Abstract
Several patterns of coherent activity have been described in developing cortical structures, thus providing a general framework for network maturation. A detailed timely description of network patterns at circuit and cell levels is essential for the understanding of pathogenic processes occurring during brain development. Disturbances in the expression timetable of this pattern sequence are very likely to affect network maturation. This review focuses on the maturation of coherent activity patterns in developing neocortical structures. It emphasizes the intrinsic and synaptic cellular properties that are unique to the immature neocortex and, in particular, the critical role played by extracellular glutamate in controlling network excitability and triggering synchronous network waves of activity.

Rosa Cossart is currently leading a research group in the Institut de Neurobiologie de la Méditerranée (INSERM U901, Marseille, France). Her research interests focus on the maturation of functional cortical GABAergic microcircuits. She was not initially trained as a neurobiologist but as a physicist with a strong education in mathematics, studying engineering in the Ecole Centrale de Paris. In 2001, she obtained a PhD in biophysics at Paris VI University under the supervision of Dr C. Bernard in the laboratory directed by Dr Yehezkel Ben-Ari. She next pursued her research training as a postdoctoral fellow in Professor Rafael Yuste's laboratory at Columbia University (New York, USA). There she developed a novel approach to study network dynamics in brain slices that combines two-photon calcium imaging with online mathematical analysis and targeted electrophysiological recordings. Since 2002 she has been as a permanent research fellow in the Centre National de la Recherche Scientifique. She received in 2005 a ‘Medaille de Bronze’ from the CNRS, an award that recognizes her early research achievements.
The immature brain is endowed with the ability to generate a variety of coherent activity patterns (O’Donovan et al. 1998; Roerig & Feller, 2000; Moody & Bosma, 2005; Khazipov & Luhmann, 2006; Ben-Ari et al. 2007). Several studies indicate that as development proceeds, synchronous neuronal activity displays changing dynamics and is controlled by distinct mechanisms (Syed et al. 2004; Khazipov & Luhmann, 2006; McCabe et al. 2006; Allene et al. 2008; Sibilla et al. 2009). These successive stages closely parallel the maturation of physiological and morphological cellular properties (Picken Bahrey & Moody, 2003; Moody & Bosma, 2005; Torborg & Feller, 2005; Guido, 2008; Sibilla et al. 2009). The neocortex is the structure for which probably the most compelling variety of network patterns have been described (Yuste et al. 1992; Kandler & Katz, 1998; Owens & Kriegstein, 1998; Garaschuk et al. 2000; Peinado, 2000; Voigt et al. 2001; Opitz et al. 2002; Corlew et al. 2004; Khazipov et al. 2004; Weissman et al. 2004; Adelsberger et al. 2005; Dupont et al. 2005; McCabe et al. 2006; Milh et al. 2007b). This variety not only reflects an endogenous developmental programme, but also the multiplicity of experimental approaches and animal models used to study network oscillations. Hence, network patterns which were named differently because they were measured in different experimental conditions (e.g. in vivo vs. in vitro, calcium imaging vs. electrophysiology, etc.) may actually refer to the same biological phenomenon. In an attempt to assign a function to each synchronous neuronal activity pattern it is therefore essential to review and compare their different mechanisms and conditions of observation. Cortical early network oscillations (cENOs) are large-scale oscillatory calcium waves occurring immediately after birth at low frequency and providing most of the coherent activity during the first postnatal week in the developing rodent neocortex (Garaschuk et al. 2000). These were described both in vitro and in vivo using imaging techniques (Garaschuk et al. 2000; Adelsberger et al. 2005) and more recently using electrophysiological approaches (Allene et al. 2008; Yang et al. 2009). Many developmental network patterns are mediated by GABAergic transmission given its early excitatory actions and advanced maturation compared to glutamatergic synapses (Ben-Ari et al. 2007). Remarkably, cENOs were shown to be generated by the activation of NMDA receptors (NMDA-Rs) and are critically dependent on extracellular glutamate concentration (Garaschuk et al. 2000; Allene et al. 2008; Yang et al. 2009). This feature imparts to the immature cerebral cortex a critical sensitivity to pathological transmitter accumulations. It also confers on glutamate a critical role in early cortical development. Furthermore, cENOs are preferentially observed under specific conditions such as mild anoxia. This observation questions the physiological relevance of cENOs. In this review, we will discuss the mechanisms, developmental profile and dynamics specific to cENOs in order to propose a relevant function for this network pattern and NMDA-R-driven oscillations in general during brain maturation.
A general sequence for the maturation of coherent activity patterns in cortical structures
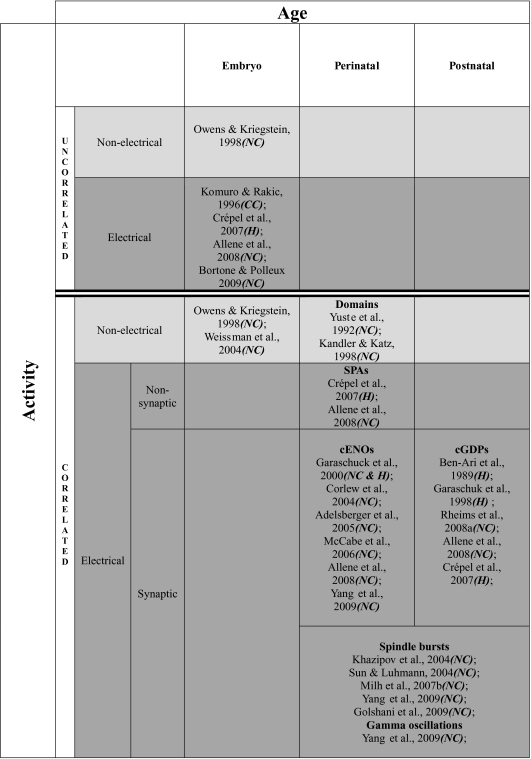
Most developing peripheral and central neurons are spontaneously active. In the cortex, neuronal activity is associated with an intracellular calcium rise that can either be produced by a membrane potential depolarization measurable with electrophysiological approaches or be produced intracellularly without any electrical signature, although measurable with optical approaches (see Fig. 1). Spontaneous activity is further subdivided into uncorrelated and coherent activity patterns (see Fig. 1). Coherent electrical activity patterns progressively emerge during cortical development.
Figure 1. Spontaneous activity patterns in the developing rodent cortex.
NC: neocortex; CC: cerebellar cortex; H: hippocampus.
Calcium activity at embryonic stages consists of either uncorrelated membrane potential spikes (Komuro & Rakic, 1996; Crépel et al. 2007; Allene et al. 2008; Bortone & Polleux, 2009) or synchronous ‘non-electrical’ calcium rises (Owens & Kriegstein, 1998; Weissman et al. 2004; see Fig. 1). Embryonic calcium activity in cortical structures has been suggested to play a role in the regulation of neurogenesis (Owens & Kriegstein, 1998; Weissman et al. 2004) in neuronal differentiation and migration (Komuro & Rakic, 1996; Bortone & Polleux, 2009). Primitive forms of activity in embryonic cortical structures are mostly uncorrelated calcium rises that participate in the maturation of intrinsic neuronal properties.
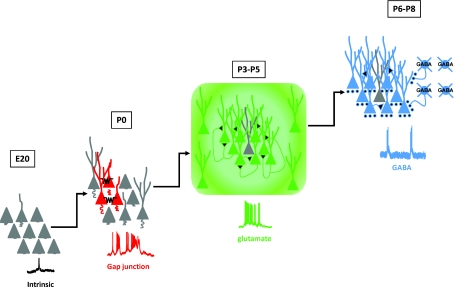
Around birth in rodents, neuronal activity becomes coherent in cortical structures. Several patterns of synchronous neuronal activity have been described (see Figs 1 and 2). With the exception of cortical domains (Yuste et al. 1992; Kandler & Katz, 1998), all of them are associated with electrical activity (see Fig. 1). There is a robust timetable in the mechanisms responsible for the synchronization of neuronal activity: population coherence first relies on gap-junction coupling and on the activation of intrinsic voltage-dependent conductances before becoming mostly synapse-driven (see Fig. 1). We have recently established in both the neocortex (Allene et al. 2008) and the hippocampus (Crépel et al. 2007) that the earliest coherent electrical activity pattern emerges at birth in the form of synchronous plateau assemblies (SPAs), so named because of their characteristic spatial–temporal dynamics: SPAs involve small groups of neurons producing synchronous calcium plateaus. Each calcium plateau is associated with sustained intrinsic membrane potential oscillations. SPAs are therefore a step of coherent electrical activity common to hippocampal and neocortical networks that precedes the emergence of synapse-driven network oscillations (Fig. 2).
Figure 2. A general sequence for the maturation of coherent electrical activity patterns.
Schematic representation of the sequential maturation of synchronized electrical activity patterns from late embryonic stages to the end of the first postnatal week in neocortical rodent slices (Allene et al. 2008). At embryonic stages electrical activity is uncorrelated. At birth it becomes synchronized through gap junctions and is supported by the activation of voltage-gated intrinsic conductances (SPAs). Later, network patterns are synapse-driven by glutamatergic (ENOs) or GABAergic (GDPs) transmission. Note that extrasynaptic glutamate receptors are also likely to be involved in the generation of ENOs. With the exception of ENOs, the same sequence was found in the developing hippocampus (Crépel et al. 2007).
At early postnatal stages, two spontaneous synapse-driven network patterns have been extensively described in developing neocortical slices: giant depolarizing potentials (GDPs) driven by GABAergic transmission (Ben-Ari et al. 1989; Garaschuk et al. 1998; Crépel et al. 2007; Allene et al. 2008; Rheims et al. 2008a) and cortical early network oscillations (cENOs) driven by glutamatergic transmission (Garaschuk et al. 2000; Corlew et al. 2004; McCabe et al. 2006; Allene et al. 2008). Relying on the apparent similarities between these patterns, cENOs were initially thought to be the neocortical counterpart of hippocampal GDPs. However, we have recently found that NMDA-R-driven ENOs and GABAAR-driven GDPs are indeed two distinct patterns in the neocortex, characterized by different spatiotemporal dynamics both in electrical and optical recordings. Whereas cENOs are low-frequency oscillations (0.01 Hz) displaying slow kinetics that gradually involve the entire network, cGDPs are recurrent oscillations (0.1 Hz) that repetitively synchronize localized neuronal assemblies. Moreover, ENOs and cGDPs are sequentially expressed in the immature neocortex since cENOs precede cGDPs. Interestingly, a recent in vivo study describing the maturation of coordinated electrical activity patterns in the rat somatosensory cortex has reported two patterns of oscillatory activity, ‘spindle bursts’ and ‘long oscillations’, with dynamics very similar to cGDPs and cENOs, respectively (Yang et al. 2009). It is therefore very likely that the sequence established in vitro will also apply in vivo (Golshani et al. 2009).
What would be the main function carried by this precise sequence for the maturation of cortical networks? Given the largely documented role of activity in the maturation of cortical neurons (Moody & Bosma, 2005; Cancedda et al. 2007; Lin et al. 2008; Wang & Kriegstein, 2008) and circuits (Katz & Shatz, 1996; Huang, 2009; Pfeffer et al. 2009), it is easy to speculate that the robust timetable for the maturation of network patterns is not merely the emergent expression of a precise sequence in the development of individual neuronal properties but something that also participates in proper cell maturation. In other words, activity itself would create a feedback loop that triggers the changes in neuronal and circuit properties that serve to terminate one network pattern and start the next. Experiments selectively preventing the expression of a given network pattern within a precise sequence indirectly support this hypothesis.
This sequence is particularly well-documented in the maturing retina, a structure in which spontaneous retinal waves will develop in three stereotyped sequential steps characterized by different dynamics and mechanisms (Syed et al. 2004). While stage I waves are mostly mediated by gap-junction coupling, stage II and III rely on nicotinic and glutamatergic receptor activation, respectively (Syed et al. 2004; Torborg & Feller, 2005). Waves at a given stage could be restored to the previous stage by blocking their specific neurotransmission system (McLaughlin et al. 2003; Syed et al. 2004; Stacy et al. 2005; Blankenship et al. 2009). For example, mice lacking the enzyme that synthesizes acetylcholine will exhibit stage I gap junction-dependent retinal waves at a period of development normally dominated by stage II cholinergic waves (Stacy et al. 2005). Similarly, at the next stage of retinal activity pattern maturation, an absence of glutamatergic signalling in VGLUT1 KO mice was shown to delay the termination of stage II waves (Blankenship et al. 2009). Also, β2−/− mice lacking the nicotinic receptor subunits mediating stage II waves will not display nicotinic-dependent correlated activity while stage III glutamatergic waves will begin earlier (McLaughlin et al. 2003). A precisely timed handover of synchrony between different network activity patterns also applies to the developing hippocampus (Crépel et al. 2007). Indeed, we have shown in this structure that the occurrence of synapse-driven GDPs actively shuts off the expression of the earlier gap-junction mediated SPA oscillations. Moreover these two patterns are mutually exclusive within the same network and blocking GDPs will restore SPAs during the first postnatal week (Crépel et al. 2007). It is possible that the activation of NMDA-Rs occurring during GDPs (Leinekugel et al. 1997) could produce a long-term down-regulation of connexin expression (Arumugam et al. 2005) that would silence SPAs. The handover of synchrony and direct interaction between SPAs, ENOs and GDPs has not yet been investigated in the neocortex. It is therefore at present difficult to claim whether a similar direct interaction between co-existing patterns also applies to the neocortex.
If such a precise timetable for synchronous neuronal activity maturation applies to the neocortex, it implies that a proper maturation of cortical structures will be highly sensitive to environmental factors. Indeed, coherent activity patterns can be largely modulated by environmental factors. For example, both in the neocortex and hippocampus, the emergence of SPAs is determined by the hormone oxytocin, which is released by the mother during delivery (Crépel et al. 2007; Allene et al. 2008). The effect of oxytocin on SPAs directly results from the action of the hormone on GABAergic transmission (Tyzio et al. 2006) since SPAs were shown to be favoured by an inhibitory GABA polarity, for example produced by NKCC1 blockade (Crépel et al. 2007). Hence, the same environmental change (i.e. oxytocin release) will create conditions that favour the emergence of SPAs but prevent GDPs. Interestingly, it is worth mentioning that probably several other environmental factors, including stress (Shen et al. 2007) and energy supply (Rheims et al. 2008b), will also ultimately impact network activity patterns by their direct action on the GABAergic system. In fact, we have shown that an anoxic episode occurring in a given network will boost the occurrence of cENOs while preventing the emergence of GDPs, the later network oscillation (Fig. 3; Allene et al. 2008). This differential sensitivity of network patterns to environmental factors is directly determined by the cellular mechanism underlying their generation. In conclusion, the same environmental change can have different consequences on the network depending on which type of activity is dominant at the time it occurs.
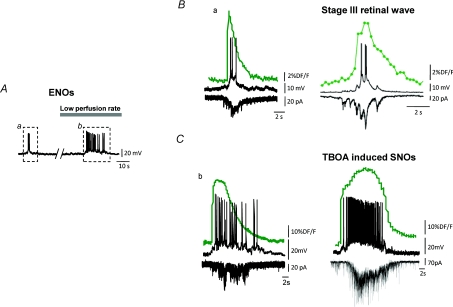
Figure 3. ENOs present striking similarities with both a physiological and a pathological network pattern.
A, ENO-associated membrane potential depolarisations recorded in current clamp mode before (a) and after (b) decreasing the rate of the perfusion from 4 to 1 ml min−1 (data taken from Allene et al. 2008). Similar effects were found in anoxic/aglycaemic perfusion conditions (see Allene et al. 2008). B, comparison between the membrane potential depolarization (top black trace), the calcium fluorescence signal (green) and the spontaneous excitatory postsynaptic currents (sEPSCs, bottom black, Vm=−60 mV) associated with the spontaneous ENO illustrated in (Aa) and with a stage III retinal wave (reprinted from Blankenship et al. (2009) with permission from Elsevier). Note the similarity between the two patterns. C, same as in B but comparing ENOs produced by a mild anoxic condition occurring when decreasing the perfusion rate (Ab) with slow network oscillations (SNOs) induced by pharmacological EEAT blockade with DL-TBOA (unpublished data from L. Aniksztejn & A.A. Cattani).
NMDA-R driven early network oscillations: a role in synapse maturation?
As discussed above, ENOs are the dominant network pattern in neocortical slices at early postnatal stages. In contrast to most synapse-driven oscillations in other developing structures including the hippocampus, ENOs are mediated by the activation of NMDA-Rs rather than GABAA-Rs (Garaschuk et al. 2000; Dupont et al. 2005; McCabe et al. 2006; Sun & Luhmann, 2007; Allene et al. 2008). Accordingly, oscillations recorded in vivo in the neonatal rat barrel cortex are also largely dependent on NMDA-R activation (Minlebaev et al. 2007; Yang et al. 2009). Therefore NMDA-Rs specifically have a major network function at early developmental stages in the neocortex. In contrast, in the hippocampus, GABAergic synapses are established before glutamatergic ones (Ben-Ari et al. 2004). Accordingly, the first synapse-driven network pattern in this region is the GABAA-R-driven GDPs. The sequence of synapse maturation in the neocortex might be different even though GABAergic transmission also critically modulates neocortical activity through its complex excitatory/shunting action (Rheims et al. 2008a). Several observations indicate that NMDA-R signalling operates early in cortical development, notably to regulate the synaptic recruitment of AMPA-Rs (Feldmeyer & Cull-Candy, 1996; Zhu et al. 2000; Shi et al. 2001; Radnikow et al. 2002; Voigt et al. 2005; Brill & Huguenard, 2008; Wang & Kriegstein, 2008). A detailed morpho-functional description of the sequential maturation of GABAergic and glutamatergic synapses in the neocortex will undoubtedly be helpful to understand the differences between the neocortex and hippocampus.
It is important to stress that the network function of NMDA-Rs in the neocortex does not necessarily imply synaptic activation of these receptors. Indeed, we have recently shown that NMDA-Rs also contribute to neuronal excitability in the neocortex by mediating a tonic current that supports membrane potential depolarization (Allene et al. 2008). In the same study, we established the critical involvement of extracellular glutamate concentrations in the generation of cENOs. It is therefore possible that the generation of cENOs partly originates in the activation of extrasynaptic NMDA-Rs by ambient glutamate (Allene et al. 2008). The analogy between retinal and neocortical activity patterns is striking, in particular regarding the involvement of extrasynaptic glutamate receptors. Indeed, increases in ambient levels of glutamate were recently shown to be critically involved in generating stage III retinal waves (Blankenship et al. 2009). In addition, the dynamics underlying NMDA-R-driven stage III retinal waves is remarkably similar to cortical ENOs (Blankenship et al. 2009). The kinetics of both calcium and electrophysiological events associated with stage III retinal waves are as slow as those occurring during cENOs (Fig. 3). Interestingly, stage III retinal waves were shown to appear at a period when the glutamatergic synaptic system is not yet mature in the retina (Syed et al. 2004; Blankenship et al. 2009) supporting a role for extrasynaptic NMDA-R activation in the maturation of synaptic circuits.
Cortical ENOs may support the conversion of ‘silent’ to ‘active’ synapses and regulate the recruitment of AMPA-Rs into functional synapses. Indeed this network pattern occurs just before the shift of ‘silent’ or ‘labile’ synapses to functional ones (Groc et al. 2006) and glutamate spillover was shown to be critical for the activation of ‘silent synapses’ (Balland et al. 2008). In agreement with this hypothesis, it was recently shown that GABAA-R and NMDA-R synaptic currents can be recorded prior to AMPA-R EPSCs in the neocortex and jointly contribute to the development of AMPA-R mediated transmission (Wang & Kriegstein, 2008). Still, the regulation of AMPA-Rs by NMDA-R-driven ENOs is probably not so straightforward. Indeed, while some reports suggested that NMDA-R signalling early in development negatively regulates the recruitment of functional AMPA-Rs into synapses (Hall & Ghosh, 2008), others proposed a positive regulation of AMPA-Rs by NMDA-R-mediated transmission (Zhu et al. 2000; Shi et al. 2001; Voigt et al. 2005; Brill & Huguenard, 2008). In fact, this controversy belongs to a more general one, as other studies have indicated that the maturation of these two types of receptors might be independent (Meguro et al. 1992; Okabe et al. 1998; Zhu & Malinow, 2002; Colonnese et al. 2003). This debate underlies the complex role of NMDA-R signalling during development and makes it difficult to attribute a single function to early NMDA-R-driven oscillations.
The developing neocortex: a network in a ‘critical state’?
ENO dynamics are characterized by a massive recruitment of neuronal populations throughout cortical subregions irrespective of anatomical boundaries (Garaschuk et al. 2000; Adelsberger et al. 2005; Yang et al. 2009). Interestingly, during a restricted developmental period of 1 or 2 days, these large synchronizations co-exist with local events in the form of GDPs (Allene et al. 2008). The coexistence of these two network events with very different sizes could be the sign of an ‘avalanche’ mode of activity in developing neocortical slices (Plenz & Thiagarajan, 2007; Werner, 2007). The term ‘neuronal avalanche’ was recently introduced to describe the fact that the size of spontaneous neuronal synchronizations can follow a power-law distribution implying that both rare massive events and frequent local ones can occur in the same network (Plenz & Thiagarajan, 2007; Werner, 2007). An avalanche type of organization is the sign of a network in a critical state and was reported in immature organotypic cortical cultures (Stewart & Plenz, 2008).
By definition, a critical state is at the edge of stability, and any small perturbation would break it. Several observations could indeed indicate that network dynamics in the developing neocortex can rapidly switch to a pathological state. Maybe the most striking one is the fact that the neocortex is exceptionally prone to seizures at early developmental stages (Ben-Ari & Holmes, 2006; Bender & Baram, 2007; Holmes et al. 2007; Scantlebury et al. 2007). For example, it was recently shown that GDPs in that region rapidly evolve towards interictal and ictal-like seizures if synaptic activity levels are pharmacologically increased (Rheims et al. 2008b). Likewise, the blockade of excitatory amino acid transporters (EAAT) that remove glutamate from the extracellular space induces an epileptiform ‘suppression burst’ activity pattern (Demarque et al. 2004). These slow network oscillations (SNOs) induced by EAAT blockade share striking features with hypoxia-induced-ENOs (Allene et al. 2008) regarding their dynamics and mechanisms (Fig. 3). SNOs appear as an amplified form of ENOs in regard to individual event kinetics and network dynamics. Interestingly, hypoxic–ischaemic encephalopathy in human neonates is very often associated with discontinuous EEG patterns including ‘suppression bursts’, in which dynamics and suggested cellular mechanisms can also be intriguingly similar to cENOs (Biagioni et al. 1999; Ohtahara & Yamatogi, 2003; Milh et al. 2007a). Moreover, hypoxic conditions both facilitate ENOs (Allene et al. 2008) and impair glutamate transporter function (Dallas et al. 2007). Altogether, this would suggest that ENOs could be a network pattern critically close to a pathological state.
To conclude, we propose a robust sequence for the maturation of coherent activity patterns in cortical structures with the role of NMDA-R driven ENOs remaining an open question. Because of their resemblance to both physiological patterns like stage III retinal waves or neuronal avalanches, and pathological oscillations like the bursts induced by an impairment of EAAT, it is at present difficult to assign a definite developmental function to ENOs. Finding the in vivo electrical pattern corresponding to cENOs is a difficult task. It will require combining multineuron imaging with electrophysiological recordings but it will certainly help to address this issue.
References
- Adelsberger H, Garaschuk O, Konnerth A. Cortical calcium waves in resting newborn mice. Nat Neurosci. 2005;8:988–990. doi: 10.1038/nn1502. [DOI] [PubMed] [Google Scholar]
- Allene C, Cattani A, Ackman JB, Bonifazi P, Aniksztejn L, Ben-Ari Y, Cossart R. Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J Neurosci. 2008;28:12851–12863. doi: 10.1523/JNEUROSCI.3733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam H, Liu X, Colombo PJ, Corriveau RA, Belousov AB. NMDA receptors regulate developmental gap junction uncoupling via CREB signalling. Nat Neurosci. 2005;8:1720–1726. doi: 10.1038/nn1588. [DOI] [PubMed] [Google Scholar]
- Balland B, Lachamp P, Kessler JP, Tell F. Silent synapses in developing rat nucleus tractus solitarii have AMPA receptors. J Neurosci. 2008;28:4624–4634. doi: 10.1523/JNEUROSCI.5355-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–1063. doi: 10.1016/S1474-4422(06)70626-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Represa A, Gozlan H. Interneurons set the tune of developing networks. Trends Neurosci. 2004;27:422–427. doi: 10.1016/j.tins.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Bender RA, Baram TZ. Epileptogenesis in the developing brain: what can we learn from animal models? Epilepsia. 2007;48(Suppl 5):2–6. doi: 10.1111/j.1528-1167.2007.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagioni E, Bartalena L, Boldrini A, Pieri R, Cioni G. Constantly discontinuous EEG patterns in full-term neonates with hypoxic-ischaemic encephalopathy. Clin Neurophysiol. 1999;110:1510–1515. doi: 10.1016/s1388-2457(99)00091-7. [DOI] [PubMed] [Google Scholar]
- Blankenship AG, Ford KJ, Johnson J, Seal RP, Edwards RH, Copenhagen DR, Feller MB. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron. 2009;62:230–241. doi: 10.1016/j.neuron.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill J, Huguenard JR. Sequential changes in AMPA receptor targeting in the developing neocortical excitatory circuit. J Neurosci. 2008;28:13918–13928. doi: 10.1523/JNEUROSCI.3229-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L, Fiumelli H, Chen K, Poo MM. Excitatory GABA action is essential for morphological maturation of cortical neurons in vivo. J Neurosci. 2007;27:5224–5235. doi: 10.1523/JNEUROSCI.5169-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT, Shi J, Constantine-Paton M. Chronic NMDA receptor blockade from birth delays the maturation of NMDA currents, but does not affect AMPA/kainate currents. J Neurophysiol. 2003;89:57–68. doi: 10.1152/jn.00049.2002. [DOI] [PubMed] [Google Scholar]
- Corlew R, Bosma MM, Moody WJ. Spontaneous, synchronous electrical activity in neonatal mouse cortical neurones. J Physiol. 2004;560:377–390. doi: 10.1113/jphysiol.2004.071621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crépel V, Aronov D, Jorquera I, Represa A, Ben-Ari Y, Cossart R. A parturition-associated nonsynaptic coherent activity pattern in the developing hippocampus. Neuron. 2007;54:105–120. doi: 10.1016/j.neuron.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Dallas M, Boycott HE, Atkinson L, Miller A, Boyle JP, Pearson HA, Peers C. Hypoxia suppresses glutamate transport in astrocytes. J Neurosci. 2007;27:3946–3955. doi: 10.1523/JNEUROSCI.5030-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarque M, Villeneuve N, Manent JB, Becq H, Represa A, Ben-Ari Y, Aniksztejn L. Glutamate transporters prevent the generation of seizures in the developing rat neocortex. J Neurosci. 2004;24:3289–3294. doi: 10.1523/JNEUROSCI.5338-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 2005;439:79–83. doi: 10.1038/nature04264. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Cull-Candy S. Functional consequences of changes in NMDA receptor subunit expression during development. J Neurocytol. 1996;25:857–867. doi: 10.1007/BF02284847. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol. 1998;507:219–236. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3:452–459. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- Golshani P, Goncalves JT, Khoshkhoo S, Mostany R, Smirnakis S, Portera-Cailliau C. Internally mediated developmental desynchronization of neocortical network activity. J Neurosci. 2009;29:10890–10899. doi: 10.1523/JNEUROSCI.2012-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Gustafsson B, Hanse E. AMPA signalling in nascent glutamatergic synapses: there and not there! Trends Neurosci. 2006;29:132–139. doi: 10.1016/j.tins.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Guido W. Refinement of the retinogeniculate pathway. J Physiol. 2008;586:4357–4362. doi: 10.1113/jphysiol.2008.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BJ, Ghosh A. Regulation of AMPA receptor recruitment at developing synapses. Trends Neurosci. 2008;31:82–89. doi: 10.1016/j.tins.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Harden C, Liporace J, Gordon J. Postnatal concerns in children born to women with epilepsy. Epilepsy Behav. 2007;11:270–276. doi: 10.1016/j.yebeh.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Huang ZJ. Activity-dependent development of inhibitory synapses and innervation pattern: role of GABA signalling and beyond. J Physiol. 2009;587:1881–1888. doi: 10.1113/jphysiol.2008.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J Neurosci. 1998;18:1419–1427. doi: 10.1523/JNEUROSCI.18-04-01419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996;17:275–285. doi: 10.1016/s0896-6273(00)80159-2. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABAA and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe AK, Chisholm SL, Picken-Bahrey HL, Moody WJ. The self-regulating nature of spontaneous synchronized activity in developing mouse cortical neurones. J Physiol. 2006;577:155–167. doi: 10.1113/jphysiol.2006.117523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O’Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40:1147–1160. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumanishi T, Arakawa M, Sakimura K, Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Milh M, Becq H, Villeneuve N, Ben-Ari Y, Aniksztejn L. Inhibition of glutamate transporters results in a ‘suppression-burst’ pattern and partial seizures in the newborn rat. Epilepsia. 2007a;48:169–174. doi: 10.1111/j.1528-1167.2006.00839.x. [DOI] [PubMed] [Google Scholar]
- Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, Khazipov R. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb Cortex. 2007b;17:1582–1594. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Ben-Ari Y, Khazipov R. Network mechanisms of spindle-burst oscillations in the neonatal rat barrel cortex in vivo. J Neurophysiol. 2007;97:692–700. doi: 10.1152/jn.00759.2006. [DOI] [PubMed] [Google Scholar]
- Moody WJ, Bosma MM. Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol Rev. 2005;85:883–941. doi: 10.1152/physrev.00017.2004. [DOI] [PubMed] [Google Scholar]
- O’Donovan MJ, Wenner P, Chub N, Tabak J, Rinzel J. Mechanisms of spontaneous activity in the developing spinal cord and their relevance to locomotion. Ann N Y Acad Sci. 1998;860:130–141. doi: 10.1111/j.1749-6632.1998.tb09044.x. [DOI] [PubMed] [Google Scholar]
- Ohtahara S, Yamatogi Y. Epileptic encephalopathies in early infancy with suppression-burst. J Clin Neurophysiol. 2003;20:398–407. doi: 10.1097/00004691-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Okabe S, Vicario-Abejon C, Segal M, McKay RD. Survival and synaptogenesis of hippocampal neurons without NMDA receptor function in culture. Eur J Neurosci. 1998;10:2192–2198. doi: 10.1046/j.1460-9568.1998.00233.x. [DOI] [PubMed] [Google Scholar]
- Opitz T, de Lima AD, Voigt T. Spontaneous development of synchronous oscillatory activity during maturation of cortical networks in vitro. J Neurophysiol. 2002;88:2196–2206. doi: 10.1152/jn.00316.2002. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Patterns of intracellular calcium fluctuation in precursor cells of the neocortical ventricular zone. J Neurosci. 1998;18:5374–5388. doi: 10.1523/JNEUROSCI.18-14-05374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado A. Traveling slow waves of neural activity: a novel form of network activity in developing neocortex. J Neurosci. 2000;20:RC54. doi: 10.1523/JNEUROSCI.20-02-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer CK, Stein V, Keating DJ, Maier H, Rinke I, Rudhard Y, Hentschke M, Rune GM, Jentsch TJ, Hubner CA. NKCC1-dependent GABAergic excitation drives synaptic network maturation during early hippocampal development. J Neurosci. 2009;29:3419–3430. doi: 10.1523/JNEUROSCI.1377-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picken Bahrey HL, Moody WJ. Early development of voltage-gated ion currents and firing properties in neurons of the mouse cerebral cortex. J Neurophysiol. 2003;89:1761–1773. doi: 10.1152/jn.00972.2002. [DOI] [PubMed] [Google Scholar]
- Plenz D, Thiagarajan TC. The organizing principles of neuronal avalanches: cell assemblies in the cortex? Trends Neurosci. 2007;30:101–110. doi: 10.1016/j.tins.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Radnikow G, Feldmeyer D, Lubke J. Axonal projection, input and output synapses, and synaptic physiology of Cajal-Retzius cells in the developing rat neocortex. J Neurosci. 2002;22:6908–6919. doi: 10.1523/JNEUROSCI.22-16-06908.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheims S, Minlebaev M, Ivanov A, Represa A, Khazipov R, Holmes GL, Ben-Ari Y, Zilberter Y. Excitatory GABA in rodent developing neocortex in vitro. J Neurophysiol. 2008a;100:609–619. doi: 10.1152/jn.90402.2008. [DOI] [PubMed] [Google Scholar]
- Rheims S, Represa A, Ben-Ari Y, Zilberter Y. Layer-specific generation and propagation of seizures in slices of developing neocortex: role of excitatory GABAergic synapses. J Neurophysiol. 2008b;100:620–628. doi: 10.1152/jn.90403.2008. [DOI] [PubMed] [Google Scholar]
- Roerig B, Feller MB. Neurotransmitters and gap junctions in developing neural circuits. Brain Res Brain Res Rev. 2000;32:86–114. doi: 10.1016/s0165-0173(99)00069-7. [DOI] [PubMed] [Google Scholar]
- Scantlebury MH, Heida JG, Hasson HJ, Veliskova J, Velisek L, Galanopoulou AS, Moshe SL. Age-dependent consequences of status epilepticus: animal models. Epilepsia. 2007;48(Suppl 2):75–82. doi: 10.1111/j.1528-1167.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Aamodt SM, Townsend M, Constantine-Paton M. Developmental depression of glutamate neurotransmission by chronic low-level activation of NMDA receptors. J Neurosci. 2001;21:6233–6244. doi: 10.1523/JNEUROSCI.21-16-06233.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibilla S, Fabbro A, Grandolfo M, D’Andrea P, Nistri A, Ballerini L. The patterns of spontaneous Ca2+ signals generated by ventral spinal neurons in vitro show time-dependent refinement. Eur J Neurosci. 2009;29:1543–1559. doi: 10.1111/j.1460-9568.2009.06708.x. [DOI] [PubMed] [Google Scholar]
- Stacy RC, Demas J, Burgess RW, Sanes JR, Wong RO. Disruption and recovery of patterned retinal activity in the absence of acetylcholine. J Neurosci. 2005;25:9347–9357. doi: 10.1523/JNEUROSCI.1800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CV, Plenz D. Homeostasis of neuronal avalanches during postnatal cortex development in vitro. J Neurosci Methods. 2008;169:405–416. doi: 10.1016/j.jneumeth.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JJ, Luhmann HJ. Spatio-temporal dynamics of oscillatory network activity in the neonatal mouse cerebral cortex. Eur J Neurosci. 2007;26:1995–2004. doi: 10.1111/j.1460-9568.2007.05819.x. [DOI] [PubMed] [Google Scholar]
- Syed MM, Lee S, Zheng J, Zhou ZJ. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol. 2004;560:533–549. doi: 10.1113/jphysiol.2004.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hubner CA, Represa A, Ben-Ari Y, Khazipov R. Maternal oxytocin triggers a transient inhibitory switch in GABA signalling in the fetal brain during delivery. Science. 2006;314:1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- Voigt T, Opitz T, de Lima AD. Synchronous oscillatory activity in immature cortical network is driven by GABAergic preplate neurons. J Neurosci. 2001;21:8895–8905. doi: 10.1523/JNEUROSCI.21-22-08895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt T, Opitz T, de Lima AD. Activation of early silent synapses by spontaneous synchronous network activity limits the range of neocortical connections. J Neurosci. 2005;25:4605–4615. doi: 10.1523/JNEUROSCI.3803-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR. GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J Neurosci. 2008;28:5547–5558. doi: 10.1523/JNEUROSCI.5599-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Werner G. Brain dynamics across levels of organization. J Physiol Paris. 2007;101:273–279. doi: 10.1016/j.jphysparis.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Yang JW, Hanganu-Opatz IL, Sun JJ, Luhmann HJ. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J Neurosci. 2009;29:9011–9025. doi: 10.1523/JNEUROSCI.5646-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science. 1992;257:665–669. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Malinow R. Acute versus chronic NMDA receptor blockade and synaptic AMPA receptor delivery. Nat Neurosci. 2002;5:513–514. doi: 10.1038/nn0602-850. [DOI] [PubMed] [Google Scholar]