Abstract
Glycine receptors are, in several ways, the member of the nicotinic superfamily that is best-suited for single-channel recording. That means that they are ideal for testing ideas about how activation proceeds in a ligand-gated ion channel from the binding of the agonist to the opening of the channel. This review describes the quantitative characterization by single-channel analysis of a novel activation mechanism for the glycine receptor. The favourable properties of the glycine receptor allowed the first detection of a conformation change that follows the binding of the agonist but precedes the opening of the channel. We used the term ‘flipping’ to describe this pre-opening conformational change. The ‘flipped’ state has a binding affinity higher than the resting state, but lower than the open state. This increased affinity presumably reflects a structural change near the agonist binding site, possibly the ‘capping’ of the C-loop. The significance of the ‘flip’ activation mechanism goes beyond understanding the behaviour and the structure–function relation of glycine channels, as this mechanism can be applied also to other members of the superfamily, such as the muscle nicotinic receptor. The ‘flip’ mechanism has thrown light on the question of why partial agonists are not efficacious at keeping the channel open, a question that is fundamental to rational drug design. In both muscle nicotinic and glycine receptors, partial agonists are as good as full agonists at opening the channel once flipping has occurred, but are not as effective as full agonists in eliciting this early conformational change.

Lucia Sivilotti (University College London, UK) qualified as a Pharmaceutical Chemist at the University of Ferrara in Italy. After postgraduate work in Ferrara and Milan on transmitter release in the CNS, she was awarded travelling fellowships by the Royal Society and the Italian Ministry of Education to work at St Bartholomew's College in London. Her project there (on field potentials recorded from the in vitro frog visual system) led to the characterization of the GABAC receptor and the award of a PhD in 1988. From her days as a postdoc in David Colquhoun's lab in the Pharmacology Department at University College London, the focus of her research has been on channels in the nicotinic superfamily and how they work as single molecules.
Introduction
It is tempting to consider glycine receptors as a ‘minor’ member of the nicotinic superfamily of ligand-gated ion channels. For instance, glycine channels do not have the profuse diversity of subunit genes that neuronal nicotinic receptors have, as only four subunit isoforms are known in man, α1–3 and β (mice and chickens have also α4). Any of the α subunits can form functional homomeric channels or αβ heteromeric channels. Furthermore, the synaptic role of glycine is not as widespread as that of GABA, and glycinergic synapses are important only in restricted areas of the adult nervous system, such as the spinal cord, brainstem and retina (for comprehensive surveys of the literature on the molecular properties, pharmacology and synaptic function of glycine receptors see Legendre, 2001 and Lynch, 2004). In addition, few of the agents that act on glycine receptors are specific and none is in therapeutic use. Glycine receptors can be activated by many common amino acids, such as β-alanine, taurine, sarcosine, β-aminobutyric acid, β-aminoisobutyric acid and GABA and these, except for β-alanine (which is somewhat intermediate, Lewis et al. 2003), have a very much lower efficacy than glycine, as they elicit a much smaller maximum current. The most potent antagonist is strychnine which is selective, has a competitive mechanism of action and a high affinity for both native and recombinant receptors (about 20 nm, Young & Snyder, 1973; Lewis et al. 1998). Picrotoxin, a mixture of picrotoxinin and picrotin which is a potent blocker of GABAA receptors, does block glycine receptors too and can help in identifying homomeric receptors, being at least 20-fold more potent on homomeric than on heteromeric channels (Pribilla et al. 1992; Burzomato et al. 2003). The GABAA competitive antagonist gabazine also blocks glycine receptors (Wang & Slaughter, 2005). Because of its low affinity (0.2 mm), and its fast dissociation from the glycine receptor (Beato et al. 2007), it has proved useful in probing the time course of glycine in the cleft of glycinergic synapses onto motoneurones (Beato, 2008). Relatively few compounds that act on glycine channels are sufficiently subunit-selective to be of any use as experimental tools, even though recent work has identified promising candidates such as 5,7-dichlorokynurenic acid (Han et al. 2004), ginkgolides and cannabinoids (Hawthorne et al. 2006; Yang et al. 2008). Glycine channel activity can be modulated by many of the agents that affect GABAA channels, such as zinc, steroids, alcohol and general anaesthetics (Lynch, 2004), but none of these is selective for glycine receptors. Finally, inherited defects of the glycine channel are one of the causes of human congenital startle disease or hyperekplexia (together with defects in other components of glycinergic synapses, such as the glycine transporter and receptor-clustering proteins such as gephyrin and collybistin; Harvey et al. 2008). Human hyperekplexia is a rare, mostly autosomal dominant disease, and it can be successfully treated with benzodiazepines, which presumably increase the signal at GABAergic synapses, compensating for the decrease in glycinergic transmission (Bakker et al. 2006).
Nevertheless, it happens that glycine receptors have properties that make them particularly useful for the investigation of channel-opening mechanisms. This sort of study ultimately relies on the analysis of single-channel recordings, because these give greater resolution and avoid some of the ambiguities of interpretation that whole-cell current measurements are subject to (Colquhoun, 1998). Although most investigations have been done with muscle-type nicotinic acetylcholine receptor channels, glycine receptors are better in two ways. Firstly, unlike nicotinic channels, they are not blocked by the agonist itself and that makes investigations with high agonist concentrations simpler, particularly for low-potency agonists (Lape et al. 2008). Secondly, valuable information on mechanism comes from the concentration dependence of single-channel activations: longer bursts made of several openings become increasingly common, replacing shorter ones, as agonist concentration is increased (and more binding sites on the channel become bound). At low concentrations, partially liganded shorter bursts are more common (and therefore easier to characterise) in glycine receptors than they are in muscle nicotinic recordings. In addition to that, glycine receptors express well in heterologous systems, where they produce channels that resemble native ones (Takahashi et al. 1992; Beato & Sivilotti, 2007), have a high conductance (86 pS for α1 homomers and 44 pS for α1β heteromers (Bormann et al. 1993) and, at least in cell-attached recordings, rarely open to subconductance levels (Beato et al. 2004; Burzomato et al. 2004). This relatively high conductance makes it easier to obtain single-channel records with good signal-to-noise ratio, in turn ensuring good time resolution in detecting and measuring channel openings and closings. These characteristics make glycine channels (at least in their α1 or α1β form) a useful system for testing our ideas about the molecular mechanisms of receptor activation in this superfamily, by fitting mechanisms to single channel records.
Mechanisms: a user's guide
What is the purpose of fitting a mechanism to the data? A mechanism represents the process of channel activation as a chemical reaction that takes several reversible steps. First and foremost it is a way of writing down in a clear and compact form what you think physically happens when the channel is activated and as such it should help in formulating this question and thinking about it.
The modern age for modelling ligand-gated ion channels began in 1957 when del Castillo and Katz working at University College London on frog endplate nicotinic receptors proposed ‘as a working hypothesis, that the receptor … reacts by a … two-step process, first forming an intermediate inactive compound which is then changed into an active, depolarizing form… whose nature and transformation are, at present, unknown’ (del Castillo & Katz, 1957). At the time, the existence, let alone the nature, of ion channels as aqueous pores in the membrane was indeed controversial (reviewed in Hille, 2001). Starting from a simple, reasonable assumption of what the physical process must entail (in modern terms, binding of the agonist A to the receptor R, followed by agonist-bound channel AR opening to AR*), the del Castillo–Katz mechanism
| (1) |
elegantly accounts for a range of observations that include the effects of partial agonists (agonists that are less effective at keeping the channel open once they are bound) and competitive antagonists (compounds that bind but cannot produce the subsequent conformational change that opens the channel).
A reaction mechanism, like that of del Castillo & Katz, postulates that the receptor–channel molecule can exist only in one or another of a relatively small number of discrete states. For example it may be open (AR*) or shut (R and AR), or it may have 0, 1, …n molecules of ligand A bound to it. Looking at the sharp transitions between shut and open states provides perhaps the most direct evidence ever for the assumption of all chemical kinetics that there are discrete states and that the time taken to move from one state to another can be very small. Of course, the postulated ‘discrete states’ are an approximation: they represent minima in the energy profile. Any sufficiently deep well represents a state that, for practical purposes, can be considered discrete. The rate for the transitions between the states (the number on the arrows in the reaction diagram) represents the frequency of the transitions and the next step in understanding the channel activation is to get values for these rates, and hence for the equilibrium constants for each reaction step. In the simple scheme in eqn (1), a binding step, described by an equilibrium dissociation constant Kd (the ratio between koff and kon) is followed by an opening or gating step, described by an equilibrium constant for the conformational change, often referred to as efficacy E (the ratio between the opening rate β and the closing rate α; note that in this review we use the term gating to refer only to the final shut–open transition, and not to the overall activation process).
Mechanisms like this have an immediate usefulness for the interpretation of single-channel records, as the average time that is spent in any of the states is given by the reciprocal of the sum of the rates of exit from the state. For instance, in the del Castillo–Katz mechanism, the mean lifetime of the open-state AR* is the reciprocal of the closing rate α, and is not affected by the binding rate constants (or by the concentration of A). As we will see, this is a first approximation, for two reasons. Firstly, our experimental observations do not measure the lifetimes of each individual state, because we can only know whether the channel is open or closed, but cannot tell which of the open or shut states the channel is visiting (typically there are several open and shut states in a mechanism). Secondly, the simple interpretation would work only if we could be sure that we can measure each single opening or closing in isolation, a condition that is hardly ever met in practice, where things are complicated by the finite bandwidth of our recordings, i.e. by the fact that we will miss the shortest events. If we do not detect a shutting between two openings, because it is too short, we will think that we have one long opening rather than two shorter ones (this is the missed event problem, see below).
The mechanism in eqn (1) also makes predictions that, with even greater caution (see Colquhoun, 1998), can be useful in the interpretation of macroscopic dose–response curves. The maximum open probability at equilibrium will be E/(E+ 1), a function of efficacy alone, but the EC50 will depend on both the binding and the gating steps. If you want to know whether a mutation affects binding or gating, then EC50 measurements cannot tell you the answer. Single-channel analysis is the only way that is known to separate the reaction steps in the mechanism. That is why we need to estimate the rate constants (and hence the equilibrium constants) for each step in the mechanism and to find how each value is affected by mutations.
Over the years, the del Castillo–Katz mechanism provided a conceptual frame for interpreting new observations, such as the voltage dependence in both the decay of the endplate current and the apparent open time of the channel (Magleby & Stevens, 1972; Anderson & Stevens, 1973). It also gave a basis for clear thinking about what to expect experimentally, see for instance the prediction that channel openings occur in bursts if the binding step is not much faster than the opening step (Colquhoun & Hawkes, 1977). Mechanistic thinking about the receptor has allowed the application of linear-free energy relations to the investigation of how the perturbation introduced by agonist binding spreads to the channel gate (Grosman et al. 2000; Auerbach, 2007; Purohit et al. 2007). Over time, the simple 1957 mechanism evolved to incorporate the presence of two agonist binding sites for acetylcholine (Katz & Thesleff, 1957; Adams, 1975), the existence of monoliganded (Colquhoun & Sakmann, 1981) or unliganded openings (Jackson et al. 1990; Purohit & Auerbach, 2009) and the possibility that the two binding steps are different either because the binding sites are physically different to start with or because the affinity of the sites appears to change as binding progresses (Colquhoun & Sakmann, 1985; Sine et al. 1990). This review aims to examine how in our own work we have applied the mechanistic approach to glycine receptors.
Back to glycine: the startle disease mutation K276E
In our lab, work on glycine receptors started in 1996, when our colleagues Michele Rees and Mark Gardiner from the UCL Paediatrics Department identified a new human startle disease mutation, in the M2–M3 loop of the α1 subunit. In recombinant receptors, this mutation, K276E, produced the typical ‘startle phenotype’, a large decrease in both the potency of glycine and its maximum effect (Lewis et al. 1998). These effects are very similar to those of other startle mutations, such as K271L/Q. This, the first startle disease mutation to be discovered (Shiang et al. 1993), is located nearby, at the extracellular end of M2, the pore lining domain, and not only produces the startle phenotype, but had been reported also to decrease the average channel conductance (Langosch et al. 1994). Our first exploratory single-channel recordings showed that the new K276E mutation did not affect channel conductance, but reduced the duration of apparent openings (Lewis et al. 1998). As we saw above, the simplest explanation for this is that the mutation must affect the channel gating step, rather than the agonist binding. This was not an obvious conclusion at the time. The structural information available was limited to a 9 Å structure of the Torpedo nicotinic receptor (Unwin, 1993), and the position of the M2–M3 loop with respect to the binding domain was unknown. Indeed, it was still thought possible that this domain was part of the agonist binding site, and any mutation here would have to impair receptor function by impairing glycine binding. Our data, however, showed that the binding site could not be grossly distorted in the mutant, as the mutation did not change the affinity of strychnine (whose binding site must overlap that of glycine, because strychnine is a competitive antagonist). Both the decrease in apparent open time and the macroscopic startle phenotype can be explained by a change in agonist efficacy E, the effectiveness with which the channel opens once it binds the agonist. This was in agreement with the observations of Lynch et al. (1997), that a startle phenotype could be produced by mutations at several positions, in either M1–M2 or M2–M3 and suggested that these loops could serve as a hinge in the channel opening. M1–M2 is unlikely to participate directly in agonist binding as it is on the cytoplasmic face of the membrane.
However plausible our conclusions, they had to be taken with caution, as they relied in part on interpreting the absolute magnitude of the maximum response observed in a macroscopic dose–response curve in an oocyte. This is a measurement that is affected not just by agonist efficacy, but also by other factors that can be changed by a mutation and are difficult to quantify in an oocyte, such as the rate and extent of desensitisation and the level of channel expression (Colquhoun, 1998). The other evidence for an effect on channel gating relied on interpreting apparent open times in the absence of an established mechanism. This is a potential problem and the reason is clear if we look at the del Castillo–Katz mechanism in eqn (1) again (even though, as we shall see, a realistic activation mechanism for glycine is much more complicated than that). Once the open channel AR* has closed, the channel – now in AR – can either open again, or lose the agonist molecule and go back to the resting unbound state, R. If the opening rate is fast enough, the channel will often return to AR* after pausing a very brief time in AR and will continue to oscillate between AR and AR*, opening and closing until the agonist dissociates (Colquhoun & Hawkes, 1977). This is known to happen in other ligand-gated channels, such as the muscle nicotinic receptor, where openings occur in groups or bursts. The duration of bursts is determined not only by the average length of an opening (i.e. by the channel gating) but also by the number of times that the channel reopens before the agonist dissociates and that depends on the binding step, i.e. on how tightly the agonist binds to the low-affinity, resting state of the channel. In our hyperekplexia recordings, if some or all of the apparent open times that we measured were really channel bursts, rather than single openings (because we missed the short shut times between the openings), they could be shorter either because the openings are shorter (i.e. the mutation reduces the stability of the open channel through an effect on gating) or because there are fewer openings per burst. The latter could occur if the mutation speeds up agonist dissociation and therefore affects binding rather than gating. At the time we concluded that it was likely that K276E affected mostly the transduction from agonist binding to gating. Obtaining stronger evidence for this conclusion would require a full correction for the limited recording bandwidth. The only way to solve this problem (and therefore to identify the role of this domain) is to establish a detailed mechanism for glycine activation, so that we could include in our analysis a full correction for missed events.
Fitting mechanisms to glycine channels
We therefore set out on a systematic investigation of the single-channel behaviour of the glycine receptor in its wild-type form, by obtaining recordings of channel activity at a range of glycine concentrations at equilibrium. There had already been a report (Twyman & Macdonald, 1991) that the behaviour of glycine channels is too complex to be explained by a simple del Castillo–Katz scheme with a single open state, even if the scheme is adjusted for two or three binding sites. In outside-out patches from mouse spinal cord neurones, the length of openings and bursts was seen to be concentration dependent, with longer events becoming more common as glycine concentration increases. A similar thing happens for muscle nicotinic receptors (Colquhoun & Sakmann, 1985), but for glycine the effect occurs over a wider concentration range. As Twyman & Macdonald (1991) insightfully point out, this must mean that the channel can open not only when saturated by the agonist, but also when only some of its binding sites are occupied, and that the resulting bursts look different. A simple sequential mechanism with one open state is therefore not sufficient.
Some practical problems were encountered early in our work. The native channels in this study are likely to be α1β heteromers, so we started investigating in a systematic way how to express recombinant heteromeric channels that were as pure as possible, given that the α subunit can also form functional homomeric channels. Homomers can be identified by their higher conductance, but this is not always possible at low agonist concentrations, when the openings may not be long enough to reach full amplitude (Colquhoun & Sigworth, 1995). This means that if we want to use low concentration records in our analysis, we have to eliminate contamination by homomeric channels. Earlier work (including our own) had just assumed that a 1:4 or a 1:10 ratio of α to β subunits in the transfection mix was sufficient to do this, but we found that in HEK293 cells the ratio needed is more extreme, as high as 1:40 (Burzomato et al. 2003). In the same work we investigated the number of α subunits in the heteromeric pentamer, by inserting a reporter mutation (L9′T, Labarca et al. 1995) in the channel lining M2 domain of the α or the β subunit. This was found to have a greater effect on the agonist EC50 when inserted in the α subunit. This suggests that α is the more numerous subunit in the pentamer, if the effect of mutations in the different subunits is equivalent (as shown for the muscle nicotinic receptor and for some neuronal nicotinic receptors by Labarca et al. 1995 and Boorman et al. 2000; but see contrary results for GABAA receptors by Chang et al. 1996 and for some neuronal nicotinic receptors by Groot-Kormelink et al. 2001, 2006). If we assume that α is the principal binding subunit, this finding implies that there are three agonist binding sites in the heteromeric glycine receptor. Receptor stoichiometry is still controversial. Our finding of three α copies agrees with the results of Kuhse et al. (1993), but later data by the same group (Grudzinska et al. 2005) favour two α subunits in the receptor. Nevertheless, what matters is the number (and nature) of the agonist binding sites and Grudzinska et al. (2005) conclude that there is a minimum of three agonist binding sites. Some of the uncertainty may derive from differences between expression systems, as we find that in oocytes heavy homomeric contamination persists even with extreme (1:40) α:β transfection ratios (P. Krashia, unpublished observations). None of these problems would have been apparent in experiments with whole-cell currents.
Since the invention of patch clamping, steady-state single-channel records have provided the richest source of functional information, as the number of components that can be distinguished in the open and closed time distributions is much greater than the number of components that can be resolved in macroscopic responses and it directly provides a minimum value for the number of open and closed states. Indications can also be gleaned on which of the steps in the mechanism are concentration dependent and, by looking at open–shut correlations, on how open states and closed states are connected. Once you have a mechanism, you try to estimate the values of the rate constants for each of the steps from the time constants of the distributions, including approximate corrections for missed events.
This approach gave us much of our understanding of the activation of muscle nicotinic receptors (Colquhoun & Sakmann, 1985), but suffers from several limitations. Analysing distributions of dwell times means that the information contained in the order of events in the original records is not used. Furthermore, it is often impossible to be sure about how many components a distribution contains and an exact correction for the missed event problem is only possible after a mechanism is postulated (Hawkes et al. 1990, 1992). Finally, the relation between the time constants and areas of the experimental dwell-time distributions and the values of the mechanism rate constants (that we wish to estimate) is not straightforward. Even in the absence of missed events, each time constant is not the expression of a single rate constant but the reciprocal of the minus eigenvalues of the appropriate submatrix of the transition matrix Q (areas are considerably more complicated, see Colquhoun & Hawkes, 1995). That makes it difficult to estimate rate constant values one by one, in a piecemeal approach.
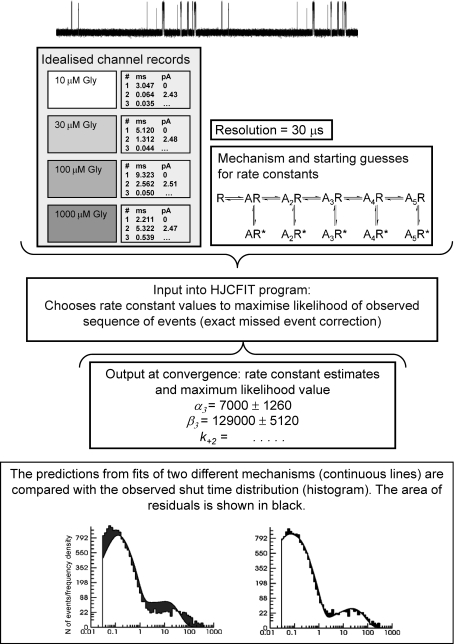
The modern approach is to fit mechanisms to the whole sequence of openings and closings recorded for a set of experimental conditions: the procedure is shown diagrammatically in Fig. 1. Analysis programs that do that have been developed by David Colquhoun and co-workers in London http://www.ucl.ac.uk/Pharmacology/dc.html (the software we used in our work) and by Tony Auerbach, Fred Sachs and colleagues at Buffalo http://www.qub.buffalo.edu. In the case of ligand-gated channels, we need to fit stretches of data at several different agonist concentrations (after idealisation, in our case by time course fitting with the SCAN program). Idealised data are analysed initially to choose portions of the recording that are likely to reflect the activity of a single ion channel molecule. In practice this means dividing the records at low agonist concentrations into bursts (groups of openings separated by shuttings during which the channel has lost its bound agonist) and those at high concentrations into clusters (groups of bursts delimited by desensitised intervals). These data, together with an estimate of the time resolution (i.e. the shortest event that can be unambiguously resolved), is input into HJCFIT, the fitting program (‘HJC’ stands for Hawkes, Jalali and Colquhoun, from the 1990 and 1992 papers which showed how to calculate the distributions of apparent open and shut times, which are what we actually observe). In order to calculate the likelihood of the data (Colquhoun & Hawkes, 1995), and in order to correct for missed short events, it is necessary to postulate a mechanism at the outset (the adequacy of this mechanism will be judged later from the quality of the fit). The program calculates the likelihood of the set of data, given the chosen mechanism and the estimated resolution, and optimises the values for the rate constants (from the starting guesses) until it converges to a maximum value for the likelihood and outputs a set of rate constant values (Colquhoun et al. 2003; Hatton et al. 2003).
Figure 1.
A diagrammatic example of how an activation mechanism is fitted to sets of single-channel data with the HJCFIT program.
The next step is to assess whether the fit to the data is good. The actual data in different displays (dwell-time distributions, Popen curves, open–shut time correlations) are examined, together with the predictions calculated from the mechanism and the best-fit rates for the same distributions or plots (see Colquhoun et al. 2009). Figure 1 (bottom) shows one such display for a shut time distribution and the fit of two different models to the data. The difference between the observations (histogram) and the predictions of the model (continuous line), highlighted by the dark fill, is much greater for the plot on the left and suggests that the model fitted on the left is worse at describing the data. The continuous line superimposed on the histogram was not fitted to the histogram, but is the predicted HJC distribution that is calculated from the output of HJCFIT. The good match of the HJC distribution with the observed histogram shows that the model fitted on the right is capable of describing the data.
The first glycine receptor we investigated with this approach was the α1 homomeric receptor (from rat subunits expressed in HEK293 cells and recorded in the cell-attached configuration; Beato et al. 2004). Homomers contain five possible agonist binding sites, but how many of these participate in activation? We found that the binding of three glycine molecules is sufficient for the channel to reach its very high maximum open probability (98%), but could not determine whether a fourth and a fifth glycine molecule can bind to the other sites. If they do bind, they cannot further increase the channel open probability. Maximum activation at incomplete binding saturation may be a property common to other homomeric receptors in this superfamily, given the similar conclusions reached for the homomeric α7-5HT3 chimeric receptor (Rayes et al. 2009). This elegant work expressed mixtures of control and binding-defective subunits and identified the number of mutant subunits in each opening in the single-channel record by adding a ‘reporter’ conductance mutation.
We also applied direct mechanism fitting to the heteromeric α1β glycine receptor, and systematically attempted to fit a number of physically plausible reaction schemes to sets of records. Each set contained four records obtained at four different concentrations from the foot of the Popen concentration curve to its top. Most of the 30 schemes that we tested failed to provide a good description of the data and it became clear that, in order to obtain a reasonable fit, the mechanisms had to incorporate several specific features (Burzomato et al. 2004).
First of all, the receptor can bind several agonist molecules and our data, in particular the value of the slope of the Popen–concentration curve (3.4), suggests at least three. Openings can occur with measurable frequency when one, two or three glycine molecules are bound and there are three open states. Similar results were obtained for homomeric channels (Beato et al. 2004), whose burst distributions have several components. As the agonist concentration is increased, the longer burst components (which reflect higher levels of ligation) become more prevalent, but their time constant does not change.
So far, our work confirmed the insights of Twyman & Macdonald (1991). However, we next found that the mechanism they proposed, which is the simplest mechanism that can be constructed with three binding steps and three open states, is not sufficient to describe the data. The three bound shut states in this scheme are not enough to account for the observed shut time distributions of α1β channels. In order to achieve good fits, we had to add more shut states, and this posed the question of how to connect these states to the mechanism. Shut states in addition to the ones required by simple sequential schemes had already been postulated in order to explain the time course of recovery of GABAA currents from paired-pulse desensitisation and the concentration dependence of the biphasic decay of currents elicited by fast agonist applications (Jones & Westbrook, 1995). GABAA receptors have two agonist binding sites and the scheme proposed by Jones & Westbrook incorporates two binding steps, two open states and two additional shut states (which they term desensitised) connected to the resting shut states.
A similar scheme with three binding steps (Fig. 2) described our data from heteromeric receptors very well, but the similarity with GABAA receptors is limited to the shape of the mechanism. For the GABAA receptor, the additional shut states are relatively long (tens of milliseconds) and it is the rapid entrance into these states that gives the burst (and therefore the current in response to a short, synaptic-like transmitter pulse, see Wyllie et al. 1998) its long duration and relatively low open probability. This does not occur for glycine receptors, as first pointed out by Legendre (1998), who modelled the macroscopic kinetics of zebrafish glycine channels, testing various schemes with two binding sites and two open states on the time course of currents evoked by fast applications of glycine. The ‘reluctant gating’ scheme that Legendre proposed connects both open states to the fully saturated resting state, one directly and one via an additional (reluctant) shut state, which is relatively short lived (sub-millisecond) and explains well both the sigmoidal rise of current onset after a step rise in agonist concentration and the biphasic decay of the current after the glycine pulse ends.
Figure 2.
The extended version of a Jones & Westbrook-type mechanism (Jones & Westbrook, 1995) used to fit the single-channel activity of glycine heteromeric receptors. Up to three molecules of agonist A can bind to the resting receptor R (middle row, black). After binding glycine, the receptor can open (AR* states, bottom row, red) or desensitise (AD states, top row, green). The equilibrium constants are K (equilibrium dissociation constant), E (the equilibrium constants for the conformational change from resting to open, ratio between the opening and the closing rate) and D (the equilibrium constants for the conformational change from resting to desensitised, ratio between the rate of entry and that of exit from the desensitised states); numbers in small print shown by the arrows of the scheme are the values of the rates (or rate constants; units are s−1 or s−1m−1, as appropriate) obtained by Burzomato et al. (2004).
The results of our fits of the Jones and Westbrook-type model confirmed Legendre's finding that the maximum Popen of glycine channels is high. Consequently, the additional shut states needed to explain the equilibrium behaviour of heteromeric channels have to be short (in fact we found them to be short enough to be often missed in recordings). This also means that the open probability during a burst is high and this is relevant for the response to a short, synaptic-like application of transmitter. Note that in our channel analysis, long-lasting desensitised states are used to excise individual clusters of openings, but are not characterised or incorporated in the mechanism. This procedure can be justified by testing it with simulations (Colquhoun et al. 2003). Long desensitised states occur for glycine receptors exposed to long agonist pulses and must be separately characterised by appropriate concentration jump protocols with long applications (Legendre et al. 2002; Beato et al. 2007; Beato, 2008; Pitt et al. 2008).
We also tested another way of adding the shut states to the activation scheme, i.e. between the resting closed states and the open states (the example here has these additional, intermediate shut states also connected by binding steps).
This mechanism, which we termed ‘flip’ (Fig. 3), describes very well the single-channel data, despite a lower number of free parameters than the Jones & Westbrook-type mechanism.
Figure 3.
The ‘flip’ mechanism (Burzomato et al. 2004) used to fit the single-channel activity of glycine heteromeric receptors. R denotes the resting conformation (black, top row), F flipped (dark red, middle row, see text) and F* open (red, bottom row). The equilibrium constants are KR and KF (equilibrium dissociation constant for the resting states or the flipped states, respectively), F (the equilibrium constants for the conformational change from resting to flipped, ratio between the rate of entry and that of exit to the flipped states) and E (the equilibrium constants for the conformational change from flipped to open, ratio between the opening and the closing rates); the numbers in small print shown by the arrows of the scheme are the values of the rates (or rate constants; units are s−1 or s−1m−1, as appropriate) obtained by Burzomato et al. 2004).
If the Jones & Westbrook type model were a correct representation, then fitting it to data suggests that the affinity for binding to the resting conformation increases progressively by 65-fold from the first to the third glycine molecule that binds. This implies that a vacant binding site can sense whether the other ones are occupied or not, and change its affinity accordingly. That seems improbable because the binding sites are quite a long way apart (at least 40 Å apart if contiguous). However, the appearance of increasing affinity for the shut conformation can be produced, just as in oxygen binding to haemoglobin, if there exist two different shut conformations, one with a higher affinity for glycine that the other. In the presence of glycine, a shift of the equilibrium to the higher affinity conformation is expected. This additional, higher affinity shut state is explicitly incorporated in the flip model (Fig. 3), where the channel can exist in three different conformations, resting (R), flipped (F) and open (F*) with increasing affinity for the agonist. Note that the binding sites in this mechanism are assumed to be independent of each other and the binding affinity is the same for the first, second and third binding to the resting state (R). Likewise the affinity is the same for the first, second and third binding to the flipped conformation. The first two rows of the flip mechanism have thus exactly the same form as the Monod–Wyman–Changeux mechanism for the binding of oxygen to haemoglobin, where oxygen, just like the agonist, selects and stabilises the higher affinity form of the protein (Monod et al. 1965). All that has been added here is the opening reactions.
We could not get good fits with any mechanism if we allowed binding or unbinding from the open states, but, given how tightly the agonist binds to the open channel (for examples from muscle nicotinic receptors see Jackson, 1989; Grosman & Auerbach, 2001) estimating binding and unbinding rates for the open states is hard. The reason for the poorer fit when open states are connected directly is that the ratio of E3/E2 is constrained by microscopic reversibility to be the same as the ratio of E2/E1. Another possibility to explain the poorer fit when open states are joined is that microscopic reversibility is breached for the open–shut reaction, as might be the case if the presence of ions in the pore influenced opening and shutting rates (Läuger, 1995), a possibility suggested by our finding that the intracellular concentration of chloride strongly affects the time course of glycine channel deactivation (Pitt et al. 2008).
Given that the flipped conformations are not distal, unconnected shut states, but are on the path between resting and open states, it is tempting to attribute a physical meaning to these states, viewing them as an intermediate point in the process of activation. The channel has changed conformation in response to the agonist and is no longer resting but is not open yet. It is plausible that one (or more) such intermediate states should be visited in the channel trajectory from resting to open (Auerbach, 2005), as the wave of conformational change initiated in the extracellular domain by the binding of the agonist spreads to several groups of residues in succession until it reaches the channel and causes it to open (Grosman et al. 2000; Chakrapani et al. 2004; Purohit et al. 2007). In the nicotinic superfamily, the details of what happens near the binding site when an agonist binds are still uncertain, but it is well established that, in the acetylcholine binding protein, at least one of the loops that surround the site, loop C, moves to a ‘capped’ conformation when the site binds an agonist (usually a small molecule) but not when an antagonist is bound (Hansen et al. 2005). This domain closure occurs at the interface between subunits and is a smaller motion than that of the extracellular domain of AMPA receptor subunits (reviewed in Mayer, 2006). Our observations give no direct information about the structure of the ‘flipped’ conformation, but the idea that binding affinity is higher in the flipped conformation because the C-loop caps the binding site is very attractive.
A crucial feature of our mechanism is that it has no need to postulate interaction between the agonist binding sites. A good fit is obtained when we assume that binding affinity depends only on the receptor conformation (i.e. resting, flipped or open), but is not influenced by how many ligands are already bound. A somewhat similar mechanism has been postulated recently for the muscle nicotinic receptor by Mukhtasimova et al. (2009) who also include pre-open shut states which they refer to as ‘primed’ rather than ‘flipped’. They suggest that, before opening, one or both of the C-loops of the principal binding subunit α must move to cap the binding site and ‘prime’ the channel for gating. If only one site is primed, the open channel is relatively unstable and openings are brief, if both sites are primed, the classical long high Popen bursts appear. The structural interpretation is elegantly substantiated by showing that cross-linking the tip of the C-loop to the other side of the binding site has an effect similar to that of adding agonist. Agonist binding facilitates priming, but is not necessary for it to occur, especially if the channel bears a gain-of-function mutation in the pore. In these mutant channels, both brief and long spontaneous openings are seen in the absence of agonist (see also Grosman & Auerbach, 2000) and addition of agonist produces a progressively larger proportion of long openings. The flip model can describe spontaneous openings by adding unliganded flipped and open states but would predict only one sort of opening (because, in its extended form, it only has one unliganded open state). On the other hand, the ‘primed’ mechanism can account for the existence of more than one sort of opening in spontaneously active mutants because the priming transition occurs independently in each binding site. But direct testing of the concerted versus independent flipping postulates is not possible with the resolution that is achievable now, because it is not possible to estimate all the rate constants in the primed mechanism. The flip mechanism as applied to the wild-type receptor (where spontaneous openings are too rare to be characterised) has 10 states and all of its 14 free rate constants could be estimated from single-channel data (Burzomato et al. 2004). The primed mechanism for the glycine receptor would have 28 states and more than twice as many free rate constants as the flip mechanism. Even when applied to the muscle nicotinic receptor (where there are two binding sites), the primed model cannot be fitted in its entirety because of the number of free parameters, and that forced Mukhtasimova et al. (2009) to fit only a somewhat arbitrary subset of the full primed model.
A more subtle question arises if we ask whether the flipping reaction is concerted (i.e. whether it occurs simultaneously for all binding sites). All we can say about that is that our mechanism, which postulates a converted flipping reaction, describes the data quite well. This question highlights the fact that – no matter how detailed – the mechanisms that we fit to our data are bound to be simplified approximations of the real activation process (which is likely to include several steps from agonist binding to channel opening, Purohit et al. 2007). However, we do not have structural ‘snapshots’ of the channel frozen at different points in its activation trajectory. Until then, analysing single-channel data by using mechanisms as detailed as can be robustly fitted and characterised is the best tool we have to identify the essential features of the physical behaviour of the channel molecule in time.
The mechanism of partial agonism
Fitting the detailed flip model to our glycine data has also allowed us to revisit one of the questions that gave rise to the del Castillo–Katz scheme, namely what makes an agonist partial or full. Until recently it has always been supposed that a partial agonist is partial because it is poor at opening the channel, i.e. that the open–shut equilibrium constant, E, is small, as proposed by del Castillo & Katz. In the flip model, overall agonist efficacy is split into two steps, the initial conformational change (flipping) and the actual opening of the channel. Thus a partial agonist could be poor either at opening the flipped channel (low E) or at causing the channel to flip (low F), or both. A priori, we did not know what to expect. It may be useful to look at another receptor superfamily, that of glutamate channels, where a compelling model for agonist efficacy has been proposed for AMPA channels, and see what this model would predict if it applied to our channel. For glutamate receptors, closure of a ‘clamshell’ ligand-binding core around the agonist is well documented structurally in constructs of the extracellular domains that do not include the channel. Functional data with macroscopic currents suggests that AMPA receptors go through a pre-opening conformational change (Zhang et al. 2008), but these channels have a relatively small conductance and verifying this conclusion by our sort of single-channel modelling is impracticable. For AMPA channels, the model for agonist efficacy comes from elegant work with a chemically related series of agonists and links efficacy to the degree of closure of the ligand-binding domain in the agonist-bound structures (Jin et al. 2003; Mayer, 2006; but see Zhang et al. 2008). This relation does not apply to the NR1 subunit of NMDA receptors (Inanobe et al. 2005) and may not hold for kainate receptors (see Mayer, 2005; Hald et al. 2007; Frydenvang et al. 2009; Fay et al. 2009). If we assume for the sake of argument that nicotinic superfamily channels behave like AMPA receptors, and that each agonist moves the extracellular domain to a different extent, depending on its overall efficacy, each agonist would be expected to give rise to a physically different flipped conformation in the bound channel. It would be reasonable to expect these different intermediates to connect to the open state with different opening rates. As a consequence, you might see that agonists differ both in the average durations of the sojourns in the flipped state and in the opening and closing rates they produce. This is not what we found (Lape et al. 2008).
Both the partial agonist taurine (which keeps the fully bound channel open approximately 50% of the time) and the full agonist glycine spend a very short time in the flipped state (about 8 μs). This happens largely because the mean lifetime of the flipped state is dominated by the opening rate and this is very fast for both agonists (Fig. 4B). The rate of leaving the flipped state for the resting state is about 6-fold faster for taurine than for glycine, so the flipped state structures must be somewhat different. However, most of the difference in the flipping equilibrium constant F stems from the rate of leaving the resting state for the flipped state, which reflects the structure of the resting state. Once the flipped state is reached, both glycine-bound and taurine-bound channels open at almost the same, high rate. The channels also stay open for much the same time, on average, for both agonists, so the gating equilibrium constant is much the same for the full and partial agonist, contrary to what has always been supposed until now. The deficiency in the partial agonist lay almost entirely in its ability to make the channel flip, and hardly at all in the ability of the channel to open once flipped. This is very clear if we express our findings as an energy diagram for the two agonists (Fig. 4A): once the intermediate (flipped) conformation is reached, the energy barrier and energy change to go to the open states are similar for both agonists. It is the first step, the flipping, that accounts for almost all the difference between full and partial agonists.
Figure 4.
Energy diagram and activation mechanism for glycine heteromeric receptors fully bound to the full agonist glycine (red) or to the partial agonist taurine (blue). In the energy diagram (A), the main difference between the two agonists is in the first step, the transition from resting to flipped. This is downhill for glycine, but uphill for taurine. The calculations used a frequency factor of 10−7 s−1 (Andersen, 1999) and the lines are shifted vertically so they meet at the open state. B, the activation mechanism shows the values for the rates (numbers near the arrows, expressed in s−1) and the equilibrium constants F3 and E3 for flipping and gating in the receptor saturated by glycine or taurine (abbreviated as Gly and Tau; values from Burzomato et al. 2004 and Lape et al. 2008).
Taurine and glycine differ in the time they spend in the resting bound state (because the resting to flipped rate constant is different). In the saturated receptor the partial agonist takes a long time to produce a flip (almost 1.5 ms on average), whereas the glycine-bound channel only takes an average of about 50 μs to flip. Similar results were obtained for the effects of acetylcholine and tetramethylammonium on muscle nicotinic receptors. Work on this channel at negative potentials was technically much more difficult because of channel block by the agonist, but our finding that efficacy is mostly due to differences in flipping was confirmed when we analysed the effect of tetramethylammonium and acetylcholine at positive holding potentials (where block is effectively absent).
We have begun to apply the flip mechanism to study the effect of mutations, starting with the spasmodicαA52S mutation, known to produce startle disease in mice. The αA52 residue is in loop 2, near the interface between the extracellular part of the channel and its transmembrane domains, an area that may mediate the transduction of the binding perturbation in channel opening (Lee & Sine, 2005; Dougherty, 2008). In heteromeric channels, we found that A52S also impairs flipping (Plested et al. 2007). This effect is too small to result in a noticeable decrease in the maximum Popen (which is given by F3E3/(1 +F3+F3E3), but is enough to increase the glycine EC50 by about 5-fold.
The examples discussed in our case history for the glycine receptor show that single-channel analysis can throw light on a variety of questions on the behaviour of the channel as a molecular nanoswitch activated by a neurotransmitter. These questions range from the precise role of particular amino acid residues in the protein, to the nature and time course of the conformational wave that couples binding to channel opening, to the number of agonist molecules that are necessary for full activation. As the mechanisms that we fit get closer to physical reality we can expect progress to be made on relating structure to function, a problem that has proved to be remarkably difficult.
Acknowledgments
The work on glycine receptors was supported by the Medical Research Council (Project grants G9629178, G9819400 and Programme Grant G0400869). I am grateful to David Colquhoun for commenting on a draft of the manuscript.
References
- Adams PR. An analysis of the dose-response curve at voltage-clamped frog-endplates. Pflugers Arch. 1975;360:145–153. doi: 10.1007/BF00580537. [DOI] [PubMed] [Google Scholar]
- Andersen OS. Graphic representation of the results of kinetic analyses. J Gen Physiol. 1999;114:589–590. doi: 10.1085/jgp.114.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CR, Stevens CF. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973;235:655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A. Gating of acetylcholine receptor channels: brownian motion across a broad transition state. Proc Natl Acad Sci U S A. 2005;102:1408–1412. doi: 10.1073/pnas.0406787102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A. How to turn the reaction coordinate into time. J Gen Physiol. 2007;130:543–546. doi: 10.1085/jgp.200709898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker MJ, Van Dijk JG, Van Den Maagdenberg AM, Tijssen MA. Startle syndromes. Lancet Neurol. 2006;5:513–524. doi: 10.1016/S1474-4422(06)70470-7. [DOI] [PubMed] [Google Scholar]
- Beato M. The time course of transmitter at glycinergic synapses onto motoneurons. J Neurosci. 2008;28:7412–7425. doi: 10.1523/JNEUROSCI.0581-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M, Burzomato V, Sivilotti LG. The kinetics of inhibition of rat recombinant heteromeric α1β glycine receptors by the low-affinity antagonist SR-95531. J Physiol. 2007;580:171–179. doi: 10.1113/jphysiol.2006.126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. The activation of α1 homomeric glycine receptors. J Neurosci. 2004;24:895–906. doi: 10.1523/JNEUROSCI.4420-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M, Sivilotti LG. Single-channel properties of glycine receptors of juvenile rat spinal motoneurones in vitro. J Physiol. 2007;580:497–506. doi: 10.1113/jphysiol.2006.125740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman JP, Groot-Kormelink PJ, Sivilotti LG. Stoichiometry of human recombinant neuronal nicotinic receptors containing the β3 subunit expressed in Xenopus oocytes. J Physiol. 2000;529:567–578. doi: 10.1111/j.1469-7793.2000.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J, Rundström N, Betz H, Langosch D. Residues within transmembrane segment M2 determine chloride conductance of glycine receptor homo- and hetero-oligomers. EMBO J. 1993;12:3729–3737. doi: 10.1002/j.1460-2075.1993.tb06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzomato V, Beato M, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. Single-channel behaviour of heteromeric α1β glycine receptors: an attempt to detect a conformational change before the channel opens. J Neurosci. 2004;24:10924–10940. doi: 10.1523/JNEUROSCI.3424-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzomato V, Groot-Kormelink PJ, Sivilotti LG, Beato M. Stoichiometry of recombinant heteromeric glycine receptors revealed by a pore-lining region point mutation. Receptors Channels. 2003;9:353–361. doi: 10.3109/714041016. [DOI] [PubMed] [Google Scholar]
- Chakrapani S, Bailey TD, Auerbach A. Gating dynamics of the acetylcholine receptor extracellular domain. J Gen Physiol. 2004;123:341–356. doi: 10.1085/jgp.200309004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: The interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:923–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Hatton CJ, Hawkes AG. The quality of maximum likelihood estimates of ion channel rate constants. J Physiol. 2003;547:699–728. doi: 10.1113/jphysiol.2002.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc Roy Soc Lond B Biol Sci. 1977;199:231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG. The principles of the stochastic interpretation of ion-channel mechanisms. In: Sakmann B, Neher E, editors. Single-Channel Recording. New York: Plenum Press; 1995. pp. 397–482. [Google Scholar]
- Colquhoun D, Lape R, Sivilotti L. Single ion channels. In: Knight AE, editor. Single Molecule Biology. San Diego: Academic Press; 2009. pp. 223–251. [Google Scholar]
- Colquhoun D, Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981;294:464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single-channel records. In: Sakmann B, Neher E, editors. Single-Channel Recording. New York: Plenum Press; 1995. pp. 483–587. [Google Scholar]
- del Castillo J, Katz B. Interaction at end-plate receptors between different choline derivatives. Proc Roy Soc Lond B Biol Sci. 1957;146:369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Dougherty DA. Cys-loop neuroreceptors: structure to the rescue? Chem Rev. 2008;108:1642–1653. doi: 10.1021/cr078207z. [DOI] [PubMed] [Google Scholar]
- Fay AM, Corbeil CR, Brown P, Moitessier N, Bowie D. Functional characterization and in silico docking of full and partial GluK2 kainate receptor agonists. Mol Pharmacol. 2009;75:1096–1107. doi: 10.1124/mol.108.054254. [DOI] [PubMed] [Google Scholar]
- Frydenvang K, Lash LL, Naur P, Postila PA, Pickering DS, Smith CM, Gajhede M, Sasaki M, Sakai R, Pentikainen OT, Swanson GT, Kastrup JS. Full domain closure of the ligand-binding core of the ionotropic glutamate receptor iGluR5 induced by the high affinity agonist dysiherbaine and the functional antagonist 8,9-dideoxyneodysiherbaine. J Biol Chem. 2009;284:14219–14229. doi: 10.1074/jbc.M808547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Boorman JP, Sivilotti LG. Formation of functional α3β4α5 human neuronal nicotinic receptors in Xenopus oocytes: a reporter mutation approach. Br J Pharmacol. 2001;134:789–796. doi: 10.1038/sj.bjp.0704313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Broadbent S, Beato M, Sivilotti L. Constraining the expression of nicotinic acetylcholine receptors using pentameric constructs. Mol Pharmacol. 2006;69:558–563. doi: 10.1124/mol.105.019356. [DOI] [PubMed] [Google Scholar]
- Grosman C, Auerbach A. Kinetic, mechanistic, and structural aspects of unliganded gating of acetylcholine receptor channels: a single-channel study of second transmembrane segment 12′ mutants. J Gen Physiol. 2000;115:621–635. doi: 10.1085/jgp.115.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosman C, Auerbach A. The dissociation of acetylcholine from open nicotinic receptor channels. Proc Natl Acad Sci U S A. 2001;98:14102–14107. doi: 10.1073/pnas.251402498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosman C, Zhou M, Auerbach A. Mapping the conformational wave of acetylcholine receptor channel gating. Nature. 2000;403:773–776. doi: 10.1038/35001586. [DOI] [PubMed] [Google Scholar]
- Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. The β subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Hald H, Naur P, Pickering DS, Sprogoe D, Madsen U, Timmermann DB, Ahring PK, Liljefors T, Schousboe A, Egebjerg J, Gajhede M, Kastrup JS. Partial agonism and antagonism of the ionotropic glutamate receptor iGLuR5: structures of the ligand-binding core in complex with domoic acid and 2-amino-3-[5-tert-butyl-3-(phosphonomethoxy)-4-isoxazolyl]propionic acid. J Biol Chem. 2007;282:25726–25736. doi: 10.1074/jbc.M700137200. [DOI] [PubMed] [Google Scholar]
- Han Y, Li P, Slaughter MM. Selective antagonism of rat inhibitory glycine receptor subunits. J Physiol. 2004;554:649–658. doi: 10.1113/jphysiol.2003.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 2005;24:3635–3646. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Topf M, Harvey K, Rees MI. The genetics of hyperekplexia: more than startle! Trends Genet. 2008;24:439–447. doi: 10.1016/j.tig.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Shelley C, Brydson M, Beeson D, Colquhoun D. Properties of the human muscle nicotinic receptor, and of the slow-channel myasthenic syndrome mutantɛL221F, inferred from maximum likelihood fits. J Physiol. 2003;547:729–760. doi: 10.1113/jphysiol.2002.034173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes AG, Jalali A, Colquhoun D. The distributions of the apparent open times and shut times in a single channel record when brief events can not be detected. Philos Trans R Soc Lond A. 1990;332:511–538. doi: 10.1098/rstb.1992.0116. [DOI] [PubMed] [Google Scholar]
- Hawkes AG, Jalali A, Colquhoun D. Asymptotic distributions of apparent open times and shut times in a single channel record allowing for the omission of brief events. Phil Trans R Soc Lond B. 1992;337:383–404. doi: 10.1098/rstb.1992.0116. [DOI] [PubMed] [Google Scholar]
- Hawthorne R, Cromer BA, Ng HL, Parker MW, Lynch JW. Molecular determinants of ginkgolide binding in the glycine receptor pore. J Neurochem. 2006;98:395–407. doi: 10.1111/j.1471-4159.2006.03875.x. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3rd edn. Sunderland, MA, USA: Sinauer Associates; 2001. [Google Scholar]
- Inanobe A, Furukawa H, Gouaux E. Mechanism of partial agonist action at the NR1 subunit of NMDA receptors. Neuron. 2005;47:71–84. doi: 10.1016/j.neuron.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Jackson MB. Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1989;86:1–6. doi: 10.1073/pnas.86.7.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Imoto K, Mishina M, Konno T, Numa S, Sakmann B. Spontaneous and agonist-induced openings of an acetylcholine receptor channel composed of bovine muscle α- β- and δ-subunits. Pflugers Arch. 1990;417:129–135. doi: 10.1007/BF00370689. [DOI] [PubMed] [Google Scholar]
- Jin R, Banke TG, Mayer ML, Traynelis SF, Gouaux E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nat Neurosci. 2003;6:803–810. doi: 10.1038/nn1091. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Katz B, Thesleff S. A study of the ‘desensitization’ produced by acetylcholine at the motor end-plate. J Physiol. 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhse J, Laube B, Magalei D, Betz H. Assembly of the inhibitory glycine receptor: identification of amino acid sequence motifs governing subunit stoichiometry. Neuron. 1993;11:1049–1056. doi: 10.1016/0896-6273(93)90218-g. [DOI] [PubMed] [Google Scholar]
- Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, Lester HA. Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature. 1995;376:514–516. doi: 10.1038/376514a0. [DOI] [PubMed] [Google Scholar]
- Langosch D, Laube B, Rundstrom N, Schmieden V, Bormann J, Betz H. Decreased agonist affinity and chloride conductance of mutant glycine receptors associated with human hereditary hyperekplexia. EMBO J. 1994;13:4223–4228. doi: 10.1002/j.1460-2075.1994.tb06742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R, Colquhoun D, Sivilotti LG. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–727. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Conformational transitions of ionic channels. In: Sakmann B, Neher E, editors. Single-Channel Recording. New York: Plenum Press; 1995. pp. 651–662. [Google Scholar]
- Lee WY, Sine SM. Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature. 2005;438:243–247. doi: 10.1038/nature04156. [DOI] [PubMed] [Google Scholar]
- Legendre P. A reluctant gating mode of glycine receptor channels determines the time course of inhibitory miniature synaptic events in zebrafish hindbrain neurons. J Neurosci. 1998;18:2856–2870. doi: 10.1523/JNEUROSCI.18-08-02856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P. The glycinergic inhibitory synapse. Cell Mol Life Sci. 2001;58:560–593. doi: 10.1007/PL00000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Muller E, Badiu CI, Meier J, Vannier C, Triller A. Desensitization of homomericα1 glycine receptor increases with receptor density. Mol Pharmacol. 2002;62:817–827. doi: 10.1124/mol.62.4.817. [DOI] [PubMed] [Google Scholar]
- Lewis TM, Schofield PR, McClellan AM. Kinetic determinants of agonist action at the recombinant human glycine receptor. J Physiol. 2003;549:361–374. doi: 10.1113/jphysiol.2002.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TM, Sivilotti L, Colquhoun D, Schoepfer R, Rees M. Properties of human glycine receptors containing the hyperekplexia mutation α1(K276E), expressed in Xenopus oocytes. J Physiol. 1998;507:25–40. doi: 10.1111/j.1469-7793.1998.025bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Rajendra S, Pierce KD, Handford CA, Barry PH, Schofield PR. Identification of intracellular and extracellular domains mediating signal transduction in the inhibitory glycine receptor chloride channel. EMBO J. 1997;16:110–120. doi: 10.1093/emboj/16.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby KL, Stevens CF. A quantitative description of end-plate currents. J Physiol. 1972;223:173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML. Crystal structures of the GluR5 and GluR6 ligand binding cores: molecular mechanisms underlying kainate receptor selectivity. Neuron. 2005;45:539–552. doi: 10.1016/j.neuron.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Glutamate receptors at atomic resolution. Nature. 2006;440:456–462. doi: 10.1038/nature04709. [DOI] [PubMed] [Google Scholar]
- Monod J, Wyman J, Changeux J-P. On the nature of allosteric transitions: a plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mukhtasimova N, Lee WY, Wang HL, Sine SM. Detection and trapping of intermediate states priming nicotinic receptor channel opening. Nature. 2009;459:451–454. doi: 10.1038/nature07923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt SJ, Sivilotti LG, Beato M. High intracellular chloride slows the decay of glycinergic currents. J Neurosci. 2008;28:11454–11467. doi: 10.1523/JNEUROSCI.3890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plested AJ, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. Single channel study of the spasmodic mutation α1A52S in recombinant rat glycine receptors. J Physiol. 2007;581:51–73. doi: 10.1113/jphysiol.2006.126920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Bormann J, Betz H. The atypical M2 segment of the β subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992;11:4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit P, Auerbach A. Unliganded gating of acetylcholine receptor channels. Proc Natl Acad Sci U S A. 2009;106:115–120. doi: 10.1073/pnas.0809272106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit P, Mitra A, Auerbach A. A stepwise mechanism for acetylcholine receptor channel gating. Nature. 2007;446:930–933. doi: 10.1038/nature05721. [DOI] [PubMed] [Google Scholar]
- Rayes D, De Rosa MJ, Sine SM, Bouzat C. Number and locations of agonist binding sites required to activate homomeric Cys-loop receptors. J Neurosci. 2009;29:6022–6032. doi: 10.1523/JNEUROSCI.0627-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiang R, Ryan SG, Zhu YZ, Hahn AF, O’Connell P, Wasmuth JJ. Mutations in the α1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nat Genet. 1993;5:351–358. doi: 10.1038/ng1293-351. [DOI] [PubMed] [Google Scholar]
- Sine SM, Claudio T, Sigworth FJ. Activation of Torpedo acetylcholine receptors expressed in mouse fibroblasts. Single channel current kinetics reveal distinct agonist binding affinities. J Gen Physiol. 1990;96:395–437. doi: 10.1085/jgp.96.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A, Hirai K, Hishinuma F, Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992;9:1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Kinetic properties of the glycine receptor main- and sub-conductance states of mouse spinal cord neurones in culture. J Physiol. 1991;435:303–331. doi: 10.1113/jphysiol.1991.sp018512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 Å resolution. J Mol Biol. 1993;229:1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Wang P, Slaughter MM. Effects of GABA receptor antagonists on retinal glycine receptors and on homomeric glycine receptor α subunits. J Neurophysiol. 2005;93:3120–3126. doi: 10.1152/jn.01228.2004. [DOI] [PubMed] [Google Scholar]
- Wyllie DJA, Béhé P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol. 1998;510:1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Aubrey KR, Alroy I, Harvey RJ, Vandenberg RJ, Lynch JW. Subunit-specific modulation of glycine receptors by cannabinoids and N-arachidonyl-glycine. Biochem Pharmacol. 2008;76:1014–1023. doi: 10.1016/j.bcp.2008.07.037. [DOI] [PubMed] [Google Scholar]
- Young AB, Snyder SH. Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci U S A. 1973;70:2832–2836. doi: 10.1073/pnas.70.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Cho Y, Lolis E, Howe JR. Structural and single-channel results indicate that the rates of ligand binding domain closing and opening directly impact AMPA receptor gating. J Neurosci. 2008;28:932–943. doi: 10.1523/JNEUROSCI.3309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]