Abstract
Functional studies of the ligand gated ion channel family (nicotinic acetylcholine, serotonin Type 3, glycine and GABA receptors) along with the crystal structure of the acetylcholine binding protein (AChBP) and molecular dynamics simulations of the nAChR structure have resulted in a structural model in which the agonist-binding pocket comprises six loops (A–F) contributed by adjacent subunits. It is presumed that the binding of agonist results in a local structural rearrangement that is then transduced to the gate, causing the pore to open. Efforts are underway to better define the specific roles of the six binding loops. Several studies have suggested Loop F may play a direct role in linking the structural rearrangement within the binding pocket to the gate, although other investigations have indicated Loop F may be crucial for locking the agonist molecule into the binding site. This review will focus on the controversy surrounding the role of Loop F during GABA receptor activation.

David Weiss (University of Texas HSC at San Antonio, TX, USA) obtained his bachelor's degree from the University of North Carolina at Chapel Hill and his PhD in Neuroscience from Baylor College of Medicine. His first faculty position was at the University of South Florida in the Physiology Department followed by a 10 year stint at the University of Alabama at Birmingham in the Department of Neurobiology. Since 2005 he has been a Professor and Chairman of the Physiology Department at the University of Texas Health Science Center at San Antonio. He has spent most of his career studying the structure and function of GABA receptors and is currently on the editorial boards of Biophysical Journal, Journal of Biological Chemistry and Cell Science.
GABA receptors
The ionotropic GABA receptors (GABAA and GABAC) underlie fast inhibitory synaptic transmission in the central nervous system (Enz et al. 1995, 1996; Wegelius et al. 1998). They are members of the ligand-gated ion channel (LGIC) family, which also includes the nicotinic acetylcholine (nAChR), glycine receptors and the serotonin 5-HT3A receptor (Noda et al. 1982; Grenningloh et al. 1987; Schofield et al. 1987; Maricq et al. 1991). Overall, 19 GABA receptor subunits have been cloned to date: α1–6, β1–3, γ1–3, δ, ɛ, θ, π and ρ1–3 (Schofield et al. 1987; Barnard & Seeburg, 1988; Khrestchatisky et al. 1989; Pritchett et al. 1989; Olsen et al. 1990; Pritchett & Seeburg, 1990; Garret et al. 1997; Hedblom & Kirkness, 1997; Whiting et al. 1997; Hevers & Luddens, 1998) These subunits all have the same basic structure: a large extracellular amino terminal domain, four transmembrane domains, a large intracellular domain and a short extracellular C-terminus. Like all members of the LGIC family, the GABAA and GABAC receptors consist of five subunits with the stoichiometry of the primary GABAA receptor found in the CNS consisting of two α subunits, two β subunits, and one γ subunit (Chang et al. 1996; Tretter et al. 1997; Baumann et al. 2002). The ρ1 GABA receptor subunit, which comprises the GABAC receptor, was cloned from a retinal library and is thought to exist primarily in the retina (Cutting et al. 1991; Amin & Weiss, 1994), but has been speculated to exist in the brain (Rozzo et al. 2002; Liu et al. 2004; Schlicker et al. 2004).
The agonist binding site
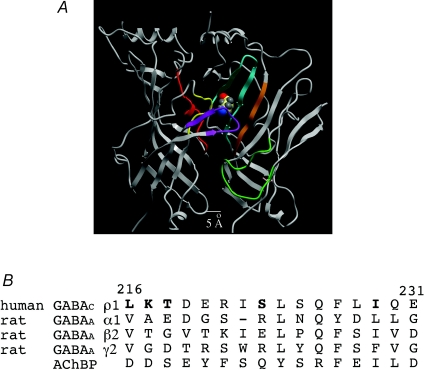
Figure 1 shows a structural model of two subunits of the GABAC receptor amino terminal domain based on the AChBP structure (Brejc et al. 2001). GABA's binding pocket is located in the subunit interface of the extracellular amino terminal domain and is formed by six loops (A–F). The loops are highlighted in Fig. 1. Loops A (red), B (yellow) and C (purple) are contributed by the left subunit, whereas Loops D (orange), E (cyan) and F (green) are contributed by the right, neighbouring subunit. In terms of the activation mechanism, the binding of GABA induces a structural rearrangement within the binding pocket. This structural rearrangement is then transduced as a conformational wave to the gate causing it to open thereby allowing chloride ions to move through the pore. Within the binding pocket, residues from Loops A–E have been postulated to interact with the ligand (Amin & Weiss, 1993; Corringer et al. 2000; Brejc et al. 2001; Boileau et al. 2002; Chang et al. 2002; Torres & Weiss, 2002). It has been hypothesized, based on structural and functional data, that Loop F (Fig. 1, green) may play a key role in linking ligand binding to channel opening (Lyford et al. 2003; Hansen et al. 2005; Thompson et al. 2006). Conflicting data not supporting this hypothesis have been recently published (Khatri et al. 2009; Pless & Lynch, 2009), so the precise role of Loop F remains a source of contention within the LGIC field. We will briefly review the Loop F field and then present our data that directly address the role of Loop F in GABAC receptor activation.
Figure 1.
The GABAC receptor extracellular domain and the sequence alignment of Loop F A, a putative structural model is shown for two subunits of the GABAC receptor extracellular domain based on the AChBP from Brejc et al. (2001). The six loops thought to form the binding pocket are coloured: Loop A, red; Loop B, yellow; Loop C, purple; Loop D, orange; Loop E, cyan; Loop F, green. GABA was docked into the GABAC receptor structural model presenting a preferred orientation of its carboxylic group facing Loop C and amino group facing Loop E. B, sequence alignment of Loop F from three subunits of the GABAA receptor, the GABAC receptor and the AChBP. Note the low sequence homology for Loop F within the GABA receptor family.
Loop F
Structural information
The initial structural resolution of the Lymnaea stagnalis acetylcholine binding protein (L-AChBP) presented a poorly resolved Loop F (Brejc et al. 2001; Hansen et al. 2005). The structure of the Aplysia californica AChBP was crystallized in the apo form (unbound) and bound by two agonists and two antagonists. Comparing these structures suggested Loop F undergoes a ligand induced structural rearrangement (Hansen et al. 2005). Not only did the agonist induce a structural rearrangement in the binding pocket, but antagonists did as well. Several other AChBP structures supported Loop F's flexibility and suggested it adopts a random coil-like structure (Bourne et al. 2005; Celie et al. 2005). The Torpedo acetylcholine receptor structure derived by electron microscopy revealed that Loop F resides in the transduction zone, just above the lipid membrane (Unwin, 2005; Dellisanti et al. 2007). Recently two structures of the prokaryotic LGIC have been published, but very little was mentioned about Loop F except that it is located in the transduction zone similar to what was observed in the Torpedo acetylcholine receptor structure (Hilf & Dutzler, 2008; Bocquet et al. 2009).
Molecular dynamics (MD) simulations
MD suggested Loop F was one of the most flexible regions in the receptor during activation, similar to what was proposed in the crystal structure of the AChBP and nAChR (Law et al. 2005; Szarecka et al. 2007). These MD simulations suggested Loop F moves into a more aqueous environment in the presence of a ligand (Law et al. 2005; Cheng et al. 2006). In addition to Loop F's proposed large range of motion, a recent MD study suggested that it could play a role in maintaining the local structure of the binding interface (Szarecka et al. 2007). Also, structural modelling of the GABAC receptor amino terminal domain based on the L-AChBP proposed that Loop F is approximately 5 Å from GABA suggesting Loop F is too distant to directly interact with GABA (Harrison & Lummis, 2006).
Functional studies
Early functional studies suggested Loop F has two distinct regions (Newell & Czajkowski, 2003; Thompson et al. 2006). The upper portion of Loop F is thought to line the binding pocket and could potentially be important for the affinity and specificity of ligand binding while the lower portion of Loop F was proposed to be a link between ligand binding and channel opening (Newell & Czajkowski, 2003; Shimomura et al. 2003; Thompson et al. 2006; Zhang et al. 2007). In the end, they hypothesized that the structural rearrangement occurring in Loop F played a direct role in controlling the actions of the gate. Studies in the αβγ GABAA receptor also suggested Loop F is important for the binding of benzodiazepines, modulators of the GABAA receptor, as well as important for the allosteric actions (potentiation) of benzodiazepines on GABA receptor activity (Sancar et al. 2007; Hanson & Czajkowski, 2008; Padgett & Lummis, 2008). On the contrary, studies of Loop F in the homomeric ρ1 GABAC receptor demonstrated that most mutations in this region did not affect receptor function. Furthermore, these studies used the substituted cysteine accessibility method (SCAM), which was originally developed to identify pore lining residues in the LGIC family (Akabas et al. 1992; Xu & Akabas, 1996; Wilson & Karlin, 1998). Cysteines are introduced into a region of interest and then bound to a sulfhydryl-reactive compound. Any change in the function of the mutant receptors in the presence of the sulfhydryl-reactive compound could signify a functionally important region of the receptor. SCAM analysis demonstrated that the residues in Loop F do not directly interact with either GABA or the competitive antagonist 3-APA. This latter study, at least for ρ1 GABA receptors, brings into question whether Loop F may play a direct role in ligand binding (Sedelnikova et al. 2005).
To further understand the role of Loop F during activation, a technique called voltage clamp fluorometry (VCF), initially developed to study the structural rearrangement of voltage-gated ion channels (Mannuzzu et al. 1996), was adapted and used in LGICs (Chang & Weiss, 2002). VCF allows real time visualization of the structural rearrangements occurring in the receptor during activation and antagonism along with the simultaneous recording of current by two-electrode voltage clamp. First, a cysteine is introduced into a region of interest in the receptor and then labelled with an environmentally sensitive fluorophore. The fluorescence intensity of this fluorophore is determined by the hydrophobicity of the surrounding environment. When the hydrophobicity surrounding the fluorophore changes then the fluorescence intensity will increase if the fluorophore moves into a more hydrophobic environment and decrease if the fluorophore moves into a more hydrophilic environment. By simultaneously detecting fluorescence and ligand-induced current, structural changes can be correlated with receptor activation. To date, there have been three VCF studies on Loop F, and one study determined that Loop F is along the transduction pathway and plays a key role in activation (Zhang et al. 2009). However, a more recent study concluded the opposite; Loop F does not play a key role in linking ligand binding to the gating of the receptor, nor is it involved in ligand specificity (Pless & Lynch, 2009). The third VCF study on Loop F was preformed by us and will be considered now.
Our work on the GABAC receptor
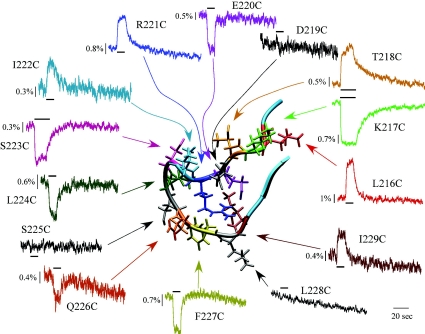
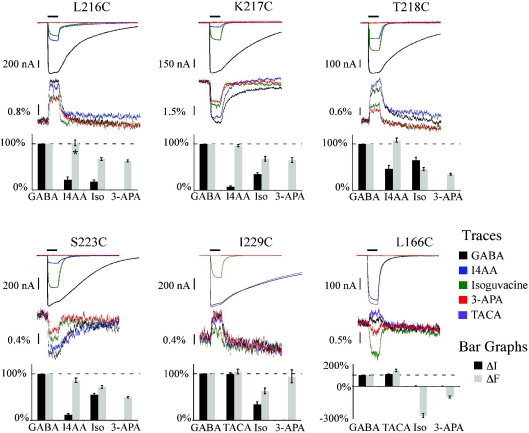
The overall focus of our study was to better define the role of Loop F during activation. The cysteine-introduced mutant receptors were expressed in Xenopus laevis oocytes and then labelled with the fluorophore Alexa 546 c5 maleimide (A5m). The oocytes were placed in a special chamber to simultaneously record a change in current (ΔI) and fluorescence (ΔF). Figure 2 shows the GABA-induced ΔF for the 14 residues that form the putative structure of Loop F (corresponding currents are not shown). The ΔF for L216C, T218C, R221C, I222C and I229C increases upon receptor activation whereas K217C, E220C, S223C, L224, S225C, Q226C and E227C exhibit a decrease in ΔF. It is remarkable that nearly all the residues (11 out of 14) in Loop F generate a ΔF with GABA application. Residues S223C–F227C of Loop F exhibited a decrease in the ΔF suggesting they move into a more aqueous environment and this agrees with the MD simulations discussed earlier (Law et al. 2005). If Loop F is a link between ligand binding and channel opening, we reasoned that the degree of activation of the receptor should be reflected in the ΔF. To control the degree of activation, we employed full, partial and competitive antagonists. A partial agonist is defined as a ligand that can bind the same site as a full agonist, in this case GABA, but not fully activate the receptor. It is possible that the reduced current induced by the partial agonist is the result of a subconductance state of the GABAC receptor, rather than a decreased open probability. Testing this possibility would be difficult since the dominant conductance state of the GABAC receptor is less then 1 pS (Wotring et al. 1999). However, at least for the highly homologous GABAA receptor, partial agonists do not alter the conductance, but rather decrease the open probability (Mistry & Hablitz, 1990). A competitive antagonist is an extreme example of a partial agonist; it binds the same site as GABA but does not activate the receptor at all. Some type of a correlation between ΔI and ΔF for these ligands would support the hypothesis that Loop F is involved in the transduction of the structural rearrangement from the binding pocket to the gate of the receptor. For these experiments, we focused on five residues that provided the most consistent and robust fluorescence signals in Loop F. Since these residues are distributed in Loop F, their ΔF is assumed to reflect the structural changes throughout the whole domain. Figure 3 compares the ΔI and ΔF recordings for two full agonists (GABA and Trans-aminocrotonic acid (TACA)), two partial agonists (isoguvacine and imidazole-4-acetic acid, or I4AA) and a competitive antagonist (3-aminopropylphosphonic acid, or 3-APA) at saturating concentrations. The top panel is the ligand-induced currents, and below are the corresponding ΔFs. The bar graphs for the ΔI and ΔF for each ligand are normalized to GABA. In general, for all the data, there was no clear correlation between the current and fluorescence. For example, consider the application of I4AA on L216C, K217C, T218C and S223C. In this case, I4AA induced a smaller current compared to GABA whereas the I4AA induced a ΔF similar in magnitude to the GABA-induced ΔF. The ΔF does not seem to correlate with the ligand induced current for either I4AA or isoguvacine. Quite a different picture is observed for L166C of Loop E. In this case, agonists cause an increase in ΔF, while antagonists decrease ΔF. Isoguvacine behaves as a competitive antagonist in this mutant and does not induce a current, but does cause a large decrease in the ΔF, as does the competitive antagonist 3-APA.
Figure 2.
Fluorescence changes for the residues of Loop F The 14 residues of Loop F were individually mutated to cysteines and then labelled with the fluorophore A5m (see text). The application of a saturating concentration GABA induced a ΔF for 11 of the 14 residues. The corresponding currents are not shown. Reproduced from Khatri et al. (2009) with permission from Elsevier.
Figure 3.
Correlation of agonists (GABA and TACA), partial agonists (I4AA and isoguvacine), and a competitive antagonist (3-APA) induced ΔI and ΔF for residues L216C, K217C, T218C, S223C and I229C of Loop F as well as L166C of Loop E The top panel shows the ligand-induced currents with the corresponding ΔF below. The bar graphs depict the ΔI (grey) and ΔF (black) normalized to GABA for each residue. In general, there was no clear correlation between the fluorescence and the functional effects of the ligand. Reproduced from Khatri et al. (2009) with permission from Elsevier.
Conclusions
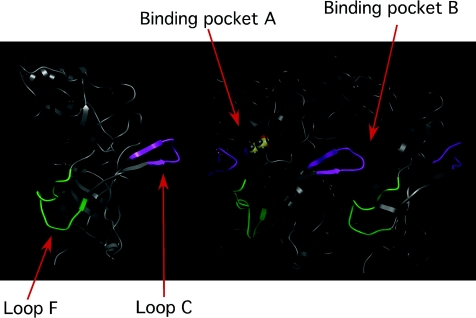
As discussed above, structural information, MD simulations, as well as functional studies have suggested Loop F plays a direct role in linking ligand binding to channel opening. Zhang et al. (2009) also employed VCF to examine the role of Loop F of the GABAC receptor. While there was clear overlap with our studies, they concluded Loop F had two functional regions, with the first half of Loop F coupled to binding and the second half coupled to activation. There are two noteworthy differences in methodology. First, they employed a different fluorophore. And second, their conclusion was based on a SCAM analysis of Loop F as well as the actions of picrotoxin. It is widely accepted that this non-competitive antagonist binds to the pore region (Gurley et al. 1995; Xu et al. 1995; Das & Dillon, 2005; Sedelnikova et al. 2006). These particular results, while certainly intriguing, are difficult to interpret since the mechanism of picrotoxin block remains an enigma (Constanti, 1978; Newland & Cull-Candy, 1992; Pribilla et al. 1992; Erkkila et al. 2008). There have been two recent VCF studies (including ours) that have challenged the hypothesis that Loop F plays a role in channel activation. They both concluded that Loop F is moving in response to ligand binding, but does not seem to play a direct role in linking ligand binding to channel opening (e.g. transduction). These studies have been performed on an array of receptors within the LGIC family and have resulted in a mixed understanding of Loop F's role during activation. It is possible that Loop F could play a different role during activation for each receptor subtype. However, given the regions that form the ligand binding site and the gate are conserved, as well as other regions across the LGIC family, we have assumed that the activation structures and mechanisms are conserved. One possible role we favour for Loop F is that it may be part of the machinery that serves to lock the ligand into the binding pocket. The structural model in Fig. 4 shows that Loop C and Loop F are adjacent in the primary sequence of the subunit with approximately six ‘connecting’ residues. It is conceivable that these two loops are tightly coupled and therefore any structural rearrangements occurring in Loop C would be directly transferred to Loop F (and vice versa). Also note from Fig. 4 that the connected Loop F and Loop C span adjacent binding pockets. Although highly speculative, this connection could functionally couple binding sites leading to changes in affinity in Site A when Site B is agonist-bound, for example. Again, highly speculative, but this feature may underlie the agonist ‘cooperativity’ reported for the activation of these LGICs (Edelstein & Changeux, 1996). Nevertheless, we are now focusing our attention on other domains of the GABA binding pocket as possible links between the rearrangements in the binding pocket and the opening of the gate.
Figure 4.
View of the structural model depicting the link between Loop C and Loop F Note how the connected Loop C and Loop F each exist in distinct (neighbouring) binding sites. Although speculation, this feature could serve to functionally couple the adjacent binding sites.
References
- Akabas HM, Stauffer DA, Xu M, Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Amin J, Weiss DS. Homomeric ρ1 GABA channels: activation properties and domains. Receptors Channels. 1994;2:227–236. [PubMed] [Google Scholar]
- Barnard E, Seeburg P. Structural Basis of the GABA-Activated chloride channel: Molecular biology and molecular electrophysiology. Adv Biochem Psychopharmacol. 1988;45:1–18. [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Forced subunit assembly in α1β2γ2 GABAA receptors: insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Newell JG, Czajkowski C. GABA(A) receptor beta 2 Tyr97 and Leu99 line the GABA-binding site. Insights into mechanisms of agonist and antagonist actions. J Biol Chem. 2002;277:2931–2937. doi: 10.1074/jbc.M109334200. [DOI] [PubMed] [Google Scholar]
- Bourne Y, Talley TT, Hansen SB, Taylor P, Marchot P. Crystal structure of a Cbtx-AChBP complex reveals essential interactions between snake alpha-neurotoxins and nicotinic receptors. EMBO J. 2005;24:1512–1522. doi: 10.1038/sj.emboj.7600620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, Van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotininc receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Celie PH, Klaassen RV, van Rossum-Fikkert SE, van Elk R, van Nierop P, Smit AB, Sixma TK. Crystal structure of acetylcholine-binding protein from Bulinus truncatus reveals the conserved structural scaffold and sites of variation in nicotinic acetylcholine receptors. J Biol Chem. 2005;280:26457–26466. doi: 10.1074/jbc.M414476200. [DOI] [PubMed] [Google Scholar]
- Chang Y, Ghansah E, Chen Y, Ye J, Weiss D. Desensitization mechanism of GABA receptors revealed by single oocyte binding and receptor function. J Neurosci. 2002;22:7982–7990. doi: 10.1523/JNEUROSCI.22-18-07982.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Weiss DS. Site specific fluorescence reveals distinct structural changes with GABA receptor activation and antagonism. Nat Neurosci. 2002;5:1163–1168. doi: 10.1038/nn926. [DOI] [PubMed] [Google Scholar]
- Cheng X, Lu B, Grant B, Law RJ, McCammon JA. Channel opening motion of alpha7 nicotinic acetylcholine receptor as suggested by normal mode analysis. J Mol Biol. 2006;355:310–324. doi: 10.1016/j.jmb.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Constanti A. The ‘mixed’ effect of picrotoxin on the GABA dose/conductance relation recorded from lobster muscle. Neuropharmacology. 1978;17:159–167. doi: 10.1016/0028-3908(78)90095-3. [DOI] [PubMed] [Google Scholar]
- Corringer P-J, Le Novère N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- Cutting GR, Lu L, O’Hara BF, Kasch LM, Montrose-Rafizadeh C, Donovan DM, Shimada S, Antonarakis SE, Guggino WB, Uhl GR, Kazazian HH. Cloning of the γ-aminobutyric acid (GABA) ρ1 cDNA: A GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci U S A. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Dillon GH. Molecular determinants of picrotoxin inhibition of 5-hydroxytryptamine type 3 receptors. J Pharmacol Exp Ther. 2005;314:320–328. doi: 10.1124/jpet.104.080325. [DOI] [PubMed] [Google Scholar]
- Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, Chen L. Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 A resolution. Nat Neurosci. 2007;10:953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- Edelstein SJ, Changeux J-P. Allosteric proteins after thirty years: the binding and state functions of the neuronal α7 nicotinic acetylcholine receptors. Experientia. 1996;52:1083–1090. doi: 10.1007/BF01952106. [DOI] [PubMed] [Google Scholar]
- Enz R, Brandstatter JH, Hartveit E, Wassle H, Bormann J. Expression of GABA receptor rho 1 and rho 2 subunits in the retina and brain of the rat. Eur J Neurosci. 1995;7:1495–1501. doi: 10.1111/j.1460-9568.1995.tb01144.x. [DOI] [PubMed] [Google Scholar]
- Enz R, Brandstatter JH, Wassle H, Bormann J. Immunocytochemical localization of the GABAc receptor rho subunits in the mammalian retina. J Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkkila BE, Sedelnikova AV, Weiss DS. Stoichiometric pore mutations of the GABAAReceptor reveal a pattern of hydrogen bonding with picrotoxin. Biophys J. 2008;94:4299–4306. doi: 10.1529/biophysj.107.118455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garret M, Bascles L, Boue-Grabot E, Sartor P, Charron G, Bloch B, Margolskee RF. An mRNA encoding a putative GABA-gated chloride channel is expressed in the human cardiac conduction system. J Neurochem. 1997;68:1382–1389. doi: 10.1046/j.1471-4159.1997.68041382.x. [DOI] [PubMed] [Google Scholar]
- Grenningloh G, Rienitz A, Schmitt B, Methfessel C, Zensen M, Beyreuther K, Gundelfinger ED, Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987;328:215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Gurley D, Amin J, Ross PC, Weiss DS, White G. Point mutations in the M2 region of the α, β, or γ subunit of the GABAA channel that abolish block by picrotoxin. Receptors Channels. 1995;3:13–20. [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 2005;24:3635–3646. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Czajkowski C. Structural mechanisms underlying benzodiazepine modulation of the GABA(A) receptor. J Neurosci. 2008;28:3490–3499. doi: 10.1523/JNEUROSCI.5727-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NJ, Lummis SC. Molecular modelling of the GABA(C) receptor ligand-binding domain. J Mol Model. 2006;12:317–324. doi: 10.1007/s00894-005-0034-6. [DOI] [PubMed] [Google Scholar]
- Hedblom E, Kirkness EF. A novel class of GABAA receptor subunit in tissues of the reproductive system. J Biol Chem. 1997;272:15346–15350. doi: 10.1074/jbc.272.24.15346. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- Khatri A, Sedelnikova A, Weiss DS. Structural Rearrangements in Loop F of the GABA Receptor Signal Ligand Binding, Not Channel Activation. Biophys J. 2009;96:45–55. doi: 10.1016/j.bpj.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khrestchatisky M, Maclennan J, Chiang M, Xu W, Jackson M, Brecha N, Sternini C, Olsen R, Tobin A. A novel α subunit in rat brain GABAA receptors. Neuron. 1989;3:745–753. doi: 10.1016/0896-6273(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Law RJ, Henchman RH, McCammon JA. A gating mechanism proposed from a simulation of a human alpha7 nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 2005;102:6813–6818. doi: 10.1073/pnas.0407739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hattori N, Jiang B, Nakayama Y, Zhang NY, Wu B, Kitagawa K, Taketo M, Matsuda H, Inagaki C. Single cell RT-PCR demonstrates differential expression of GABAC receptor rho subunits in rat hippocampal pyramidal and granule cells. Brain Res Mol Brain Res. 2004;123:1–6. doi: 10.1016/j.molbrainres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Lyford LK, Sproul AD, Eddins D, McLaughlin JT, Rosenberg RL. Agonist-induced conformational changes in the extracellular domain of alpha 7 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;64:650–658. doi: 10.1124/mol.64.3.650. [DOI] [PubMed] [Google Scholar]
- Mannuzzu LM, Moronne MM, Isacoff EY. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science. 1996;271:213–216. doi: 10.1126/science.271.5246.213. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–436. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Mistry DK, Hablitz JJ. Activation of subconductance states by γ-aminobutyric acid and its analogs in chick cerebral neurons. Pflugers Arch. 1990;416:454–461. doi: 10.1007/BF00370754. [DOI] [PubMed] [Google Scholar]
- Newell JG, Czajkowski C. The GABAA receptor alpha 1 subunit Pro174-Asp191 segment is involved in GABA binding and channel gating. J Biol Chem. 2003;278:13166–13172. doi: 10.1074/jbc.M211905200. [DOI] [PubMed] [Google Scholar]
- Newland C, Cull-Candy S. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurons of the rat. J Physiol. 1992;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Takahashi H, Tanabe T, Toyosato M, Furutani Y, Hirose T, Asai M, Inayama S, Miyata T, Numa S. Primary structure of alpha subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982;299:793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Bureau M, Khrestchatisky M, MacLennan AJ, Chiang M-Y, Tobin AJ, Xu W, Jackson M, Sternini C, Brecha N. Isolation of pharmacologically distinct GABA-BENZODIAZEPINE receptors by protein chemistry and molecular cloning. Adv Biochem Psychopharmacol. 1990;46:35–49. [PubMed] [Google Scholar]
- Padgett CL, Lummis SC. The F-loop of the GABAA receptor γ2 subunit contributes to benzodiazepine modulation. J Biol Chem. 2008;283:2702–2708. doi: 10.1074/jbc.M705699200. [DOI] [PubMed] [Google Scholar]
- Pless SA, Lynch JW. Ligand-specific conformational changes in the α1 glycine receptor ligand-binding domain. J Biol Chem. 2009;284:15847–15856. doi: 10.1074/jbc.M809343200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribilla I, Takagi T, Langosch D, Bormann J, Betz H. The atypical M2 segment of the β subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992;11:4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Luddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Seeburg PH. γ-Aminobutyric acidA receptor α5-subunit creates a novel type II benzodiazepine receptor pharmacology. J Neurochem. 1990;54:1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- Rozzo A, Armellin M, Franzot J, Chiaruttini C, Nistri A, Tongiorgi E. Expression and dendritic mRNA localization of GABAC receptor rho1 and rho2 subunits in developing rat brain and spinal cord. Eur J Neurosci. 2002;15:1747–1758. doi: 10.1046/j.1460-9568.2002.02013.x. [DOI] [PubMed] [Google Scholar]
- Sancar F, Ericksen SS, Kucken AM, Teissere JA, Czajkowski C. Structural determinants for high-affinity zolpidem binding to GABA-A receptors. Mol Pharmacol. 2007;71:38–46. doi: 10.1124/mol.106.029595. [DOI] [PubMed] [Google Scholar]
- Schlicker K, Boller M, Schmidt M. GABAC receptor mediated inhibition in acutely isolated neurons of the rat dorsal lateral geniculate nucleus. Brain Res Bull. 2004;63:91–97. doi: 10.1016/j.brainresbull.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Schofield PR, Darlison MG, Fujita N, Burt DR, Stephenson FA, Rodriguez H, Rhee LM, Ramachandran J, Reale V, Glencorse TA, Seeburg PH, Barnard EA. Sequence and functional expression of the GABA-A receptor shows a ligand-gated receptor superfamily. Nature. 1987;328:221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Sedelnikova A, Erkkila BE, Harris H, Zakharkin SO, Weiss DS. Stoichiometry of a pore mutation that abolishes picrotoxin-mediated antagonism of the GABAA receptor. J Physiol. 2006;577:569–577. doi: 10.1113/jphysiol.2006.120287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelnikova A, Smith CD, Zakharkin SO, Davis D, Weiss DS, Chang Y. Mapping the rho1 GABAC receptor agonist binding pocket. Constructing a complete model. J Biol Chem. 2005;280:1535–1542. doi: 10.1074/jbc.M409908200. [DOI] [PubMed] [Google Scholar]
- Shimomura M, Yokota M, Okumura M, Matsuda K, Akamatsu M, Sattelle DB, Komai K. Combinatorial mutations in loops D and F strongly influence responses of the α7 nicotinic acetylcholine receptor to imidacloprid. Brain Res. 2003;991:71–77. doi: 10.1016/j.brainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Szarecka A, Xu Y, Tang P. Dynamics of heteropentameric nicotinic acetylcholine receptor: implications of the gating mechanism. Proteins. 2007;68:948–960. doi: 10.1002/prot.21462. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Padgett CL, Lummis SC. Mutagenesis and molecular modelling reveal the importance of the 5-HT3 receptor F-loop. J Biol Chem. 2006;281:16576–16582. doi: 10.1074/jbc.M601265200. [DOI] [PubMed] [Google Scholar]
- Torres VE, Weiss DS. Identification of a tyrosine in the agonist binding site of the homomeric ρ1 GABA receptor that, when mutated, produces spontaneous opening. J Biol Chem. 2002;277:43741–43748. doi: 10.1074/jbc.M202007200. [DOI] [PubMed] [Google Scholar]
- Tretter T, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Wegelius K, Pasternack M, Hiltunen JO, Rivera C, Kaila K, Saama M, Reeben M. Distribution of GABA receptor r subunit transcripts in the rat brain. Eur J Neurosci. 1998;10:350–357. doi: 10.1046/j.1460-9568.1998.00023.x. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, McAllister G, Vassilatis D, Bonnert TP, Heavens RP, Smith DW, Hewson L, O’Donnell R, Rigby MR, Sirinathsinghji DJ, Marshall G, Thompson SA, Wafford KA, Vassilatis D. Neuronally restricted RNA splicing regulates the expression of a novel GABAA receptor subunit conferring atypical functional properties. J Neurosci. 1997;17:5027–5037. doi: 10.1523/JNEUROSCI.17-13-05027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GG, Karlin A. The location of the gate in the acetylcholine receptor channel. Neuron. 1998;20:1269–1281. doi: 10.1016/s0896-6273(00)80506-1. [DOI] [PubMed] [Google Scholar]
- Wotring VE, Chang Y, Weiss DS. Permeability and selectivity of human homomeric ρ1 GABAC receptors. J Physiol. 1999;521:327–336. doi: 10.1111/j.1469-7793.1999.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Akabas MH. Identification of channel-lining residues in the M2 membrane-spanning segment of the GABAA receptor α1 subunit. J Gen Physiol. 1996;107:195–205. doi: 10.1085/jgp.107.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Covey DF, Akabas MH. Interaction of picrotoxin with GABAA receptor channel-lining residues probed in cysteine mutants. Biophys J. 1995;69:1858–1867. doi: 10.1016/S0006-3495(95)80056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xue F, Chang Y. Agonist- and antagonist-induced conformational changes of loop F and their contributions to the rho1 GABA receptor function. J Physiol. 2009;587:139–153. doi: 10.1113/jphysiol.2008.160093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wen X, Militante J, Hester B, Rhubottom HE, Sun H, Leidenheimer NJ, Yan D, White MM, Machu TK. The role of loop F residues in determining differential d-tubocurarine potencies in mouse and human 5-hydroxytryptamine 3A receptors. Biochemistry. 2007;46:1194–1204. doi: 10.1021/bi0616100. [DOI] [PubMed] [Google Scholar]