Abstract
This article reviews especially the early history of glutamate and GABA as neurotransmitters in vertebrates. The proposal that some amino acids could mediate synaptic transmission in the CNS initially met with much resistance. Both GABA and its parent glutamate are abundant in the brain; but, unlike glutamate, GABA had no obvious metabolic function. By the late 1950s, the switch of interest from electrical to chemical transmission invigorated the search for central transmitters. Its identification with Factor I, a brain extract that inhibited crustacean muscle, focused interest on GABA as a possible inhibitory transmitter. In the first microiontophoretic tests, though GABA strongly inhibited spinal neurons, these effects were considered ‘non-specific’. Strong excitation by glutamate (and other acidic amino acids) led to the same conclusion. However, their great potency and rapid actions on cortical neurons convinced other authors that these endogenous amino acids are probably synaptic transmitters. This was partly confirmed by showing that both IPSPs and GABA greatly increased Cl− conductance, their effects having similar reversal potentials. Many anticonvulsants proving to be GABA antagonists, by the 1970s GABA became widely accepted as a mediator of IPSPs. Progress was much slower for glutamate. Being generated on distant dendrites, EPSPs could not be easily compared with glutamate-induced excitation, and the search for specific antagonists was long hampered by the lack of blockers and the variety of glutamate receptors. These difficulties were gradually overcome by the application of powerful techniques, such as single channel recording, cloning receptors, as well as new pharmacological tools.

Krešimir Krnjević is Emeritus professor of Physiology, at McGill University, Montreal. Born in Zagreb, he obtained his MBChB at the University of Edinburgh; went on to a PhD in Physiology (under D. Whitteridge). After two post docs in Seattle and Canberra (the latter with J. C. Eccles) he returned to the UK, to spend 5 years at Babraham (under J. H. Gaddum). He has been at McGill since 1964, where he was head of Anaesthesia Research until retirement in 2000, as well as chairman of the Physiology department for 10 years (1978–1987). For pioneering research on brain neurotransmitters, he received a Gairdner International award and the Wilder Penfield prize (Quebec). He is a fellow of the Royal Society of Canada, a member of the Croatian Academy of a Arts and Sciences, as well as an officer of the Order of Canada.
‘we have no adequate idea how one nerve cell provokes or restrains the activity of another’
(W. D. M. Paton, 1959)
Introduction
In several ways, amino acids are primordial units of structure and function in all living matter. First and foremost, chains of amino acids make up the proteins from which tissues are constructed and enzymes developed to operate the intricate biochemical pathways that acquire and dispense energy, the essential characteristic of living organisms. A half-century ago, when neuroscientists began to accept the idea that chemical transmitter might mediate the transfer of information between nerve cells at synapses, no one seriously thought that amino acids were the likely agents. According to the general opinion, like peripheral junctions, central synapses probably utilize unusual molecules, specialized for this function, and not much good for anything else: indeed, very likely the same transmitters (acetylcholine and monoamines) as at nerve–muscle and ganglionic junctions. This mind-set governed the direction of much of the thinking and research for several decades (Paton, 1959). Hence, the question, when and why were amino acids first proposed as neurotransmitters?
Early history
Now we know that both glutamate and its close derivative GABA (readily obtained from glutamate by α-decarboxylation) are chemical signals at various peripheral and central sites throughout the metazoan series (Gerschenfeld, 1973; Majewska, 2007). For example, in hydra (coelenterates) they modulate pacemaker activity (Kass-Simon et al. 2003); in sea-fans. GABA mediates nematocyst discharge (Girosi et al. 2007). Even in protozoa, in the amoeba Dictyostelium, glutamate and GABA compete at a GABAB-like receptor in the process of sporulation (Anjard & Loomis, 2006); and GABA may induce bacterial spore germination (Foerster & Foerster, 1973). Both glutamate and GABA may well be signalling molecules in plants (Forde & Lea, 2007): indeed, GABA was identified in potato tubers (Dent et al. 1947) before it was discovered in the mammalian brain. The compelling evidence for GABA- and glutamate-mediated inhibition and excitation, respectively, at the crustacean neuromuscular junction, thoroughly reviewed by Gerschenfeld (1973), will not be further discussed here.
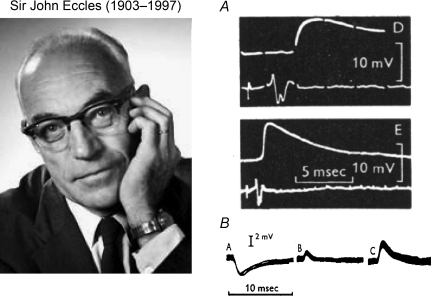
Most of the research on central transmitters in vertebrates was prompted by some key events of the 1950s. One was the discovery in 1950 (Awapara et al. 1950, and two other groups) that the brain contains large amounts of GABA, an unusual, omega amino acid of no known function. Another, quite unconnected, was J. C. Eccles’ conversion to the idea that central synapses operate not electrically but chemically (Eccles, 1953). Until that time, most neurophysiologists believed that rapid communication between nerve cells could only be mediated by fast electrical currents (Fulton, 1949). Wasn't transmission most easily and cleanly explained if the axon's electrical signal simply jumped across the tiny gap at the synapse (no need to postulate a messy chemical process…)? The electrical hypothesis had for many years been vehemently promoted by Eccles. But now, on the basis of his pioneering microelectrode recordings in the spinal cord, he became even more outspoken in favour of chemical transmission: the discrepancy between the observed large, prolonged synaptic potentials and the minute fields generated by presynaptic spikes clearly was not compatible with simple electrical transmission (Fig. 1).
Figure 1. Pioneering recordings by Eccles and collaborators of synaptic potentials evoked in spinal cord by afferent stimulation.
A, EPSP recorded inside motoneuron and presynaptic field potential, both evoked by afferent volley in biceps semitendinosus (BSt) nerve (at higher sweep speed in panel labelled with small D; note, millisecond gaps in traces) (from Fig. 9 in Brock et al. 1952). B, IPSPs, recorded through double-barrelled KCl microelectrode, generated in a BSt motoneuron by Ia afferent volley from antagonist muscle (quadriceps). Traces (small A–C) show progressive reversal of IPSP as Cl− diffuses from microelectrode, though resting potential remained at −59 mV (part of Fig. 3 in Coombs et al. 1955).
One question then arose. Could the very different synaptic actions – excitation and inhibition, respectively manifested by membrane depolarization and hyperpolarization – be mediated by a single chemical transmitter, binding to receptors having opposite effects on postsynaptic excitability? Such a scheme, in some ways analogous to electrical transmission, would simplify transmitter chemistry; but it would require specific targeting of the presynaptic axon to the correct receptor (no simple task, especially in the complex circuitry of the CNS). At any rate, this solution has not been generally adopted. Broadly speaking, fast excitation is mediated by glutamate-induced Na+ and Ca2+ influx, and inhibition by GABA- or glycine-induced Cl− influx. There must have been some advantage in having separate agents, released by different cells: probably greater flexibility in circuit design and operation, especially of the inhibitory systems, which are notoriously subject to modulation by endogenous factors or by drugs.
Eccles’ conversion to the chemical hypothesis was soon reinforced by his discovery that acetylcholine (ACh), released by intraspinal collateral branches of cholinergic motor axons, excites inhibitory Renshaw cells (Eccles et al. 1954). But pharmacological tests showed that ACh could not be the mediator of the prominent excitatory and inhibitory synaptic actions recorded in most spinal neurons. The transmitter(s) had to be some other agent(s).
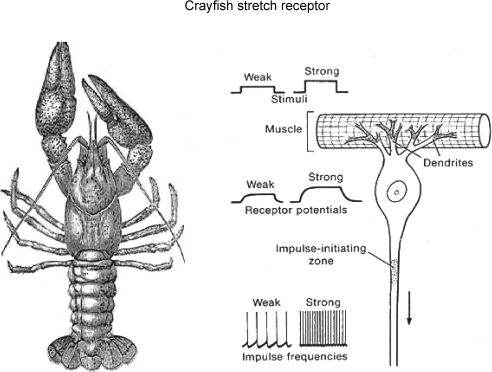
Two crucial findings pioneered the shift of synaptic studies towards amino acids. In both, Ernst Florey (Fig. 2), a young Austrian scientist, played a key role. As a student in Graz, he had been taught by Umrath, who had worked under Biedermann, the discoverer of inhibitory nerves to crustacean muscle (Biedermann, 1887). This laid the foundation of Florey's life-long interest in the inhibitory mechanisms in crustacea (as vividly described in Florey, 1991). The first was the result of Florey's visit, as Fulbright Fellow, to C. A. Wiersma's laboratory in California. The California Institute of Technology (CalTech) lab was studying the properties of the recently discovered stretch receptor neuron of crayfish – on which Florey proceeded to test extracts of mammalian CNS. He thus found that extracts of cortex and spinal cord (but not peripheral nerves) contained an unknown inhibitory agent (‘Factor I’) that suppressed firing of the stretch receptor neuron (Florey, 1954). Being highly sensitive to various chemicals, the crayfish stretch receptor (Fig. 3) was ‘..an ideal test object… Since the nerve impulses are nearly perfectly rhythmical, even the slightest changes in their frequencies can be detected easily.’ (as described by Florey, 1954). It undoubtedly played an essential role in the early studies on GABA and its identification. At any rate, having an action so similar to that of inhibitory nerves in crustacea, Factor I might well be the chemical released by these nerves; but its identity was an open question (Florey also found an excitatory Factor E in his extracts, whose identity was never elucidated).
Figure 2.
Ernst Florey, the Austrian zoologist, whose discovery of ‘Factor I’ in mammalian brain (Florey, 1954) opened the road towards GABA and other amino acid neurotransmitters.
Figure 3.
On the left, sketch of crayfish (Astacus) (Fig. 10, Ritchie, 1944, by permission of Oxford University Press); right, diagrammatic representation of stretch receptor and increased firing of afferent axon as receptor muscle is stretched (from Fig. 7-2, in Eckert, 1988). Owing to its great sensitivity to Factor I, this crayfish preparation was a major tool in the identification of GABA as a/the major transmitter at inhibitory synapses in the brain.
When Florey came again to North America, to work at the Montreal Neurological Institute, he continued his investigations of Factor I in K. A. C. Elliott's Neurochemistry laboratory, a leading centre of research on brain chemistry (Fig. 4). After testing numerous brain chemicals on the crayfish stretch receptor, Bazemore et al. (1956) concluded that GABA fully accounted for the inhibitory activity of purified Factor I. By a curious twist of fate, Florey almost immediately rejected the idea that GABA was the synaptic inhibitor: at least partly because GABA was reported to be absent in chromatograms of Factor I (McLennan, 1958) and because GABA and Factor I differed in some of their effects on intestinal muscle (Florey & McLennan, 1959). In spite of all the later developments, he never fully accepted GABA as the inhibitory transmitter, especially in crustacea (Florey, 1991). Nevertheless, it must be remembered that Florey's pioneering studies did set the stage for all the subsequent research on signalling by amino acids.
Figure 4.
Allan Elliott, head of the research group at the Montreal Neurological Institute which first showed that endogenous GABA could account for the inhibitory action of Factor I (Bazemore et al. 1956).
Until the end of the 1950s, further progress was stalled by several, apparently negative, findings. It was clear that the EPSPs or IPSPs recorded in the spinal cord were not mediated by ACh or monoamines, the well-known peripheral transmitters. But topical applications of GABA to the brain yielded only inconclusive results (Iwama & Jasper, 1957; Purpura et al. 1957). Much more suggestive were the effects produced by microiontophoretic applications, sharply localized to only a few cells (Fig. 5). By passing known currents through a micropipette containing a solution of a given agent, small amounts can be released in a controlled manner by an electric current (the main drawback is that, though the amount of substance released is fairly well known, its concentration at the site of action is a steep function of the effective diffusion distance, a variable that cannot be readily controlled). Using multibarrelled pipettes – from which several compounds could be released – David Curtis and colleagues in Eccles’ laboratory systematically tested GABA and related amino acids in the spinal cord (Curtis et al. 1959).
Figure 5. Diagrammatic representation of microiontophoretic electrodes.
A, multibarrelled for extracellular recording. B, twin electrodes for combined intracellular recording and external iontophoresis (Figs 3 and 7 from Krnjević, 1971a). This technique was a major advance over systemic or topical applications: by releasing different agents from separate barrels, their effects on individual nerve cells could be compared directly. With careful positioning of the electrode tip close to a cell, release by very short current pulses approached the kinetics of synaptic transmission. Significant pitfalls include current artifacts (iontophoretic current directly changing neuronal excitability), possible indirect effects mediated by neighbouring cells, and ongoing leakage of active agents, especially from larger tips – most of which can be controlled. Though not strictly quantitative, the delivery of agents should be linearly related to the iontophoretic current, and approximately calculable if the relevant transport number is known.
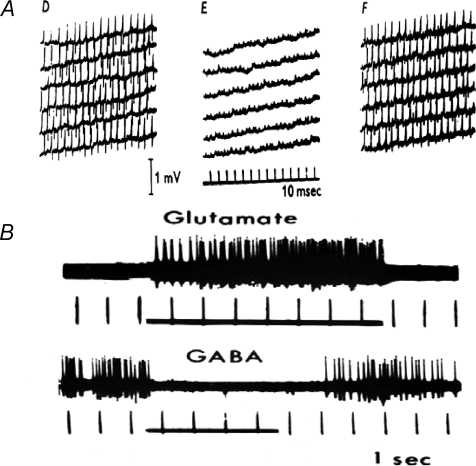
Their main finding was that GABA and β-alanine (another omega amino acid) consistently inhibited all types of spinal neurons (Curtis et al. 1959) (Fig. 6A). However, for several reasons – that GABA and β-alanine had similar effects; that they were not blocked by strychnine and not associated with hyperpolarization – the authors dismissed their actions as ‘non-specific’. The same team went on to test GABA's parent molecule, l-glutamate – as well as some other acidic amino acids, such as aspartate and cysteate – also on spinal neurons (Curtis et al. 1960). All were powerful excitants; but the authors again concluded that these were non-specific actions, mainly because blockers of enzymes that catabolize these amino acids did not alter their potency or time course of action (as the authors expected, by analogy with cholinergic transmission, which is normally curtailed by cholinesterase-mediated hydrolysis of acetylcholine – they did not take into consideration amino acid removal by cellular uptake, already reported by Stern et al. 1949).
Figure 6. Early iontophoretic tests of inhibitory amino acids.
A, spikes evoked by steady application of glutamic acid to a dorsal horn cell by an anionic current (50 nA). During ‘E’, β-alanine was also applied by current (80 nA) from another barrel of the same multibarelled electrode. Individual sweeps are virtually continuous (from Fig. 3 in Curtis et al. 1959). B, typical microiontophoretic excitation and inhibition of cortical neurons by l-glutamate and GABA. Times of applications are indicated by horizontal lines (Fig. 2 from Krnjević, 1971b, with kind permission from Springer Science+Business Media).
A contrasting view was taken by Krnjević & Phillis (1961, 1963). In similar tests on cortical and cerebellar neurons, they were impressed by the very fast, strong and highly reproducible excitation produced by glutamate and related acidic amino acids, and the equally powerful inhibitory actions of GABA and related omega amino acids (Fig. 6B). The quick time course of action, especially when compared with the slow and prolonged excitation produced by acetylcholine on the same cells, and the minute amounts of amino acid needed to induce or block neuronal firing seemed just what would be expected of synaptic transmitters. In view of the abundance of both glutamate and GABA in the brain – which guaranteed a ready supply – and their active uptake from extracellular space – which would account for the quickly reversible actions –Krnjević & Phillis (1963) concluded that these amino acids were probably the main central synaptic transmitters, about which there had been so much speculation. For some years, unsupported by further evidence, this idea gained little in popularity.
Rapid progress on the inhibitory front
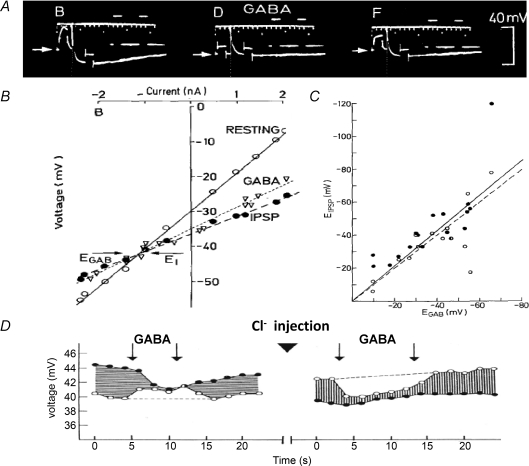
A more searching comparison of IPSPs and GABA's actions on membrane properties proved decisive. Combining intracellular recording and GABA iontophoresis, Krnjević & Schwartz (1967), Obata et al. (1967) and Dreifuss et al. (1969) showed that IPSPs and GABA induce similar changes in membrane potential and conductance – clearly an increase in Cl− conductance – even after reversal of the transmembrane Cl− gradient (Fig. 7). Similar effects of glycine on spinal neurons, as well as its distribution within the spinal cord, led Werman et al. (1968) to propose that glycine was the main spinal inhibitory transmitter. These results were confirmed pharmacologically by the selective block of both glycine and spinal IPSPs by strychnine, on the one hand, and of GABA and cerebral IPSPs by bicuculline and picrotoxin (Curtis & Johnston, 1970; Curtis et al. 1970) on the other. These antagonists became valuable tools for the identification of GABAergic and glycinergic pathways in the CNS: the latter mainly in the spinal cord and brainstem. By the early 1970s, there was wide agreement that the inhibitory amino acids were indeed major transmitters.
Figure 7. Comparing actions of GABA and IPSPs on membrane potential and conductance of cortical neurons.
A, for each trace, initial (‘resting’) potential is indicated by a horizontal arrow and two 20 ms inward current pulses were injected to show marked increase in membrane conductance near negative peak of IPSP (evoked by surface stimulation; separate traces monitoring current pulses are displaced to right). Traces were obtained with potassium citrate microelectrode before (small B), during (small D) and 40 s after end of (small F) iontophoretic application of GABA (140 nA). Note during GABA release much higher ‘resting’ conductance, small hyperpolarization and minimal IPSP, but little further conductance increase during IPSP. B, voltage–current plots obtained by applying several such pairs of current pulses: open circles give membrane potential at rest, filled circles near IPSP peak, and triangles, ‘resting’ potential during GABA application. Note similar large-conductance increases produced by IPSP and GABA and virtually identical reversal potentials (indicated by horizontal arrows). C, comparing reversal potentials for IPSPs and GABA action recorded (as illustrated above) in each of 18 such series from 13 different cortical neurons. Least squares plot (continuous line) did not differ significantly from that of ideal fit (dashed line). Closed and open circles are values obtained before and after corresponding GABA applications (A–C taken from Figs 5, 6 and 8, in Dreifuss et al. 1969, with kind permission from Springer Science+Business Media). D, reversal of IPSPs and GABA effect by intracellular injection of Cl−: open circles, resting potential; filled circles, potential at peak of IPSP (Fig. 3 in Krnjević & Schwartz, 1968).
GABAAvs. GABAB actions
Though widely effective, bicuculline did not always prevent the effects of GABA. Following up on this clue, Bowery et al. (1980) discovered a second, slower mode of action of GABA – resistant to the usual convulsants – presumably mediated by a different membrane receptor: thus was born the notion of GABAA- and GABAB-type actions. As further studies revealed (Bowery & Enna, 2000), unlike the pentameric A-type receptor built around a Cl− channel (Macdonald & Olsen, 1994; Jones-Davis et al. 2005), the dimeric GABAB receptor is not directly linked to an ion channel. Instead, it belongs to the family of receptors – with seven cross-membrane segments – which interact with G-proteins. One such G-protein opens some K+ channels, causing postsynaptic inhibition; by blocking presynaptic Ca2+ channels, it also depresses transmitter release (notably, but not exclusively, of GABA). Even slower effects are mediated by G-protein-triggered activation of adenylate cyclase and the formation of cyclic AMP. Via protein kinase A-induced changes in gene expression, this signalling pathway can initiate much longer-term modulations in cellular functions. The contrasting modes of GABAA and GABAB actions fitted well into the classification of two types of neurotransmission: fast, ionotropic and indirect slower metabotropic (Eccles & McGeer, 1979).
Could glutamate be the excitatory transmitter?
Further progress on the glutamate front was not nearly so rapid. That glutamate might be involved in synaptic transmission went against the grain. Already a well-established biochemical, abundant in all cells, its proposed, quite unexpected new role as synaptic transmitter would require overcoming a much greater psychological barrier. And there were greater technical obstacles in the way. Unlike GABA, glutamate's effects were sensitive to membrane voltage. It seemed to induce only very small increases (or even decreases) in membrane conductance, but large depolarizations that were difficult to reverse. Moreover, typically situated on dendritic spines, electrotonically far from the cell body, excitatory synases were not amenable to close examination by the techniques then available. All these properties of EPSPs precluded any reasonably accurate estimates of reversal potentials, considered the criterion for identification. The difficulties were compounded by the lack of any antagonists. In this respect, the contrast with GABA and glycine was striking: numerous convulsant drugs had long been known, and most indeed proved to be antagonists of GABA or glycine; but nothing in the pharmacopoeia suggested a selective antagonism of excitatory transmission. As it turned out, glutamate binds to a variety of receptors (Hollmann & Heinemann, 1994), all structurally different from the broad family of receptors to which GABA and glycine receptors belong (Macdonald & Olsen, 1994). Albeit generally increasing excitation, the ionotropic receptors activated by glutamate had their own characteristics. Identifying selective ligands proved especially arduous. All the specific agonists and antagonists at the various glutamate receptors, such as anthelmintics, were unsuspected ‘new’ agents, only slowly revealed by a process of almost random, several decades-long search (Mayer & Westbrook, 1987; Watkins & Jane, 2006). Reluctant to accept glutamate as the main excitant, other investigators searched extensively for a credible alternative. As none transpired, in time glutamate became generally accepted as the transmitter at most central excitatory synapses.
Further advances
At first, the picture seemed relatively straightforward. But complications soon arose. Both GABA and glutamate turned out to be anything but ‘simple’ transmitters.
GABA
Immunohistochemical markers of GABA or glutamate decarboxylase (needed to produce GABA from glutamate) revealed widely dispersed GABAergic interneurons and even more widely distributed GABAergic nerve terminals. That they are crucial for controlled brain function was made all too clear by the prominent seizures induced by any pharmacological or pathological process that interfered with their activity or efficacy. The corresponding postsynaptic receptors were cloned. They were of the same type as nicotine receptors, but with a positively charged ion channel permeable to small anions (Macdonald & Olsen, 1994; Jones-Davis et al. 2005), as were the strychnine-sensitive, anionic channels activated by glycine (Betz et al. 1999). Unexpectedly, the efficacy of the Cl−-mediated inhibition proved to be quite labile, the inhibitory potency of GABAA receptors being much enhanced by many sedatives, general anaesthetics and even some endogenous steroid hormones (Majewska, 2007); and it could be greatly reduced by changes in the transmembrane Cl− gradient.
Because Cl− currents reverse their direction at membrane potential usually not far from the cell's resting potential, even modest increases in internal [Cl−] can turn IPSPs into depolarizing potentials. Given a sufficiently positive shift in VCl, IPSPs become EPSPs (Fig. 7). Indeed, during prenatal and early postnatal development, before glutamate-mediated transmission is established, GABA induces depolarization (Fig. 8) (Cherubini et al. 1991) and GABAergic synapses provide the main excitatory signals in the brain. The determining internal [Cl−] is set by the balance between inward and outward Cl− transport (Blaesse et al. 2009). Initially, the balance favours inward transport: so internal [Cl−] is relatively high and GABA-releasing axons, which develop early, excite their target neurons. The resulting influx of Na+ and Ca2+ stimulates further synaptic and neuronal growth and functional maturation. In time, outward transport of Cl− predominates, and within a few weeks of birth, hyperpolarizing IPSPs become the norm. Thus, albeit a crucial controller, both of signalling between nerve cells and more general excitability, GABAergic inhibition is by no means a monolithic process, notably because of its dependence on the vagaries of Cl− transport. Even in the adult, decreased Cl− outward transport may be an agent of pathological hyperexcitability, manifested by epileptic seizures or chronic pain (Blaesse et al. 2009). An opposite change seems to take place transiently in the perinatal period when, under the influence of oxytocin, the depolarizing GABA-mediated synaptic events are temporally converted to hyperpolarizing IPSPs, possibly to protect the fetus’ brain from excessive excitation (Khazipov et al. 2008). Thus, fast GABAergic signalling can reverse its polarity surprisingly quickly as a result of changes in Cl− gradient.
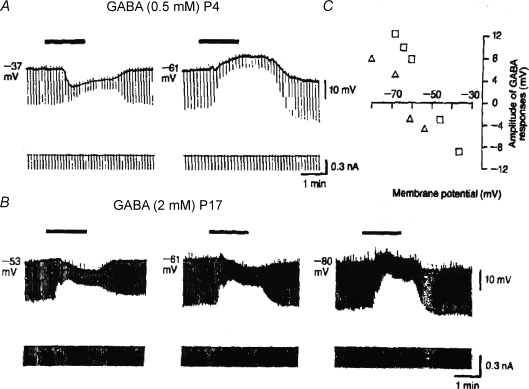
Figure 8. GABA excites in young brain: at resting potential, GABA depolarizes during first few postnatal days, and hyperpolarizes only later.
A, in hippocampal neuron at postnatal day (P) 4, GABA effect is hyperpolarizing or depolarizing when initial potential is −37 or −61 mV, respectively. B, at P17, GABA's effect is hyperpolarizing at −61 mV and becomes depolarizing only at more negative potentials. Membrane resistance was monitored throughout by brief hyperpolarizing current pulses. C, plots of GABA effects in A and B as function of membrane potential clearly show more positive reversal potential for data obtained at P4 GABA applications are indicated by horizontal bars (Fig. 2 from Cherubini et al. 1991, reproduced with permission).
Another type of synaptic modulation, presynaptic inhibition, was first proposed by Eccles (1969) to explain ‘remote’ inhibition at sensory synapses in the spinal cord: GABA-mediated depolarization of Cl−-rich terminals reduces transmitter release at the primary afferent relay. An interesting variant is presynaptic modulation of GABA release by glycine, discovered more recently at several sites in the brain stem (Turecek & Trussell, 2001; Ye et al. 2004). In the ventral tegmental area (VTA), where dopaminergic cells are inhibited by GABAergic neurons, the GABA-releasing axons are subject to presynaptic, glycine-mediated inhibition (Ye et al. 2004); thus, activation of glycine receptors on the GABAergic terminals lowers GABA release. This form of disinhibition facilitates the firing of the dopaminergic cells. An interesting point is that, in this case, the overall effect produced by glycine – facilitation – is not altered by developmental changes in neuronal [Cl−]: in early postnatal days, when internal [Cl−] is high, both GABA and glycine are depolarizing transmitters in VTA, as in the hippocampus, and glycine's presynaptic action, as in the mature animal, facilitates dopaminergic neuronal firing.
Glutamate
Glutamate-mediated excitation also turned out to be more complex than expected, but in different ways. A prominent characteristic of glutamate receptors (GluRs) is their variety: four broad types are found, of which three (ionotropic) are relatively non-specific cationic channels (like the nicotinic receptor, but structurally quite different (Hollman & Heinemann, 1999; Brockie & Maricq, 2006; Oswald et al. 2007) (Fig. 9C), and one (metabotropic) activates G-protein-triggered internal signalling (Niswender et al. 2005; Platt, 2007). But, apart from some depressant effects mediated via metabotropic receptors (especially on transmitter release), glutamate is almost exclusively a depolarizing agent. Because it is such a powerful excitant, excessive release of glutamate can have devastating effects on brain function and indeed, survival. Changes in efficacy of glutamatergic synapses typically come about through a change in the number of receptors available at the synapse for transmission. Such ‘trafficking’ of GluRs is an important aspect of ‘synaptic plasticity’: the ability of synapses to undergo very long-lasting, activity-dependent increases or decreases in efficacy (Kessels & Malinow, 2009). The different properties of ionotropic GluRs can be very briefly summarized as follows.
Figure 9. Some unusual characteristics of glutamate-induced membrane currents.
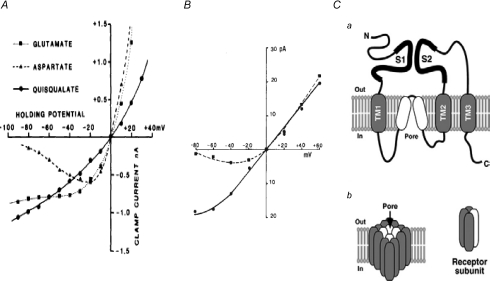
A, current–voltage (I–V) relations for responses evoked by l-glutamate, l-aspartate and quisqualate. Note monotonic I–V relation for quisqualate (typical for a non-NMDA current) and N-shaped relation (with clear negative slope between −80 and −20 mV) for the NMDA agonist l-aspartate. Because l-glutamate (the main excitatory transmitter) binds to both types of receptors, its I–V relation is of a mixed character, with a flat region between −30 and −80 mV, where there is virtually no conductance change (Fig. 2A from Westbrook & Mayer, 1984, reproduced with permission). B, N-shaped I–V relation for responses to glutamate is caused by voltage-dependent block by external Mg2+; dashed and continuous lines were fitted to data obtained in presence and absence of 0.5 mm[Mg2+], respectively (part of Fig. 1 in Nowak et al. 1984, reproduced with permission). C, ionotropic glutamate receptors (iGluRs) are formed by the heteromeric assembly of four receptor subunits. Ca, subunit membrane topology. The three transmembrane domains and the hydrophobic pore-lining region are indicated by grey and white rounded rectangles, respectively. The S1 and S2 domains form the ligand-binding domain. Cb, tetrameric assembly of a functional iGluR showing the hydrophobic regions that co-operate to form the ion channel pore (from Fig. 1 in Brockie & Maricq, 2006, reproduced with permission).
(A) Those insensitive to NMDA. (1) GluRs that bind the very specific synthetic agonist AMPA. By mediating fast EPSPs at excitatory synapses throughout the CNS, they are the workhorse of information transfer. Typically, AMPA-type channels are permeable to Na+ and K+, but not divalent cations (except for one Ca2+-permeable subtype). An important property of AMPA receptors (AMPARs) is extremely rapid desensitization (Partin et al. 1993; Raman & Trussell, 1995); therefore agonist potency can be much enhanced by drugs – or intracellular anchoring proteins – that slow down this desensitization (Partin et al. 1993; Milstein et al. 2007). (2) GluRs that bind the non-endogenous specific agonist kainate (KA). Slower acting than AMPARs, these GluRs generate a later phase of EPSPs. When situated on nerve endings, they modulate transmitter release (Lerma, 2003); for example, at mossy fibre synapses in hippocampus they mediate a cumulative increase in glutamate release, resulting in ‘frequency facilitation’ or long-term potentiation (of EPSPs (Lauri et al. 2001). Through its tonic excitatory action and enhancement of cytoplasmic [Ca2+], KA promotes seizures and excitotoxicity (Pinheiro & Mulle, 2006). The depression of GABA release at least partly accounts for KA's ability to induce seizures (Behr et al. 2002; Fritsch et al. 2009).
(B) NMDA-sensitive GluRs. Three properties make this receptor stand out (Westbrook, 1994; MacDonald et al. 2006). First, because its activation requires the presence of glycine or d-serine (as co-factor), it is sensitive to changes in local glycine or d-serine concentration or to drugs that compete with these amino acids. Second, at resting potential, external Mg ions prevent effective opening of the channel (Nowak et al. 1984) (Fig. 9B) and substantial depolarization is needed to overcome the block. As a result, the NMDAR-mediated conductance increase is voltage dependent, unlike the effect of all other ionotropic receptors (Fig. 9A). Third, the NMDAR cationic channel is very permeable to Ca ions. As cytoplasmic Ca2+ is a protean signal, NMDAR-mediated Ca2+ influx can turn on a wide variety of cellular processes – mostly beneficial, such as changes in respiration, ionic permeability, gene expression; but excessive Ca2+ influx can lead to functional disruption or even cell death (‘excitotoxicity’) (Waxman & Lynch, 2005). Of especial importance is the voltage dependence of NMDARs, which makes them sensitive detectors of correlated synaptic input and cellular firing (hence ‘coincidence detectors’), the resulting Ca2+ influx acting as an essential trigger of persistent changes in synaptic efficacy. It is now widely believed that synaptic plasticity – the ability to undergo selectively such sustained changes – is a vital aspect of learning and memory. Whether long-term potentiation or long-term depression is induced seems to depend on the intensity of the Ca2+ influx: low frequency firing is associated with modest depolarization and Ca2+ influx, which leads to synaptic depression, whereas high frequency activity induces a much greater and sharper peak of internal [Ca2+] and results in synaptic potentiation (Yang et al. 1999).
Sufficient Ca2+ influx to induce such lasting changes can also be mediated by metabotropic GluRs and some forms of AMPARs (as well as voltage-dependent Ca2+ channels and possibly other transmitters). Nevertheless, in this picture, two features remain salient: glutamate's ubiquitous function as transmitter of rapid signals, and, when acting on Ca2+-permeable receptors, its no less vital role in long-lasting modulation of the efficacy of the rapid signals.
In conclusion, amino acids have come a long way over the last half-century. At least three – glutamate, GABA and glycine – are now recognized as major transmitters throughout the animal kingdom. In vertebrates, they are the predominant transmitters at fast-operating synapses of the CNS (but not exclusively in the CNS, having a significant role at some enteric junctions (Tsai, 2005). Is it time to turn the page and focus on other newer topics? Certainly not. This conference illustrates the wide range of ongoing studies on amino acids, and this symposium reviews far from exhaustively the many directions in which the neurotransmitter functions have taken us. Their involvement in neuropathologies, in particular, is a topic with a great potential for illuminating research and life-enhancing therapeutic advances (Waxman & Lynch, 2005; Platt, 2007).
Glossary
Abbreviations
- ACh
acetylcholine
- AMP
adenosine monophosphate
- AMPA
amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
AMPA receptor
- CNS
central nervous system
- EPSP
excitatory postsynaptic potential
- GABA
γ-amino-butyric acid
- GluR
glutamate receptor
- IPSP
inhibitory postsynaptic potential
- KA
kainate
- NMDA
N-methyl-d-aspartate
- NMDAR
NMDA receptor
References
- Anjard C, Loomis WF. GABA induces terminal differentiation of Dictyostelium through a GABAB receptor. Development. 2006;133:2253–2261. doi: 10.1242/dev.02399. [DOI] [PubMed] [Google Scholar]
- Awapara J, Landua AJ, Fuerst R, Seale B. Free γ-aminobutyric acid in brain. J Biol Chem. 1950;187:35–39. [PubMed] [Google Scholar]
- Bazemore A, Elliott KA, Florey E. Factor I and γ-aminobutyric acid. Nature. 1956;178:1052–1053. doi: 10.1038/1781052a0. [DOI] [PubMed] [Google Scholar]
- Behr J, Gebhardt C, Heinemann U, Mody I. Kindling enhances kainate receptor-mediated depression of GABAergic inhibition in rat granule cells. Eur J Neurosci. 2002;16:861–867. doi: 10.1046/j.1460-9568.2002.02152.x. [DOI] [PubMed] [Google Scholar]
- Betz H, Kuhse J, Schmieden V, Laube B, Kirsch J, Harvey RJ. Structure and functions of inhibitory and excitatory glycine receptors. Ann N Y Acad Sci. 1999;868:667–676. doi: 10.1111/j.1749-6632.1999.tb11343.x. [DOI] [PubMed] [Google Scholar]
- Biedermann W. Beiträge zur allgemeinen Nerven- und Muskelphysiologie. Zwanzigste Mittheilung. Über die Innervation der Krebsschere. Sitzung ber Akad Wiss Wien Math Nahrwiss. 1887;111, 95:7–40. K1 Abt. [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Enna SJ. γ-Aminobutyric acidB receptors: first of the functional metabotropic heterodimers. J Pharmacol Exp Ther. 2000;292:2–7. [PubMed] [Google Scholar]
- Bowery NG, Hill DR, Hudson AL, Doble A, Middlemiss DN, Shaw J, Turnbull M. (–)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980;283:92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Brock LG, Coombs JS, Eccles JC. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952;117:431–460. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Maricq AV. Building a synapse: genetic analysis of glutamatergic neurotransmission. Biochem Soc Trans. 2006;34:64–67. doi: 10.1042/BST0340064. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Coombs JS, Eccles JC, Fatt P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955;130:326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Duggan AW, Felix D, Johnston GA. Bicuculline and central GABA receptors. Nature. 1970;228:676–677. doi: 10.1038/228676a0. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Johnston DA. Strychnine, glycine and vertebrate postsynaptic inhibition. Nature. 1970;225:1258–1259. doi: 10.1038/2251258a0. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Phillis JW, Watkins JC. The depression of spinal neurones by γ-amino-n-butyric acid and β-alanine. J Physiol. 1959;146:185–203. doi: 10.1113/jphysiol.1959.sp006188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Phillis JW, Watkins JC. The chemical excitation of spinal neurones by certain acidic amino acids. J Physiol. 1960;150:656–682. doi: 10.1113/jphysiol.1960.sp006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent CH, Stepka W, Steward FC. Detection of the free amino-acids of plant cells by partition chromatography. Nature. 1947;160:682–683. doi: 10.1038/160682a0. [DOI] [PubMed] [Google Scholar]
- Dreifuss JJ, Kelly JS, Krnjević K. Cortical inhibition and γ-aminobutyric acid. Exp Brain Res. 1969;9:137–154. doi: 10.1007/BF00238327. [DOI] [PubMed] [Google Scholar]
- Eccles JC. The Neurophysiological Basis of Mind. Oxford: Clarendon Press; 1953. [Google Scholar]
- Eccles JC. The Inhibitory Pathways of the Central Nervous System. Springfield, IL, USA: CC Thomas; 1969. [Google Scholar]
- Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954;126:524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, McGeer PL. Ionotropic and metabotropic neurotransmission. Trends Neurosci. 1979;2:39–40. [Google Scholar]
- Eckert R. Animal Physiology. New York: WH Freeman; 1988. p. 179. [Google Scholar]
- Florey E. An inhibitory and an excitatory factor of mammalian central nervous system, and their action on a single sensory neuron. Arch Int Physiol. 1954;62:33–53. doi: 10.3109/13813455409145367. [DOI] [PubMed] [Google Scholar]
- Florey E. GABA: history and perspectives. Can J Physiol Pharmacol. 1991;69:1049–1056. doi: 10.1139/y91-156. [DOI] [PubMed] [Google Scholar]
- Florey E, McLennan H. The effects of factor I and of gamma-aminobutyric acid on smooth muscle preparations. J Physiol. 1959;145:66–76. doi: 10.1113/jphysiol.1959.sp006127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster CW, Foerster HF. Glutamic acid decarboxylase in spores of Bacillus megaterium and its possible involvement in spore germination. J Bacteriol. 1973;114:1090–1098. doi: 10.1128/jb.114.3.1090-1098.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde BG, Lea PJ. Glutamate in plants: metabolism, regulation, and signalling. J Exp Bot. 2007;58:2339–2358. doi: 10.1093/jxb/erm121. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Qashu F, Figueiredo TH, Aroniadou-Anderjaska V, Rogawski MA, Braga MF. Pathological alterations in GABAergic interneurons and reduced tonic inhibition in the basolateral amygdala during epileptogenesis. Neuroscience. 2009;163:415–429. doi: 10.1016/j.neuroscience.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton JF. Physiology of the Nervous System. New York: Oxford University Press; 1949. p. 103. [Google Scholar]
- Gerschenfeld HM. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973;53:1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Girosi L, Ferrando S, Beltrame F, Ciarcia G, Diaspro A, Fato M, Magnone M, Raiteri L, Ramoino P, Tagliafierro G. Gamma-aminobutyric acid and related molecules in the sea fan Eunicella cavolini (Cnidaria: Octocorallia): a biochemical and immunohistochemical approach. Cell Tissue Res. 2007;329:187–196. doi: 10.1007/s00441-007-0408-4. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Iwama K, Jasper HH. The action of gamma aminobutyric acid upon cortical electrical activity in the cat. J Physiol. 1957;138:365–380. doi: 10.1113/jphysiol.1957.sp005856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Davis DM, Song L, Gallagher MJ, Macdonald RL. Structural determinants of benzodiazepine allosteric regulation of GABAA receptor currents. J Neurosci. 2005;25:8056–8065. doi: 10.1523/JNEUROSCI.0348-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass-Simon G, Pannaccione A, Pierobon P. GABA and glutamate receptors are involved in modulating pacemaker activity in hydra. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:329–332. doi: 10.1016/s1095-6433(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behaviour. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Tyzio R, Ben-Ari Y. Effects of oxytocin on GABA signalling in the foetal brain during delivery. Prog Brain Res. 2008;170:243–257. doi: 10.1016/S0079-6123(08)00421-4. [DOI] [PubMed] [Google Scholar]
- Krnjević K. Microiontophoresis. In: Fried R, editor. Methods of Neurochemistry. New York: Marcel Dekker; 1971a. pp. 129–172. chap. 4. [Google Scholar]
- Krnjević K. Synaptic transmission in the brain. Klin Wochenschr. 1971b;49:519–523. doi: 10.1007/BF01487988. [DOI] [PubMed] [Google Scholar]
- Krnjević K, Phillis JW. Sensitivity of cortical neurones to acetylcholine. Experientia. 1961;17:469. doi: 10.1007/BF02158297. [DOI] [PubMed] [Google Scholar]
- Krnjević K, Phillis JW. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K, Schwartz S. The action of γ-aminobutyric acid on cortical neurones. Exp Brain Res. 1967;3:320–336. doi: 10.1007/BF00237558. [DOI] [PubMed] [Google Scholar]
- Krnjević K, Schwartz S. Structure and Functions of the Inhibitory Neuronal Mechanisms. Oxford and New York: Pergamon Press; 1968. The inhibitory transmitter in the cerebral cortex; pp. 419–427. [Google Scholar]
- Lauri SE, Delany C, Clarke VRJ, Bortolotto ZA, Ornstein PL, Isaac JTR, Collingridge GL. Synaptic activation of a presynaptic kainate receptor facilitates AMPA receptor-mediated synaptic transmission at hippocampal mossy fibre synapses. Neuropharmacology. 2001;41:907–915. doi: 10.1016/s0028-3908(01)00152-6. [DOI] [PubMed] [Google Scholar]
- Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci. 2003;4:481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Jackson MF, Beazely MA. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol. 2006;18:71–84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- McLennan H. Absence of γ-aminobutyric acid from brain extracts containing factor I. Nature. 1958;181:1807. doi: 10.1038/1811807a0. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Steroids and ion channels in evolution: from bacteria to synapses and mind. Evolutionary role of steroid regulation of GABAA receptors. Acta Neurobiol Exp (Wars) 2007;67:219–233. doi: 10.55782/ane-2007-1650. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron. 2007;55:905–918. doi: 10.1016/j.neuron.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Jones CK, Conn PJ. New therapeutic frontiers for metabotropic glutamate receptors. Curr Top Med Chem. 2005;5:847–857. doi: 10.2174/1568026054750254. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Obata K, Ito M, Ochi R, Sato N. Pharmacological properties of the postsynaptic inhibition by Purkinje cell axons and the action of gamma-aminobutyric acid on Deiters neurons. Exp Brain Res. 1967;4:43–57. doi: 10.1007/BF00235216. [DOI] [PubMed] [Google Scholar]
- Oswald RE, Ahmed A, Fenwick MK, Loh AP. Structure of glutamate receptors. Curr Drug Targets. 2007;8:573–582. doi: 10.2174/138945007780618526. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Paton WD. Mechanisms of transmission in the central nervous system. Anaesthesia. 1959;14:3–27. doi: 10.1111/j.1365-2044.1959.tb01657.x. [DOI] [PubMed] [Google Scholar]
- Pinheiro P, Mulle C. Kainate receptors. Cell Tissue Res. 2006;326:457–482. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- Platt SR. The role of glutamate in central nervous system in health and disease – a review. Vet J. 2007;173:278–286. doi: 10.1016/j.tvjl.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Purpura DP, Girado M, Grundfest H. Selective blockade of excitatory synapses in the cat brain by γ-aminobutyric acid (GABA) Science. 1957;125:1200–1202. doi: 10.1126/science.125.3259.1200-a. [DOI] [PubMed] [Google Scholar]
- Raman IM, Trussell LO. The mechanism of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor desensitization after removal of glutamate. Biophys J. 1995;68:137–146. doi: 10.1016/S0006-3495(95)80168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JA. Thomson's Outlines of Zoology. 9th edn. London: Oxford University Press; 1944. [Google Scholar]
- Stern JR, Eggleston LV, Hems R, Krebs HA. Accumulation of glutamic acid in isolated brain tissue. Biochem J. 1949;44:410–418. [PMC free article] [PubMed] [Google Scholar]
- Tsai LH. Function of GABAergic and glutamatergic neurons in the stomach. J Biomed Sci. 2005;12:255–266. doi: 10.1007/s11373-005-1357-0. [DOI] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- Watkins JC, Jane DE. The glutamate story. Br J Pharmacol. 2006;147(Suppl. 1):S100–108. doi: 10.1038/sj.bjp.0706444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-Methyl-d-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- Werman R, Davidoff RA, Aprison MH. Inhibitory action of glycine on spinal neurons in the cat. J Neurophysiol. 1968;31:81–95. doi: 10.1152/jn.1968.31.1.81. [DOI] [PubMed] [Google Scholar]
- Westbrook GL. Glutamate receptor update. Curr Opin Neurobiol. 1994;4:337–346. doi: 10.1016/0959-4388(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Westbrook GL, Mayer ML. Glutamate currents in mammalian spinal neurons: resolution of a paradox. Brain Res. 1984;301:375–379. doi: 10.1016/0006-8993(84)91107-7. [DOI] [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol. 1999;81:781–787. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- Ye JH, Wang F, Krnjević K, Wang W, Xiong ZG, Zhang J. Presynaptic glycine receptors on GABAergic terminals facilitate discharge of dopaminergic neurons in ventral tegmental area. J Neurosci. 2004;24:8961–8974. doi: 10.1523/JNEUROSCI.2016-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]