Abstract
Ligand-gated ion channels are an important class of signalling protein that depend on small chemical neurotransmitters such as acetylcholine, l-glutamate, glycine and γ-aminobutyrate for activation. Although numerous in number, neurotransmitter substances have always been thought to drive the receptor complex into the open state in much the same way and not rely substantially on other factors. However, recent work on kainate-type (KAR) ionotropic glutamate receptors (iGluRs) has identified an exception to this rule. Here, the activation process fails to occur unless external monovalent anions and cations are present. This absolute requirement of ions singles out KARs from all other ligand-gated ion channels, including closely related AMPA- and NMDA-type iGluR family members. The uniqueness of ion-dependent gating has earmarked this feature of KARs as a putative target for the development of selective ligands; a prospect all the more compelling with the recent elucidation of distinct anion and cation binding pockets. Despite these advances, much remains to be resolved. For example, it is still not clear how ion effects on KARs impacts glutamatergic transmission. I conclude by speculating that further analysis of ion-dependent gating may provide clues into how functionally diverse iGluRs families emerged by evolution. Consequently, ion-dependent gating of KARs looks set to continue to be a subject of topical inquiry well into the future.
Derek Bowie (McGill University, Montreal, Canada) earned his PhD from the University of London in 1992 after completing an undergraduate degree in Biochemistry and Pharmacology at Strathclyde University in Scotland. He then spent the next 2 years as an Eli-Lilly postdoctoral fellow at the Université Louis Pasteur in France with a short stay at the University of Zurich, Switzerland before moving to the National Institutes of Health in the USA where he worked with Mark Mayer on the biophysics of glutamate receptor ion-channel block. After taking up a faculty position at Emory University in 1998, he turned his attention to gating mechanisms, identifying ion-dependent gating of kainate-type glutamate receptors, before moving to McGill in 2002. He is currently a tenured Associate Professor in the Department of Pharmacology and Therapeutics as well as an associate member of the Department of Neurology and Neurosurgery. He serves on the editorial boards of the European Journal of Neuroscience and Current Neuropharmacology as well as being the holder of the Canada Research Chair in Receptor Pharmacology.

Our understanding of the basic events that lead to the opening of any ligand-gated ion channel, such as AMPA- or kainate-type (KARs) ionotropic glutamate receptors (iGluRs), is still remarkably consistent with the seminal work on haemoglobin (Wyman & Allen, 1951) and nicotinic acetylcholine receptors (nAChRs) (del Castillo & Katz, 1957) in the 1950s (Colquhoun, 2006). Wyman & Allen (1951) suggested that the two known conformations of haemoglobin could represent oxygen-bound and -unbound states, the former having a higher affinity for oxygen than the latter (Monod et al. 1965). del Castillo & Katz (1957) extended this idea for ion channels by proposing that the events leading to the opening of nAChRs could be described by two separate molecular events, an initial binding step and a subsequent conformation change into the open state (del Castillo & Katz, 1957). This simplified model proposed that knowledge of two separate quantities of the ligand, affinity (i.e. binding) and efficacy (i.e. gating), were sufficient to describe the behaviour of any agonist at any ligand-gated ion channel (Clements et al. 1992; Colquhoun, 1998). Although a much greater understanding of these events has emerged over the decades (Auerbach, 2003; Colquhoun, 2006; Sivilotti, 2010), the central tenet of their model is that agonist binding is a prerequisite for channel activation.
Despite this, on occasion physiologists have noted that channel gating is affected by other factors such as the ionic conditions (Yellen, 1997). Ion effects on channel gating were first described in a study of nAChRs by Ascher and colleagues who noted that the stability of the open state was dependent on the permeant ion species (Ascher et al. 1978). This finding was later explained by a mechanism whereby permeant ions prevent channel closure whilst occupying the pore (Marchais & Marty, 1979). The importance of this observation was that it revealed that gating and permeation processes need not always behave independently and thus violated an understated assumption of the del Castillo & Katz scheme (del Castillo & Katz, 1957). When Swenson & Armstrong (1981) reported a similar observation whilst working on voltage-gated K+-channels, it became evident that protein structures which regulate channel gating and ion permeation can, in some cases, be coupled. In support of this emerging idea, a number of channel blockers were shown to hinder channel closure in much the same way as permeant ions (Armstrong, 1971; Yeh & Armstrong, 1978; Cahalan & Almers, 1979) establishing the idea that ion flow through the pore can regulate channel behaviour through a ‘foot in the door’ mechanism (Yeh & Armstrong, 1978).
The most compelling evidence supporting the existence of gating and permeation coupling at iGluRs comes from work on an NMDAR pore mutant (Schneggenburger & Ascher, 1997). Here the authors revealed that NMDARs disobey the law of microscopic reversibility (Tolman, 1938) when the external and internal monovalent cation composition is different (i.e. Cs+vs. Na+) (Schneggenburger & Ascher, 1997). Specifically, the cyclic gating scheme proposed by the authors was shown to be driven in a preferential direction determined by the cation present on the cytoplasmic side. It still remains to be determined whether strong coupling of this nature is important for wild-type NMDARs, although, recombinant receptors composed of NR1a and NR2D subunits exhibit a similar but modest degree of gating asymmetry (Wyllie et al. 1996). Since then, endeavours to establish if ions regulate the functional properties of NMDARs has been less conclusive. For example, Antonov and colleagues (1998) have shown that NMDAR gating is unaffected by the permeant ion species or its concentration whereas Yu & Salter (1998) have argued in its favour, in this case, specifically for a role of intracellular Na+.
In this review, I examine the nature of anion and cation modulation of kainate-type (KARs) iGluRs. Given the preponderance of work describing permeant ion effects on channel gating, it was initially hypothesized that ion-dependent KAR gating was somehow related to ions in the pore region. However, it has subsequently been shown to be quite different from all other reported effects of ions on ion channels. The uniqueness of this gating mechanism is that anion and cation binding are a prerequisite for KAR activation. Intriguingly, closely related NMDA- and AMPA-type iGluRs lack ion-dependence suggesting that this feature of KARs may be exploited for the development of selective ligands. The challenge for the future, I conclude, will be to harmonize the emerging structural information with the complexities of the functional properties of KARs.
Ion channel block suggested ion-dependent gating of KARs
The first indication that KARs may be regulated by external ions followed from experiments examining the mechanism of cytoplasmic polyamine block (Bowie et al. 1998). Although original findings had assumed that polyamines work by an open-channel block mechanism (Bowie & Mayer, 1995; Kamboj et al. 1995; Koh et al. 1995), a subsequent study revealed that polyamines block closed channels too and, curiously, enhance channel closure (i.e. deactivation) (Bowie et al. 1998). Interestingly, deactivation was accelerated only at membrane potentials where polyamine block occurred, demonstrating that it was the presence of polyamine in the pore that destabilized the open state of the channel (Bowie et al. 1998).
How might polyamines destabilize the open state? At least two mechanisms could be considered. The first possibility is that polyamine binding may stabilize the closed state, as recently proposed for amantadine block of NMDARs (Blanpied et al. 2005). This mechanism of course is distinct, though not mutually exclusive, from the conventional view that channel block results from occlusion of the open state. The other possibility is that polyamines promote channel closure indirectly by depleting the pore of permeant ions (Bowie et al. 1998) through a mechanism akin to C-type inactivation of voltage-gated potassium channels (Baukrowitz & Yellen, 1995; Baukrowitz & Yellen, 1996). If true, this has several important implications. First, it would suggest unexpectedly that ion occupancy of the pore stabilizes the open state. Second, it would reveal that protein structures responsible for ion flow are somehow coupled to the gating machinery of iGluRs, a property not thought to be common amongst ion channels (Yellen, 1997). Third and finally, it would suggest that iGluR and K+ channel gating may have overlapping features.
The latter possibility was particularly intriguing since an emerging view at the time it was proposed pointed to significant overlap between iGluRs and K+ channels despite their divergent roles in the vertebrate CNS. For example, it was noted that K+ channels and iGluRs share important structural features, including tetrameric subunit stoichiometry (MacKinnon, 1991; Rosenmund et al. 1998) and architectural design of the pore region (Panchenko et al. 2001) as well as possessing common ancestral proteins (Chen et al. 1999; Kuner et al. 2003). More poignantly, amino acid residues implicated in K+ channel gating (Doyle et al. 1998; Perozo et al. 1999) were found to be conserved amongst prokaryotic and eukaryotic iGluRs (Chen et al. 1999). The findings suggested that the gating mechanisms of these two important ion channel families may be shared. Consistent with this, K+ channel and iGluR activation pathways were shown to exhibit notable similarities. For example, K+ channels (Chapman et al. 1997; Zheng & Sigworth, 1997) and iGluRs (Rosenmund et al. 1998; Smith et al. 2000; Smith & Howe, 2000) were found to traverse several intermediate subconductance levels before entering the fully open state. When taken together, it seemed reasonable to also propose that the molecular events that triggered iGluR desensitization may have appreciable similarity to C-type inactivation. However as explained below, subsequent observations disproved this hypothesis but fortuitously led to the identification of a gating mechanism that was unique to KARs.
Elucidating the functional stoichiometry of AMPA and KAR desensitization
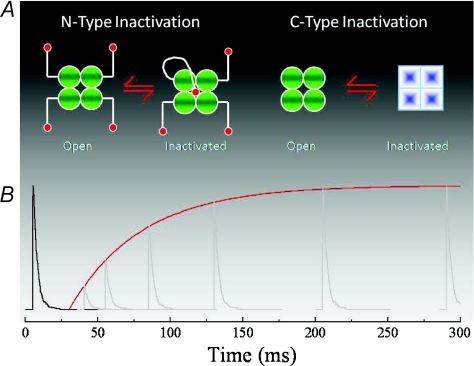
K+ channels inactivate by two distinct mechanisms called N- and C-type inactivation (Fig. 1). N-type inactivation reflects the occlusion of the open channel pore by one of four intracellular blockers tethered to individual subunits (Hoshi et al. 1990; Zagotta et al. 1990), whereas C-type inactivation represents a concerted conformation of all four subunits (Ogielska et al. 1995; Panyi et al. 1995). Importantly, C-type inactivation rates are dependent on the concentration of the external cation, K+ (Baukrowitz & Yellen, 1995). With this in mind, two experiments were designed to compare KAR (and AMPAR) desensitization with K+ channel inactivation. Firstly, the functional stoichiometry or number of kinetic steps involved in KAR desensitization (Bowie & Lange, 2002) was measured and secondly, the ion dependence of desensitization was examined (Bowie, 2002).
Figure 1. N- and C-type inactivation of K+ channels shows first order kinetics.
A, schematic diagrams showing that a single subunit and all four subunits undergo conformational change during N- and C-type inactivation, respectively. B, simulated data showing that recovery from inactivation with first order kinetics can be fitted by a single exponential function (red line).
Since both N- and C-type inactivation exhibit first order kinetics (Fig. 1), the expectation was that AMPA and KARs would behave similarly. To examine this, paired pulses of the agonist l-Glu, were applied at varying time intervals to AMPA or KARs contained in excised patches (Fig. 2). The first agonist application, or conditioning response, was used to accumulate receptors into desensitized state(s). The second application, or test response, provided information on two quantities: (i) the amplitude reported the fraction of the response that had recovered from desensitization and (ii) the time course of decay indicated the rate at which resensitized channels re-enter desensitization. From our understanding of K+-channels, it was known that rates into and out of inactivation were first order and consequently would be fitted by a single exponential function (Fig. 1). Encouragingly, earlier work had also reported similar behaviour of AMPA and KARs (Raman & Trussell, 1992; Patneau et al. 1993; Heckmann et al. 1996; Traynelis & Wahl, 1997; Wilding & Huettner, 1997; Paternain et al. 1998) suggesting that desensitization of both ion channel families was comparable in this respect.
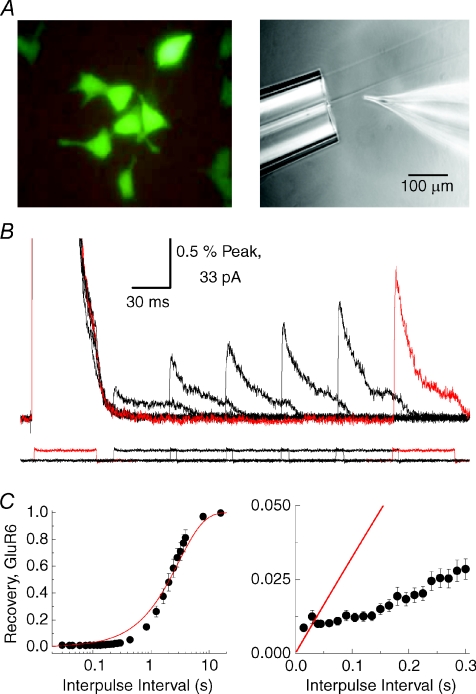
Figure 2. Kainate receptors recover from desensitization in multiple steps.
A, photomicrographs showing the green fluorescent protein (GFP)-stained tsA201 cells and arrangement of the fast agonist perfusion system used to study KAR desensitization kinetics. B, typical experiment showing conditioning and test agonist pulses used to monitor rates into and out of desensitization. A conditioning and a test pulse are highlighted in red. C, summary plot of GluR6 KAR recovery from desensitization in its entirety (left) or in the early stages (right). The continuous red line in each denotes the relationship expected of first order kinetics. Adapted from Bowie & Lange (2002) with permission from the Society for Neuroscience.
A much more complex pattern emerged, however, upon careful examination of the kinetics into and out of AMPA or KAR desensitization. Specifically, experiments revealed for the first time that individual subunits desensitize in several conformational steps making this process distinct from K+-channel inactivation (Fig. 2) (Bowie & Lange, 2002). AMPARs were shown to operate as dimer of dimers which was consistent with findings reported by others (Armstrong & Gouaux, 2000; Ayalon & Stern-Bach, 2001; Robert et al. 2001). The functional stoichiometry of KAR desensitization was unexpectedly dependent on the external ion concentration (Bowie & Lange, 2002). In solutions of high ionic strength, KARs clearly behaved as tetramers but acted as dimers and eventually as monomers as the ionic strength was lowered (Bowie & Lange, 2002). A concern at the time was that the method of counting states traversed during desensitization may underestimate the actual number of molecular events involved. This was particularly pertinent given the multiple subconductance levels associated with KAR activation (Swanson et al. 1996; Howe, 1996). Therefore, it was possible that the relationship between functional stoichiometry and external ion concentration was an inability to fully resolve all transition steps that constitute the macroscopic response. However as explained later, the finding that KAR activation has an absolute requirement for external ions (Wong et al. 2006) provided an alternative interpretation that could be explained in biological terms. In summary, this work placed significant doubt on the initial hypothesis that iGluR and K+ channel gating properties are similar. Additional experiments described below revealed a novel ion-dependent regulation of KARs that was not only distinct from that found in K+ channels but also entirely absent from other closely related iGluR subtypes.
Ion-dependent gating is unique to kainate receptors
The potential effect of ions on AMPA and/or KAR gating was examined to complement work on functional stoichiometry. To do this, deactivation and desensitization rates of each receptor subtype were compared in solutions of differing ionic strength and where Na+ and Cl− were replaced by other monovalent cations and anions, respectively (Fig. 3) (Bowie, 2002). Consistent with the hypothesis that permeant ions stabilize the open state of KARs, deactivation and desensitization rates were appreciably faster in solutions of low ionic strength (Fig. 3) (Bowie, 2002; Bowie & Lange, 2002). Interestingly however, the gating properties of AMPARs were almost entirely unaffected (Fig. 3) (Bowie, 2002; Bowie & Lange, 2002). Up until this point, it had been generally assumed that the gating properties of AMPA and KARs were similar if not identical (Dingledine et al. 1999). However this finding revealed an unappreciated distinction that clearly separated these two closely related iGluR subtypes.
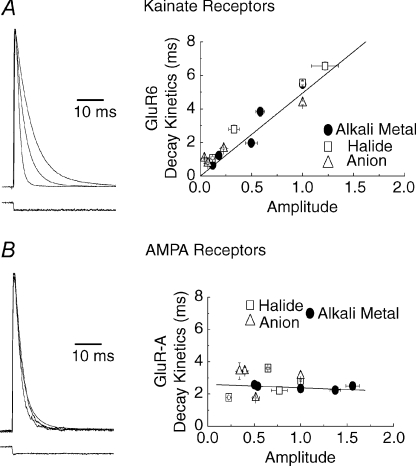
Figure 3. External anions and cations distinguish between AMPA and kainate receptors.
A, macroscopic KAR desensitization is regulated by ion concentration and ion type. Left, plot showing typical membrane currents elicited by GluR6 KARs in symmetrical solutions of 55, 150 and 405 mm NaCl. Right, plot comparing the KAR response amplitude and desensitization kinetics in different external ions. Note that the data are fitted well by a linear regression analysis showing that ions have a concomitant effect on both response amplitude and decay kinetics. B, unlike KARs, AMPAR desensitization is not regulated by changes in external ion concentration (left) nor by the type of ion present (right). Adapted from Bowie & Lange (2002) with permission from the Society for Neuroscience and Bowie (2002).
How were ions affecting the gating properties of KARs? First, the ion concentration only affected KARs when altered on the external surface and not the internal surface of the plasma membrane (Bowie, 2002) placing the binding site(s) on the extracellular portion of the KAR. Second, concentration changes in external ions were similar at all membrane potentials demonstrating that KARs were affected in a voltage-independent manner. Although these results were consistent with Na+ ions acting like a ‘foot in the door’ to prevent channel closure as described for voltage-gated ion channels (Yeh & Armstrong, 1978), a number of other mechanisms involving Na+ and/or Cl− ions could also be at play. Moreover, it was possible that what was important was not the ionic species per se but their total ionic charge or strength. Although these possibilities needed to be examined, some mechanisms could be promptly eliminated. For example, the possibility that external ions screen surface potential on KARs and/or compete with the agonist molecule at its binding site (Akk & Auerbach, 1996). First, deactivation rates were slower not faster at higher ionic strengths (Bowie, 2002). Second, the apparent agonist affinity for KARs did not decrease with increasing ionic strength as would be expected for either mechanism (Bowie, 2002).
To understand if the effects of external ions on KARs were determined by the chemical nature of individual ions or from changes in ionic strength, KAR responses were examined in solutions where the ionic strength was kept constant but the ion composition altered. As a control, ion-substitution experiments were repeated with AMPARs. The external monovalent cation, Na+, was replaced by other alkali metal ions (Li+, K+, Rb+, Cs+) that have similar permeability through non-NMDA receptors (Burnashev et al. 1996). External Cl− was replaced with a number of monovalent anions (F−, Br−, I−, propionate, nitrate, acetate) that are not permeant at unedited Q-form AMPARs or KARs (Burnashev et al. 1996). Upon completion of the experiments, it was revealed that not only were KARs modulated by external cations, they were affected by external anions too (Fig. 3). This led to two important conclusions that would impact later work. First, it demonstrated that an ion's chemical nature and not the solution's ionic strength regulated KARs. Second, it established that ion-dependent regulation of KARs is distinct from C-type inactivation since even non-permeant ions, such as anions, regulate function. Strikingly, AMPARs studied in identical ionic conditions did not exhibit this behaviour (Fig. 3), which further supported the emerging view that gating properties of AMPA and KARs exhibit fundamental distinctions. Another unexpected observation was that external ions affected both the peak response amplitude and decay kinetics of KARs in an apparently concomitant manner (Fig. 3) (Bowie, 2002). As discussed below, this finding was the first indicator that anions and cations were regulating KARs through a mechanism that was entirely novel and not found in other ion channel families.
Kainate receptors have an absolute requirement for external ions
How might external ions regulate the gating properties of KARs? Two opposing mechanisms were evaluated (Wong et al. 2006). In the first case, external ions were viewed as being essentially allosteric modulators of the basal activity of KARs. That is, ions bind to a site distinct from the agonist-binding domain and regulate activity triggered by neurotransmitter binding. This mechanism was consistent with how others had viewed anion and cation effects on other ligand- and voltage-gated ion channels (Yellen, 1997). That is, it was not a new idea from a mechanistic perspective. Importantly from an experimental view, if external ions act as allosteric modulators, KAR activity would not be abolished in circumstances where the ion-binding site(s) was not occupied (e.g. upon removal of external ions). The other possibility that was examined was an entirely novel mechanism that had not been considered for any type of ion channel. In this case, external ions were viewed not as modulators of KAR activity but instead, as an absolute requirement for activation (Wong et al. 2006). With this mechanism, KAR activity would be completely abolished if the binding site(s) for external ions was not occupied.
Having established that these opposing mechanisms could be distinguished by examining KAR responses in solutions lacking Na+ and Cl−, several features of the experimental design needed to be resolved. The primary concern was whether solutions with little ionic strength (i.e. lacking both Na+ and Cl−) would disrupt the biological integrity of the cell's plasma membrane or denature the quaternary structure of the KAR. These issues were addressed in two ways. First, all experiments were performed in excised outside-out membrane patches, which are much more resilient than intact cells to perturbations in the external solution composition (Fig. 2). Second, it was reasoned that if KARs were shown to gate normally in the absence of external ions, it would verify that quaternary structure was, at least, sufficiently undisturbed for the channel to be functional. The problem was how to show this experimentally since removal of external ions may indeed abolish receptor function. The answer was provided by an observation reported in a collaborative study by the labs of Juan Lerma and Yael Stern-Bach (Paternain et al. 2003).
Essentially they examined the ion sensitivity of AMPA–KAR chimeras and correctly identified a single methionine residue (Met770) as the molecular determinant of external cation effects on GluR6 KARs. Although the M770 residue is restricted to GluR6 and GluR7 subunits, equivalent residues in other KAR subunits also confer sensitivity to external cations (Paternain et al. 2003). Interestingly the data of Paternain et al. suggested that mutation of Met770 did not affect anion modulation, which was surprising given the great similarity between anion and cation effects on wild-type KARs (Bowie, 2002) (see below though). Based on their homology model, Met770 was located on the extracellular surface far from the conduction pore and agonist-binding pocket (Paternain et al. 2003), which was in good argument with predictions made in an earlier study (Bowie, 2002). At the time, it was not at all clear if Met770 was responsible for establishing a cation binding site. The principal concern was that methionine is neutral at physiological pH and therefore did not seem to provide the appropriate electrostatic environment to attract cations. Indeed, it was entirely possible that Met770 was located downstream of the actual cation binding site(s) where it fulfilled a role in coupling cation binding to the gating machinery. Intriguingly, though, a positively charged lysine (K759 at GluR1) was found at the homologous position on all AMPAR subunits. If the Met770 site was part of the cation binding site, replacement by a Lys residue would be expected to act as a tethered cation thus underpinning the lack of ion sensitivity of AMPARs. The actual location of cation binding site(s) would be resolved later (see below), but the identification of the Met/Lys 770 site in the meantime provided the last detail needed to examine the relationship between KARs and external Na+ and Cl− ions.
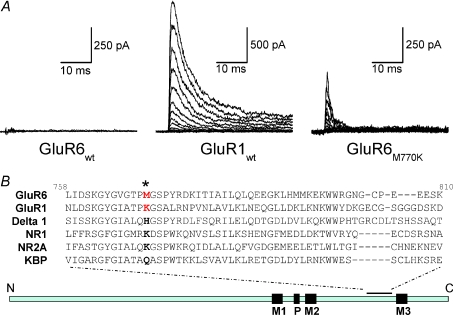
With everything in place, experiments were performed to directly test if KAR activation has an absolute requirement for external ions. For comparison, experiments were repeated on AMPARs and the KAR M770K mutant as well as wild-type GluR6 (Fig. 4). Consistent with the hypothesis that external ions are an absolute requirement for activation, wild-type KARs were entirely unresponsive in solutions lacking external ions (Fig. 4). In contrast, AMPARs were fully responsive (Fig. 4), suggesting that external ions are not an absolute requirement for the gating behaviour of this iGluR subfamily. Note that in Fig. 4, the membrane current observed represents the outward movement of permeating ions (i.e. Na+) through AMPARs from the internal solution of the patch pipette. Interestingly, the KAR M770K mutant was also responsive in solutions lacking external NaCl (Fig. 4), supporting the pivotal role of the Met/Lys site in determining KAR gating behaviour. This observation also showed that experiments performed in low ionic strength solutions did not denature the mutant KAR's quaternary structure and, by implication, the wild-type one too. It was interesting, however, that replacement of Met with a Lys reside did not restore the full activity of wild-type KARs. This observation was later explained by realizing that the tethered Lys residue closely mimicked the properties of external Rb+ and not Na+ ions (Wong et al. 2007).
Figure 4. KARs have an absolute requirement for external ions.
A, a family of membrane currents evoked by 1 mm l-Glu in the absence of all external ions at a range of membrane potentials (−100 to +110 mV, 15 mV increments). Responses evoked at GluR1 AMPARs (middle) and GluR6M770K KARs (right) are outward currents due to the outflow of Na+ ions from the internal pipette solution. In contrast, in identical solutions, GluR6 KARs are unresponsive (left). B, sequence alignment of all iGluR subfamilies around the extracellular M2–M3 linker region. The methionine-770 residue is highlighted by the asterisk and is coloured red. Adapted from Wong et al. (2006) with permission from the Society for Neuroscience.
Given the apparent uniqueness of the mechanism, it was important to identify a specific term that would immediately convey an understanding of this role of external ions. Initially the term ‘coagonist’, which had been used to describe the absolute requirement of NMDARs for extracellular glycine (Johnson & Ascher, 1987; Kleckner & Dingledine, 1988), seemed appropriate since like glycine, receptor activity was abolished in the absence of external ions. However, the glycine molecule most likely interacted with many more contact points on the NMDAR (Inanobe et al. 2005) than would be expected for Na+ and/or Cl− at KARs. Moreover, it was anticipated that the molecular details by which external ions affect KARs would turn out to be distinct from that of glycine at NMDARs. In view of this, it seemed more apt to consider the term ‘cofactor’, which was commonly used to describe non-protein chemical entities, such as ions, that are required for protein activity (e.g. enzymes) (though see Suh & Hille, 2008). The problem in adopting this term was the generality of its use, which seemed contrary to the original aim of identifying a specific term for a unique mechanism. Upon further reflection, the term ‘coactivator’ was chosen since it was reminiscent of ‘cofactor’ whilst being distinct enough to be viewed differently (Wong et al. 2006). Specifically, coactivator was used to include two possible mechanisms: (i) that ions affect KARs simply by binding or (ii) ion binding causes conformational changes in the receptor, which affects function (Wong et al. 2006). Importantly, the effect of ions was considered distinct from the agonist for the simple reason that the channel needs the agonist to gate the pore.
Removal of external ions shifts apparent agonist affinity from low to high binding states
Why do KARs fail to gate in the absence of external ions? As just discussed, it is not due to the failure of the agonist to bind since the mutant M770K KAR responds to l-Glu (Fig. 4) (Wong et al. 2006). From a mechanistic viewpoint, removal of external Na+ and Cl− leads to accumulation of KARs in a conformational state that is unresponsive to neurotransmitter; at least as measured by its ability to transport ions. Stated this way, the ‘unresponsive state’ has a key characteristic normally associated with receptor desensitization. That is, it fails to respond to successive agonist applications. Therefore is it possible that the primary effect of external Na+ and Cl− is to regulate rates into a desensitized state? Although the relationship between external ions and KAR desensitization has not been formally tested, some authors have speculated that this may indeed be the case chiefly based on structural evidence (Plested & Mayer, 2007; Plested et al. 2008). The principle argument is that external ions stabilize the dimer interface which, in turn, correlates with macroscopic desensitization rates (Chaudhry et al. 2009a,b;). There are two caveats to this argument, however. First, the dimer stability may regulate other gating processes in addition to receptor desensitization consequently the relationship between dimer stability and desensitization need not be causal. Second, macroscopic desensitization observed in excised patches may be due to other factors (e.g. deactivation) and therefore may not necessarily represent microscopic desensitization. Simply put, since we do not know the relative rates of activation and desensitization of KARs, it is not possible to infer any information about desensitization, an issue established by work on other ion channels (Colquhoun & Hawkes, 1982; Aldrich et al. 1983; Vandenberg & Horn, 1984).
But what if KARs fail to respond to l-Glu in ion-free solutions because they have desensitized? If true, then two possible mechanisms need to be considered. The first is that removal of external ions spontaneously desensitizes KARs prior to agonist binding and thus accounts for lack of functionality. This mechanism violates the classical definition of desensitization, which is thought to represent an agonist-bound state (Katz & Thesleff, 1957). KARs may not adhere to this, however, in much the same way that other channels gate in the absence of ligand (e.g. Jackson, 1984). The second possibility is that in the absence of external Na+ and Cl−, the onset of desensitization proceeds only after agonist binding. But wouldn't some channels occasionally ‘escape’ into the open state? In other words, is it possible that KARs would still exhibit some degree of functionality? It is still not clear without further experimentation; however, there are other problems with the ‘desensitization hypothesis’. For example, it has been noted that desensitized KARs recover much faster, not slower, in low external NaCl solutions (Bowie & Lange, 2002). If external ions were affecting desensitization, more subunits per KAR tetramer would desensitize as the external ion concentration is lowered. Consequently, recovery from desensitization should be slower in these conditions (see Bowie & Lange (2002) for KARs and Robert & Howe (2003) for AMPARs). However, recovery rates observed experimentally are faster in lower ionic conditions (Bowie & Lange, 2002). There is a similar inconsistency when looking at the rate into desensitization. The onset of KAR desensitization becomes faster as external NaCl is lowered (Bowie, 2002), but it would be expected to be slower since more subunits per tetramer would be desensitized. Both of these findings are inconsistent with external ions regulating KAR desensitization.
So what is the answer? Wong and colleagues have proposed that in the absence of external Na+ and Cl−, KARs become functionally uncoupled from the channel gate in a manner distinct from desensitization (Wong et al. 2007). That is, neurotransmitter binds to the agonist-binding domain but the channel never opens nor does it desensitize. This mechanism need not be at odds with structural work showing that external ions stabilize the dimer interface (Chaudhry et al. 2009a). Although more detail needs to be worked out, it is a good working hypothesis since it is consistent with experimental observations. For example, KARs would be expected to recover from desensitization faster in solutions low in NaCl (Bowie & Lange, 2002) since fewer subunits are able to desensitize in the first place. By the same reasoning, rates into desensitization would be faster in low NaCl as is observed experimentally (Bowie & Lange, 2002; Bowie, 2002). Likewise, it would also account for the changes reported in the functional stoichiometry of KARs (monomer to tetramer) in solutions of different ionic strength (Bowie & Lange, 2002). For example in low ionic conditions, fewer subunits per tetramer would be available for activation; consequently, the KAR would behave more like a monomer. The converse would be true in high ionic conditions since more subunits per tetramer would be available for activation. As a result, the KAR would operate more like a tetramer.
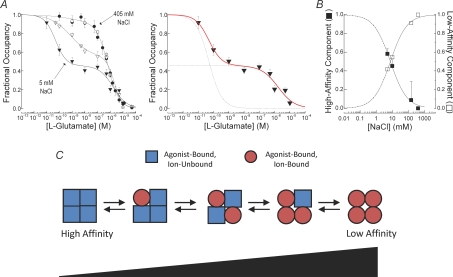
It is not clear what kinetic or conformational state this non-conducting state represents. To denote this uncertainty, Wong and colleagues have simply referred to it as a ‘novel inactive state’ (Wong et al. 2006, 2007). Interestingly, analysis of agonist-induced inhibition of KARs suggests that this state exhibits a much higher apparent agonist affinity (i.e. ion-unbound) than even the desensitized state (i.e. ion-bound). Figure 5 shows the basis of this observation with a series of inhibition curves observed in different concentrations of NaCl. In each case, the curves were generated by bathing KARs in low concentrations of l-Glu and then observing the inhibition of responses to saturating l-Glu. The curves observed in 150 and 405 mm NaCl (Fig. 5A), when almost all ion-binding sites are occupied, were best fitted with a single binding site isotherm estimating the IC50 to be about 0.5 μm. This site is designated as the desensitized state in agreement with other studies (e.g. Robert & Howe, 2003). The inhibition by low concentration of l-Glu is explained by assuming that the KAR desensitizes at agonist concentrations that fail to gate the channel. The situation becomes more complicated, however, as NaCl concentration is lowered. In this case, not all subunits bind external ions (i.e. partial occupancy) and the inhibition plots become biphasic, revealing a high affinity, NaCl-dependent binding site with IC50 values of 50 pm in 5 mm NaCl and about 1 nm in 10 mm NaCl (Fig. 5A). We have designated this site as the ‘novel inactive’ state (Wong et al. 2006). Extrapolated fits of occupancy of the high- and low-affinity states or desensitized and ‘novel inactive’ states (Fig. 5B) reveals that KARs in this high-affinity inactive state accumulate as external ions are lowered. This finding nicely explains the failure of KARs to gate in the absence of external NaCl. However, as discussed in the last sections below, the greatest challenge awaiting future work on ion-dependent gating of KARs will be to reconcile these functional observations with emerging structural information.
Figure 5. External ions regulate occupancy of a novel, high-affinity inactivated state.
A, left, family of inhibition curves to l-Glu in 5 (filled triangle), 10 (open triangle), 150 (filled circle) and 405 mm (open circle) external NaCl. Continuous lines are fits to single- or double-binding site model. Right, inhibition curve observed in 5 mm external NaCl in more detail showing contribution of high- and low-affinity states. B, effect of external NaCl on the fractional occupancy of high- and low-affinity states. Dotted lines represent fit extrapolations. C, schematic diagram to illustrate a possible interpretation of data shown in panels A and B. At saturating levels of NaCl and agonist, all four subunits of the KAR tetramer are occupied at both the neurotransmitter- and ion-binding sites. In relative terms, this state has a low affinity for the agonist and is graphically represented as a tetramer containing four circles. However, as NaCl levels are lowered, fewer and fewer subunits contain bound Na+ ions, which has two effects. First, subunits that are unbound by ions (square symbol) exhibit a significantly higher agonist affinity. Second, ion-unbinding inactivates the KAR subunit and therefore the mature tetramer fails to gate. Adapted from Wong et al. (2006) with permission from the Society for Neuroscience.
Hints at the nature of the anion/cation binding site(s)
As explained previously, the idea that ion channels fail to gate normally under different ionic conditions is not new. For voltage-dependent channels, ion-effects have been attributed to the screening of surface charge located on or in the vicinity of the voltage sensor (Kao & Stanfield, 1968; Hille et al. 1975; Dani et al. 1983). As expected for a charge-screening mechanism, the effects of cations and anions were shown to be distinct, suggesting that several non-identical, local surface charges were located on each protein structure (Dani et al. 1983). However anions and cations elicited apparently identical effects on KARs and, contrary to the charge-screening mechanism, these results favoured a common site of action for ions of opposite charge. Interestingly, it is possible to predict cation selectivity at KARs from electrostatic models developed by Eisenman (1962). Assuming that cation binding stabilizes the open state, the rank order of potency would be Na+ > Li+ > K+ > Rb+ > Cs+, which corresponds to sequence X of the Eisenman series (Bowie, 2002), favouring the binding of smaller rather than larger cations (Eisenman, 1962). Despite this interesting correlation amongst cations, it was nonetheless difficult to account for anion behaviour.
One possibility was that external anions modulate KARs from sites that are distinct from cations but regulate gating behaviour through a common pathway. An alternative possibility was that anon-cation coupling existed as suggested from experiments looking at the weak cation permeability of anion channels (Franciolini & Nonner, 1987). Specifically, Franciolini & Nonner (1987) proposed that anion channels possess a site of net negative charge (in the pore region) that attracts small cations. The pairing of the cation with this negative charge established a dipole that, in turn, attracted anions (Franciolini & Nonner, 1987). Since cations interact differently with various anions, this mechanism could also account for the observations described for KARs (Bowie, 2002).
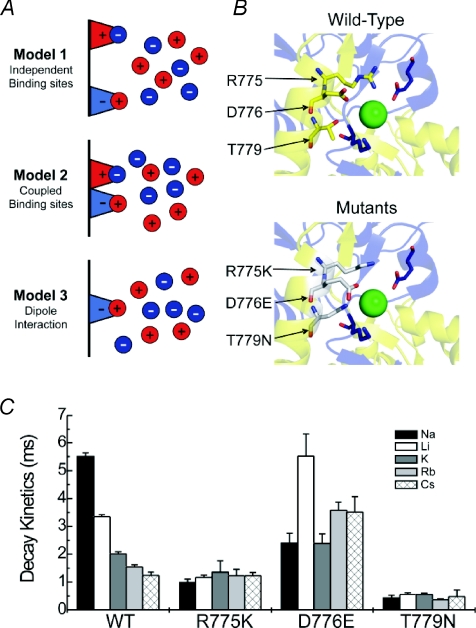
With this in mind, three simple models were considered for their ability to explain anion and cation regulation of KARs (Fig. 6) (Bowie, 2002). Although other mechanisms could be envisaged at the time, in the absence of any structural information, the focus was placed on evaluating models that could be discriminated by experimentation (Wong et al. 2007). The first possibility (Model 1) was that external anions and cations modulate KARs from discrete sites but regulate gating behaviour through a common pathway. This model would be supported experimentally if (1) KAR mutation analysis identified separate anion and cation binding sites and if (2) anions and cations were shown not to interact. The second possibility (Model 2) was that KARs possess separate binding sites that are electrostatically coupled. This model would be favoured if (1) mutation experiments identified separate anion and cation binding sites and if (2) anion–cation interactions were established. The third and final possibility is that anions and cations affect receptor function via a dipole in which either an anion or cation sets up an electric field that attracts a counter ion. Although the KAR protein may also contribute to this electric field, for simplicity and in the absence of any structural information, it would be assumed that it is determined solely by the bound ion (Wong et al. 2007). This model would be favoured if (1) mutation experiments eliminated both anion and cation effects and (2) anions and cations were shown to interact.
Figure 6. Possible mechanisms for monovalent ion interactions at KARs.
A, schematic diagram showing three distinct models to explain the effect of monovalent anions and cations on KARs. B, upper, crystal structure showing critical amino acid residues that constitute the proposed anion binding site for GluR5 KARs. Although only a single dimer is shown, the ion is also conjugated by the corresponding amino acids from the adjacent subunit. Lower, proposed anion binding pocket containing point mutations (R775K, D776E, and T779N) that interfere with both anion and cation modulation of KARs. C, summary plot showing that single point mutants which affect anion binding also have marked effects on cation modulation of KARs. Adapted from Wong et al. (2007) with permission from the Society for Neuroscience.
Structural basis of anion and cation binding
The breakthrough in understanding the structural basis of ion-dependent gating of KARs came unexpectedly with the elucidation of the anion binding pocket (Plested & Mayer, 2007). It was unexpected since prior work had primarily speculated on the nature (Bowie, 2002; Wong et al. 2006) and location (Paternain et al. 2003) of the cation binding site with little thought given to how anions may bind. Plested & Mayer revealed, however, that a single anion atom bound in a cavity established at the interface of two KAR subunits (Figs 6 and 7). Occupancy of the site was complete or saturated at physiological levels of Cl− with an EC50 of around 30 mm (Plested & Mayer, 2007). In addition, anion binding was shown to regulate open channel probability (i.e. Popen) but not the weighted unitary conductance (Plested & Mayer, 2007). This observation explained the original finding that external anions regulate peak KAR responses (Bowie, 2002). But what about their effect on decay kinetics? The authors provided an explanation for this too since mutation of residues that coordinate anion binding reduced dimer stability as well as accelerating macroscopic desensitization. Based on these findings, the authors concluded that anions regulate the fraction of KARs available for activation by regulating KAR desensitization (Plested & Mayer, 2007).
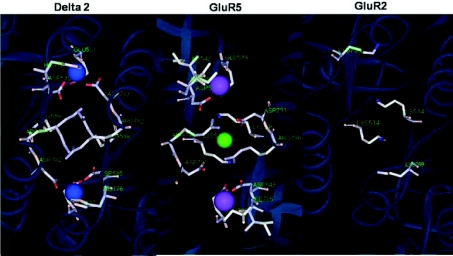
Figure 7. Variations in the cation binding pocket of iGluR families.
Composite image showing the dimer interface of the δ-2 orphan-class iGluR, the GluR5 KAR and GluR2 AMPAR. In each case, amino acids have been labelled from the first Met residue in the N-terminal. Note that δ-2 subunits bind two Ca2+ ions (blue) (Naur et al. 2007) whereas the GluR5 subunit binds two Na+ (purple) and a single Cl− (green) (Plested & Mayer, 2007). In contrast, the positively charged Lys residue acts as a tethered cation at GluR2 AMPARs. Figure is courtesy of Mark Aurousseau.
Taken together, these observations pointed to an unappreciated importance of anion binding to KAR activation. It was all the more surprising since earlier experiments by Wong and colleagues had shown that for KARs to gate in the absence of all external anions and cations, they need only a positively charged Lys tethered to the putative cation binding site (Wong et al. 2006). In other words, only cation and not anion binding was apparently essential for KAR activation. Moreover, as explained above, several important observations detract from the attractiveness of the ‘desensitization hypothesis’ proposed by Plested & Mayer (2007). So how might these data be reconciled? One possibility was that manipulations which affect anion binding may, in fact, affect cation binding too. If true, it would relegate anions to a modulatory role and underscore the preeminence of cation binding for KAR gating. Although Plested & Mayer had excluded possible interactions between anion and cation binding sites, their investigation was limited in scope (Plested & Mayer, 2007). However using a more extensive range of cations, disruption of the anion binding pocket was indeed shown to strongly affect cation binding (Fig. 6C). This finding was further supported by the fact that disruption of the putative cation binding pocket disrupted anion binding too (Wong et al. 2006, 2007). Taken together, these data demonstrated conclusively that there is indeed coupling between anion and cation binding sites (Wong et al. 2007).
With the elucidation of the cation binding pocket, several entirely new aspects of ion-dependent gating were revealed (Plested et al. 2008). First, the authors showed that the location of the cation binding site was indeed discrete from that of anions (Fig. 7). As surmised in an earlier study (Wong et al. 2007), Plested and colleagues established that the Met770 residue is in close proximity to a number of exposed carbonyl residues that contribute to the overall electronegative environment of the cation site (Plested et al. 2008). This finding resolved the conundrum that the cation binding pocket contained a critical residue (i.e. Met at GluR6 KARs) that lacked charge at physiological pH. The authors went on to show that cations bind in a tunnel where the electric field is focused and cation hydration shells are replaced by multiple protein ligands. Second, the authors’ work revealed that anion and cation binding pockets are allosterically and structurally coupled and thus provided a structural explanation of earlier electrophysiological experiments favouring functional coupling (Wong et al. 2007). Interestingly, the KAR dimer interface was shown to bind Na+ and Cl− ions at a stoichiometry of 2: 1 (Fig. 7) though estimates of cation affinity suggested that, unlike anions, cation sites were not all occupied at physiological ion levels.
Future perspectives
In closing, I would like to highlight several outstanding issues that represent some of the most pressing challenges for future work. The first of these relates to the apparent disparity between estimates of functional stoichiometry which suggest a tetramer arrangement (Bowie & Lange, 2002) and structural analysis of KARs which favour a dimer of dimers stoichiometry like AMPARs (Mayer, 2005; Nanao et al. 2005; Plested & Mayer, 2007; Plested et al. 2008; Chaudhry et al. 2009b). The issue is particularly intransigent since both observations seem to be based on quite compelling data. For example, X-ray analysis of the isolated agonist-binding domain shows a clearly defined dimer interface which, when mutated, has predictable effects on receptor function (Fleck et al. 2003; Horning & Mayer, 2004; Zhang et al. 2006; Weston et al. 2006). This observation strongly supports the notion that this is the preferred arrangement adopted by native KARs. Likewise, the conclusion that KARs function as tetramers is supported by the fact that AMPARs are correctly identified as dimer of dimers by the same method used to determining the functional stoichiometry of KARs (Bowie & Lange, 2002). Furthermore, independent biochemical analysis of KARs reports the appearance of monomers, dimers and even trimers in non-denaturating gels (Mah et al. 2005; Vivithanaporn et al. 2007), which would not be expected for a dimer of dimers assembly. Remarkably, trimer assemblies are absent from non-denaturing gels of AMPARs (M. Fleck, personal communication). The appearance of the trimers could be apparent in that the band(s) may represent KAR dimers in association with an accessory protein. The caveat, of course, is that the accessory protein would have to be coincidentally of similar molecular size to a single KAR subunit and remain bound in the different conditions used (Mah et al. 2005; Vivithanaporn et al. 2007). Can both data sets be reconciled by a common explanation? One possibility is that the pore region of KARs does not adopt the 2-fold symmetry shown by the agonist-binding domain. If true, this would have important ramifications for understanding the structural basis of KAR gating since AMPARs exhibit 2-fold rotational symmetry throughout their structure from the ligand binding domain to the outer pore region (Sobolevsky et al. 2004).
The second issue is to understand what, if any, is the physiological role of ion-dependent KAR gating. Large-scale fluctuations in the ionic composition of the extracellular milieu have been well documented (Nicholson et al. 1978; Dietzel et al. 1982; Chesler, 2003). In view of this, Paternain and colleagues (2003) have speculated that KAR function may be affected during intense neuronal activity such as spreading depression where extracellular Na+ levels drop precipitously (Somjen, 2001). Although this is an attractive hypothesis, the immediate challenge will be to design an experiment that can document this effect unambiguously. An added complication is that recombinant heteromeric KARs show significantly weaker modulation by extracellular ions (Paternain et al. 2003). Since almost all native KARs are thought to be heteromeric in nature (Lerma et al. 2001; Huettner, 2003; Pinheiro & Mulle, 2006), this finding suggests that ionic fluctuations accompanying neuronal activity may have a weaker effect than first anticipated.
Perhaps the occurrence of ion-dependent gating has its roots more in the evolution of KARs than as a mechanism to regulate their function. As noted in several studies (Wong et al. 2007; Plested et al. 2008), simpler organisms, such as Caenorhabditis elegans and Caenorhabditis briggsae, contain primitive iGluR-like sequences that possess a Lys residue at the homologous 770-position suggesting that the gating mechanism of KARs may have evolved from an ancestral iGluR protein that behaved more like AMPARs (Wong et al. 2007). A comparison of the dimer interface of KARs with that of AMPARs and the orphan-class δ-2 receptors reveals that subtle but significant changes in their structure during evolution have significantly affected cation binding (Fig. 7). As already discussed, the presence of a Lys residue at the equivalent 770 position of AMPARs circumvents their reliance on external Na+ for gating. For δ-2 receptor dimers, the Met residue at the 770 position is replaced with a histidine residue, which not only changes the architecture of secondary structure but also switches ion-binding preference to the divalent ion, Ca2+ (Naur et al. 2007; Hansen et al. 2009). These observations hint that further experimentation may provide more insight into the evolutionary origin of the various iGluR families.
And finally, is it possible to design KAR-selective drugs that would bind preferentially to the dimer interface? In principle it is, since several important AMPAR modulators, such as cyclothiazide (Sun et al. 2002), CX614 and aniracetam (Jin et al. 2005), have already been shown to bind at different sites in the dimer interface. A common feature of modulator binding is their ability to displace water molecules. For example, four and eight ordered water molecules are displaced by cyclothiazide (Sun et al. 2002) and aniracetam (and CX614) (Jin et al. 2005), respectively. Although the dimer interface of KARs has different dimensions and points of contact (Nanao et al. 2005; Zhang et al. 2006), perhaps not too surprisingly, it contains a large number of displaceable water molecules which may identify sites for exploration. Armed with the knowledge of anion and cation binding pockets and recent advances in ligand-docking software (Moitessier et al. 2008), perhaps the troubled past of KAR pharmacology is over and we can finally set course for new horizons.
Acknowledgments
This work was supported by operating grants from the Canadian Institutes for Health Research, Savoy Foundation, Fragile-X Research Foundation of Canada and the Jessica Carr Foundation. The author is the recipient of the Canada Research Chair award in Receptor Pharmacology. I am indebted to present and former lab members, especially Adrian Wong and David MacLean, for their discussions and Mark Aurousseau for help making Fig. 7. I am also grateful to Dr Mark Fleck for sharing his unpublished data. And finally, I am especially grateful to Dr Jon Johnson for providing many thoughtful comments on an earlier version of this manuscript.
References
- Akk G, Auerbach A. Inorganic, monovalent cations compete with agonists for the transmitter binding site of nicotinic acetylcholine receptors. Biophys J. 1996;70:2652–2658. doi: 10.1016/S0006-3495(96)79834-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich RW, Corey DP, Stevens CF. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983;306:436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Antonov SM, Gmiro VE, Johnson JW. Binding sites for permeant ions in the channel of NMDA receptors and their effects on channel block. Nat Neurosci. 1998;1:451–461. doi: 10.1038/2167. [DOI] [PubMed] [Google Scholar]
- Armstrong CM. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971;58:413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Ascher P, Marty A, Neild TO. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A. Life at the top: the transition state of AChR gating. Sci STKE. 2003;2003:re11. doi: 10.1126/stke.2003.188.re11. [DOI] [PubMed] [Google Scholar]
- Ayalon G, Stern-Bach Y. Functional assembly of AMPA and kainate receptors is mediated by several discrete protein-protein interactions. Neuron. 2001;31:103–113. doi: 10.1016/s0896-6273(01)00333-6. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. doi: 10.1016/0896-6273(95)90185-x. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Yellen G. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+ channel. Science. 1996;271:653–656. doi: 10.1126/science.271.5249.653. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Clarke RJ, Johnson JW. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci. 2005;25:3312–3322. doi: 10.1523/JNEUROSCI.4262-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D. External anions and cations distinguish between AMPA and kainate receptor gating mechanisms. J Physiol. 2002;539:725–733. doi: 10.1113/jphysiol.2001.013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Lange GD. Functional stoichiometry of glutamate receptor desensitization. J Neurosci. 2002;22:3392–3403. doi: 10.1523/JNEUROSCI.22-09-03392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Lange GD, Mayer ML. Activity-dependent modulation of glutamate receptors by polyamines. J Neurosci. 1998;18:8175–8185. doi: 10.1523/JNEUROSCI.18-20-08175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Villarroel A, Sakmann B. Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J Physiol. 1996;496:165–173. doi: 10.1113/jphysiol.1996.sp021674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Almers W. Interactions between quaternary lidocaine, the sodium channel gates, and tetrodotoxin. Biophys J. 1979;27:39–55. doi: 10.1016/S0006-3495(79)85201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ML, VanDongen HM, VanDongen AM. Activation-dependent subconductance levels in the drk1 K channel suggest a subunit basis for ion permeation and gating. Biophys J. 1997;72:708–719. doi: 10.1016/s0006-3495(97)78707-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry C, Plested AJ, Schuck P, Mayer ML. Energetics of glutamate receptor ligand binding domain dimer assembly are modulated by allosteric ions. Proc Natl Acad Sci U S A. 2009a;106:12329–12334. doi: 10.1073/pnas.0904175106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry C, Weston MC, Schuck P, Rosenmund C, Mayer ML. Stability of ligand-binding domain dimer assembly controls kainate receptor desensitization. EMBO J. 2009b;28:1518–1530. doi: 10.1038/emboj.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GQ, Cui C, Mayer ML, Gouaux E. Functional characterization of a potassium-selective prokaryotic glutamate receptor. Nature. 1999;402:817–821. doi: 10.1038/45568. [DOI] [PubMed] [Google Scholar]
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:924–947. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. Agonist-activated ion channels. Br J Pharmacol. 2006;147:S17–S26. doi: 10.1038/sj.bjp.0706502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982;300:1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Dani JA, Sanchez JA, Hille B. Lyotropic anions. Na channel gating and Ca electrode response. J Gen Physiol. 1983;81:255–281. doi: 10.1085/jgp.81.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Castillo J, Katz B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957;146:369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Dietzel I, Heinemann U, Hofmeier G, Lux HD. Stimulus-induced changes in extracellular Na+ and Cl− concentration in relation to changes in the size of the extracellular space. Exp Brain Res. 1982;46:73–84. doi: 10.1007/BF00238100. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Doyle DA, Morais CJ, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Eisenman G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962;2:259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck MW, Cornell E, Mah SJ. Amino-acid residues involved in glutamate receptor 6 kainate receptor gating and desensitization. J Neurosci. 2003;23:1219–1227. doi: 10.1523/JNEUROSCI.23-04-01219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciolini F, Nonner W. Anion and cation permeability of a chloride channel in rat hippocampal neurons. J Gen Physiol. 1987;90:453–478. doi: 10.1085/jgp.90.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Naur P, Kurtkaya NL, Kristensen AS, Gajhede M, Kastrup JS, Traynelis SF. Modulation of the dimer interface at ionotropic glutamate-like receptor δ2 by d-serine and extracellular calcium. J Neurosci. 2009;29:907–917. doi: 10.1523/JNEUROSCI.4081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann M, Bufler J, Franke C, Dudel J. Kinetics of homomeric GluR6 glutamate receptor channels. Biophys J. 1996;71:1743–1750. doi: 10.1016/S0006-3495(96)79375-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, Woodhull AM, Shapiro BI. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975;270:301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Horning MS, Mayer ML. Regulation of AMPA receptor gating by ligand binding core dimers. Neuron. 2004;41:379–388. doi: 10.1016/s0896-6273(04)00018-2. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Howe JR. Homomeric and heteromeric ion channels formed from the kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. J Neurophysiol. 1996;76:510–519. doi: 10.1152/jn.1996.76.1.510. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Kainate receptors and synaptic transmission. Prog Neurobiol. 2003;70:387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Inanobe A, Furukawa H, Gouaux E. Mechanism of partial agonist action at the NR1 subunit of NMDA receptors. Neuron. 2005;47:71–84. doi: 10.1016/j.neuron.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Jackson MB. Spontaneous openings of the acetylcholine receptor channel. Proc Natl Acad Sci U S A. 1984;81:3901–3904. doi: 10.1073/pnas.81.12.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Clark S, Weeks AM, Dudman JT, Gouaux E, Partin KM. Mechanism of positive allosteric modulators acting on AMPA receptors. J Neurosci. 2005;25:9027–9036. doi: 10.1523/JNEUROSCI.2567-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol. 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CY, Stanfield PR. Actions of some anions on electrical properties and mechanical threshold of frog twitch muscle. J Physiol. 1968;198:291–309. doi: 10.1113/jphysiol.1968.sp008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Thesleff S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Koh DS, Burnashev N, Jonas P. Block of native Ca2+-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol. 1995;486:305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner T, Seeburg PH, Guy HR. A common architecture for K+ channels and ionotropic glutamate receptors? Trends Neurosci. 2003;26:27–32. doi: 10.1016/s0166-2236(02)00010-3. [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Rodriguez-Moreno A, Lopez-Garcia JC. Molecular physiology of kainate receptors. Physiol Rev. 2001;81:971–998. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- Mah SJ, Cornell E, Mitchell NA, Fleck MW. Glutamate receptor trafficking: endoplasmic reticulum quality control involves ligand binding and receptor function. J Neurosci. 2005;25:2215–2225. doi: 10.1523/JNEUROSCI.4573-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchais D, Marty A. Interaction of permeant ions with channels activated by acetylcholine in Aplysia neurones. J Physiol. 1979;297:9–45. doi: 10.1113/jphysiol.1979.sp013025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML. Crystal structures of the GluR5 and GluR6 ligand binding cores: molecular mechanisms underlying kainate receptor selectivity. Neuron. 2005;45:539–552. doi: 10.1016/j.neuron.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Moitessier N, Englebienne P, Lee D, Lawandi J, Corbeil CR. Towards the development of universal, fast and highly accurate docking/scoring methods: a long way to go. Br J Pharmacol. 2008;153(Suppl 1):S7–26. doi: 10.1038/sj.bjp.0707515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Nanao MH, Green T, Stern-Bach Y, Heinemann SF, Choe S. Structure of the kainate receptor subunit GluR6 agonist-binding domain complexed with domoic acid. Proc Natl Acad Sci U S A. 2005;102:1708–1713. doi: 10.1073/pnas.0409573102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naur P, Hansen KB, Kristensen AS, Dravid SM, Pickering DS, Olsen L, Vestergaard B, Egebjerg J, Gajhede M, Traynelis SF, Kastrup JS. Ionotropic glutamate-like receptor δ2 binds d-serine and glycine. Proc Natl Acad Sci U S A. 2007;104:14116–14121. doi: 10.1073/pnas.0703718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, ten Bruggencate G, Stockle H, Steinberg R. Calcium and potassium changes in extracellular microenvironment of cat cerebellar cortex. J Neurophysiol. 1978;41:1026–1039. doi: 10.1152/jn.1978.41.4.1026. [DOI] [PubMed] [Google Scholar]
- Ogielska EM, Zagotta WN, Hoshi T, Heinemann SH, Haab J, Aldrich RW. Cooperative subunit interactions in C-type inactivation of K channels. Biophys J. 1995;69:2449–2457. doi: 10.1016/S0006-3495(95)80114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchenko VA, Glasser CR, Mayer ML. Structural similarities between glutamate receptor channels and K+ channels examined by scanning mutagenesis. J Gen Physiol. 2001;117:345–360. doi: 10.1085/jgp.117.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyi G, Sheng Z, Deutsch C. C-type inactivation of a voltage-gated K+ channel occurs by a cooperative mechanism. Biophys J. 1995;69:896–903. doi: 10.1016/S0006-3495(95)79963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain AV, Cohen A, Stern-Bach Y, Lerma J. A role for extracellular Na+ in the channel gating of native and recombinant kainate receptors. J Neurosci. 2003;23:8641–8648. doi: 10.1523/JNEUROSCI.23-25-08641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain AV, Rodriguez-Moreno A, Villarroel A, Lerma J. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology. 1998;37:1249–1259. doi: 10.1016/s0028-3908(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Vyklicky L, Jr, Mayer ML. Hippocampal neurons exhibit cyclothiazide-sensitive rapidly desensitizing responses to kainate. J Neurosci. 1993;13:3496–3509. doi: 10.1523/JNEUROSCI.13-08-03496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E, Cortes DM, Cuello LG. Structural rearrangements underlying K+-channel activation gating. Science. 1999;285:73–78. doi: 10.1126/science.285.5424.73. [DOI] [PubMed] [Google Scholar]
- Pinheiro P, Mulle C. Kainate receptors. Cell Tissue Res. 2006;326:457–482. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- Plested AJ, Mayer ML. Structure and mechanism of kainate receptor modulation by anions. Neuron. 2007;53:829–841. doi: 10.1016/j.neuron.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Plested AJ, Vijayan R, Biggin PC, Mayer ML. Molecular basis of kainate receptor modulation by sodium. Neuron. 2008;58:720–735. doi: 10.1016/j.neuron.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Trussell LO. The kinetics of the response to glutamate and kainate in neurons of the avian cochlear nucleus. Neuron. 1992;9:173–186. doi: 10.1016/0896-6273(92)90232-3. [DOI] [PubMed] [Google Scholar]
- Robert A, Howe JR. How AMPA receptor desensitization depends on receptor occupancy. J Neurosci. 2003;23:847–858. doi: 10.1523/JNEUROSCI.23-03-00847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Irizarry SN, Hughes TE, Howe JR. Subunit interactions and AMPA receptor desensitization. J Neurosci. 2001;21:5574–5586. doi: 10.1523/JNEUROSCI.21-15-05574.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Ascher P. Coupling of permeation and gating in an NMDA-channel pore mutant. Neuron. 1997;18:167–177. doi: 10.1016/s0896-6273(01)80055-6. [DOI] [PubMed] [Google Scholar]
- Sivilotti L. What single channel analysis tells us of the activation mechanism of ligand-gated channels: the case of the glycine receptor. J Physiol. 2010;588:45–58. doi: 10.1113/jphysiol.2009.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TC, Howe JR. Concentration-dependent substate behaviour of native AMPA receptors. Nat Neurosci. 2000;3:992–997. doi: 10.1038/79931. [DOI] [PubMed] [Google Scholar]
- Smith TC, Wang LY, Howe JR. Heterogeneous conductance levels of native AMPA receptors. J Neurosci. 2000;20:2073–2085. doi: 10.1523/JNEUROSCI.20-06-02073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Yelshansky MV, Wollmuth LP. The outer pore of the glutamate receptor channel has 2-fold rotational symmetry. Neuron. 2004;41:367–378. doi: 10.1016/s0896-6273(04)00008-x. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG. Effect of RNA editing and subunit co-assembly single-channel properties of recombinant kainate receptors. J Physiol. 1996;492:129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson RP, Jr, Armstrong CM. K+ channels close more slowly in the presence of external K+ and Rb+ Nature. 1981;291:427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
- Tolman RC. The Principles of Statistical Mechanics. Oxford: Oxford University Press; 1938. [Google Scholar]
- Traynelis SF, Wahl P. Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. J Physiol. 1997;503:513–531. doi: 10.1111/j.1469-7793.1997.513bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg CA, Horn R. Inactivation viewed through single sodium channels. J Gen Physiol. 1984;84:535–564. doi: 10.1085/jgp.84.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivithanaporn P, Lash LL, Marszalec W, Swanson GT. Critical roles for the M3-S2 transduction linker domain in kainate receptor assembly and postassembly trafficking. J Neurosci. 2007;27:10423–10433. doi: 10.1523/JNEUROSCI.2674-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MC, Gertler C, Mayer ML, Rosenmund C. Interdomain interactions in AMPA and kainate receptors regulate affinity for glutamate. J Neurosci. 2006;26:7650–7658. doi: 10.1523/JNEUROSCI.1519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. J Neurosci. 1997;17:2713–2721. doi: 10.1523/JNEUROSCI.17-08-02713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AY, Fay AM, Bowie D. External ions are coactivators of kainate receptors. J Neurosci. 2006;26:5750–5755. doi: 10.1523/JNEUROSCI.0301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AY, MacLean DM, Bowie D. Na+/Cl− dipole couples agonist binding to kainate receptor activation. J Neurosci. 2007;27:6800–6809. doi: 10.1523/JNEUROSCI.0284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Nassar M, Schoepfer R, Colquhoun D. Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proc Biol Sci. 1996;263:1079–1086. doi: 10.1098/rspb.1996.0159. [DOI] [PubMed] [Google Scholar]
- Wyman J, Allen DW. The problem of the heme interactions in hemoglobin and the basis of the Bohr effect. J Polym Sci. 1951;8:499–518. [Google Scholar]
- Yeh JZ, Armstrong CM. Immobilisation of gating charge by a substance that simulates inactivation. Nature. 1978;273:387–389. doi: 10.1038/273387a0. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single channel seeks permeant ion for brief but intimate relationship. J Gen Physiol. 1997;110:83–85. doi: 10.1085/jgp.110.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Salter MW. Gain control of NMDA-receptor currents by intracellular sodium. Nature. 1998;396:469–474. doi: 10.1038/24877. [DOI] [PubMed] [Google Scholar]
- Zagotta WN, Hoshi T, Aldrich RW. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990;250:568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nayeem N, Nanao MH, Green T. Interface interactions modulating desensitization of the kainate-selective ionotropic glutamate receptor subunit GluR6. J Neurosci. 2006;26:10033–10042. doi: 10.1523/JNEUROSCI.2750-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Sigworth FJ. Selectivity changes during activation of mutant Shaker potassium channels. J Gen Physiol. 1997;110:101–117. doi: 10.1085/jgp.110.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]