‘third space’ notion retain contemporary resonance. Lüttgau (1965) subsequently reported more details at a symposium in Milan (in 1963).
As to the cellular location of the antagonism, Lüttgau & Niedergerke (1958, p. 501) state: ‘It may be premature, however, to conclude that Ca and Na ions act entirely at the cell membrane. It is possible, for example, that part of the rapidly exchanging amount of cellular Ca, and also Na, is located in some cellular structures or channels which connect the fibre surface to the interior, as suggested … for the endoplasmic reticulum.’ The first inklings of the possible role of active Ca extrusion (i.e. a sarcolemmal Ca pump) were also acknowledged.
With hindsight, the attraction of studying EC coupling in cardiac rather than skeletal muscle perhaps proved unfortunate. Cardiac contraction might be highly manipulable by simply altering the bathing medium, but, in 1958, this beguiling property still hid the need to disentangle a whole array of complexities, each destined to become major research fields themselves. This worked against finding a quick, general answer to the question of how excitation triggers contraction.
The basic components for a Ca–Na exchange mechanism had nevertheless been assembled, though it fell to others (notably including Harald Reuter, Lüttgau's cousin, also to be based in Bern, see e.g. Reuter, 1974) to assemble them more explicitly. Rather neglected in the subsequent efforts to characterise Ca–Na exchange was that Ca–Na antagonism had always been related to contraction. Interest increasingly focused on the emerging role of the Ca current and later, of Ca–Na exchange both as influenced by Em and, through its electrogenicity and especially net current generation during the cardiac action potential, an influence upon Em itself.
A subsequent important step for Ca–Na antagonism was demonstrating its applicability in mammalian heart. Studies by Melchior Reiter and later by Reg Chapman, another former collaborator of Niedergerke's, thoroughly confirmed the generality of the phenomenon.
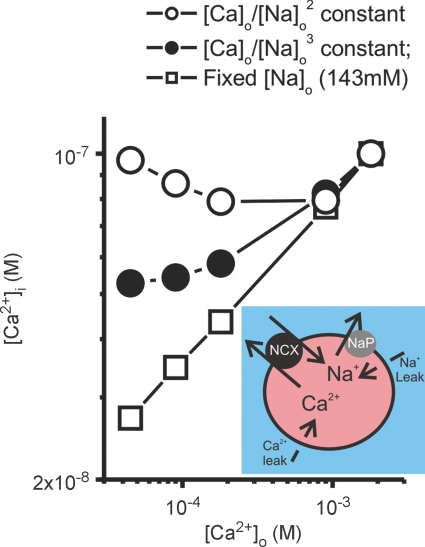
The core of Ca–Na antagonism is that tension observations can all be made to fall on a single curve when either peak twitch (Fig. 1) or contracture force is plotted against [Ca]o/[Na]o2. The shape of the curve itself was empirical, though later work showed it could be defined explicitly (Miller & Moisescu, 1976). There remain various loose ends, not least that the current paradigm of an exchanger translocating 3Na+ per Ca2+ does not immediately seem to explain the 2-to-1 antagonism observed for contraction. We have modelled the situation, utilising the description of Ca–Na exchange developed by Weber et al. (2001) which includes 3:1 stoichiometry for Na+:Ca2+ and allosteric regulation of the exchanger by intracellular Ca2+. In this simple model of the quiescent heart cell, the exchanger extrudes Ca2+ to counter its leakage into the cell. [Na+]i is dictated by influx via the Ca–Na exchanger plus a larger Na+ leak balanced by the Na+ pump (see inset in Fig. 2). Our calculations reveal (Fig. 2) that [Ca2+]i remains relatively constant over a range of [Ca]o, provided the quotient [Ca]o/[Na]o2 is kept constant. This would translate, under equivalent types of activation, to approximately the same tension being generated at each [Ca]o. By contrast, keeping [Ca]o/[Na]o3 constant fails to achieve this result, simply because the compensatory changes of [Na]o are insufficient. Thus, the earlier studies indicating a functional ‘stoichiometry’ of 2:1 ([Na]o:[Ca]o) can now be explained by a ionic/molecular stoichiometry of 3:1 for the exchanger working alongside the other processes involved.
Figure 2. The effects of extracellular Ca2+ concentration ([Ca2+]o) on intracellular [Ca2+] concentration ([Ca2+]i) in a simplified model of a quiescent cardiac muscle cell (see inset).
The intracellular concentrations of both Na and Ca ions is the result of a balance between influx and active efflux. For Ca2+ the main route for efflux is via the Ca–Na exchanger (NCX). For Na+, the magnitude of the Na+ leak was set at 10 times that of NCX-mediated Na+ influx. Both influx pathways are balanced by a Na+ pump (NaP). The data describe this relationship under the following conditions: with no adjustment of [Na+]o; with adjustment based on a constant [Ca2+]o/[Na+]o3; or [Ca2+]o/[Na+]o2. (For further details of the model, see Weber et al. 2001.)
Celebration of Lüttgau and Niedergke's paper is proper for, beyond its significance for cardiac EC coupling, it was the catalyst for Ca–Na exchange studies that revealed a fundamental cellular process, not one unique to the heart.
References
- Lüttgau HCh. Electrophysiology of the Heart. Oxford: Pergamon Press; 1965. pp. 87–95. 1963 Milan. [Google Scholar]
- Lüttgau HC, Niedergerke R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958;143:486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Moisescu DG. The effects of very low external calcium and sodium concentrations on cardiac contractile strength and calcium–sodium antagonism. J Physiol. 1976;259:283–308. doi: 10.1113/jphysiol.1976.sp011466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergerke R, Harris EJ. Accumulation of calcium (or strontium) under conditions of increasing contractility. Nature. 1957;179:1068–1069. doi: 10.1038/1791068a0. [DOI] [PubMed] [Google Scholar]
- Niedergerke R, Lüttgau HC. Antagonism between calcium and sodium ions. Nature. 1957;179:1066–1067. doi: 10.1038/1791066a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Exchange of calcium ions in the mammalian myocardium. Mechanisms and physiological significance. Circ Res. 1974;34:599–605. doi: 10.1161/01.res.34.5.599. [DOI] [PubMed] [Google Scholar]
- Weber CR, Ginsburg KS, Philipson KD, Shannon TR, Bers DM. Allosteric regulation of Na/Ca exchange current by cytosolic Ca in intact cardiac myocytes. J Gen Physiol. 2001;117:119–131. doi: 10.1085/jgp.117.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbrandt W, Koller H. DieCalciumwirkung am Froschherzen als Funktion des Ionengleichgewichts zwischen Zellmembran und Umgebung. Helv Physiol Pharm Acta. 1948;6:208–221. [PubMed] [Google Scholar]