Abstract
Activity-dependent, bidirectional control of synaptic efficacy is thought to contribute to many forms of experience-dependent plasticity, including learning and memory. Although most excitatory synapses contain both AMPA and N-methyl-d-aspartate receptors (AMPARs and NMDARs), most studies have focused on the plasticity of synaptic AMPARs, and on the pivotal role of NMDA receptors for its induction. Here we review evidence that synaptic NMDARs themselves are subject to long-term activity-dependent changes by mechanisms that may differ from that of synaptic AMPARs. The bidirectional modulation of NMDAR-mediated synaptic responses is likely to have important functional implications for NMDAR-dependent forms of synaptic plasticity.

Christophe Mulle is a cellular neurobiologist with expertise in cellular electrophysiology of synaptic transmission and plasticity, receptor cell biology and the generation of transgenic mice. Since 1995 he has worked at a CNRS laboratory he established in Bordeaux, with interests in the cellular biology and pathophysiology of glutamatergic synaptic transmission and plasticity. He trained in the laboratory of Jean-Pierre Changeux to characterize functional nicotinic receptors in the mammalian brain and in the laboratory of Mircea Steriade and Martin Deschênes, and of Steve Heinemann at the Salk Institute in San Diego. He trained in molecular biology techniques and generated knock-out mice for kainate receptor subunits, which have proved to be instrumental for the understanding the function of these elusive glutamate receptors. In addition he has provided insights into the molecular events that govern polarized trafficking of kainate receptors. More recently he has unravelled novel forms of synaptic plasticity in the hippocampus, and has ambitions to answer questions related to cell biology of glutamate receptors, and to synaptic function, integration and plasticity.
Introduction
In the central nervous system, activity-dependent bidirectional control of synaptic efficacy, as exemplified by various forms of long-term potentiation (LTP) and long-term depression (LTD), is thought to contribute to many forms of experience-dependent plasticity, including learning and memory (Malenka & Bear, 2004). At excitatory synapses, synaptic strength is regulated in great part by changes in the function and number of postsynaptic glutamate receptors, which include α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs), N-methyl-d-aspartate receptors (NMDARs) and kainate receptors (Hollmann & Heinemann, 1994). Because AMPARs are the predominant ionotropic glutamate receptors mediating basal synaptic transmission, almost all work on the mechanisms underlying LTP and LTD has focused on the modulation of AMPAR-mediated synaptic responses. The prevailing view is that NMDARs play a pivotal role in the induction of many forms of activity-dependent LTP and LTD, by acting as a coincidence detector of presynaptic and postsynaptic firing (Malenka & Bear, 2004). This property depends both on the block of NMDA receptor channels by Mg2+ at resting membrane potential and on their high permeability to Ca2+. An NMDA-mediated rise in postsynaptic Ca2+ activates kinases, notably CAMKII, PKA, PKC and mitogen-activated protein kinase (MAPK), and protein phosphatases, which ultimately results in an increase or decrease of AMPAR density and/or conductance (Kerchner & Nicoll, 2008; Newpher & Ehlers, 2009). In the context of the function of NMDARs in synaptic plasticity and memory formation (Malenka & Bear, 2004; Nakazawa et al. 2004), a critical question is whether, like synaptic AMPARs, NMDARs undergo long-lasting modulation by synaptic activity. Once thought to be relatively stable at synapses, synaptic NMDARs are slowly emerging as subjects of intense plasticity and, like the AMPARs, in an activity-dependent manner. This review focuses on recent advances on the plasticity of synaptic NMDARs induced by synaptic activity.
Properties of NMDA receptors
NMDARs are heteromeric assemblies of NR1 (GluN1, with 8 different splice variants), NR2 (GluN2A, GluN2B, GluN2C and GluN2D) and NR3 (GluN3A and GluN3B) subunits that form ligand-gated channels with various cellular, biophysical and pharmacological properties depending on the composition of subunits and splice variants (Cull-Candy & Leszkiewicz, 2004; Paoletti & Neyton, 2007). The stoichiometry of NMDA receptors has not been firmly established, but the consensus is that they are most often tetramers composed of two NR1 subunits and two NR2 subunits (Paoletti & Neyton, 2007; Ulbrich & Isacoff, 2008). NMDA receptor subunits contain a long extracellular N-terminal domain, three true transmembrane segments, a re-entrant pore loop, and an intracellular C-terminal domain of variable length (Mayer, 2005). The C-terminus of both NR1 and NR2 subunits interacts with several intracellular scaffolding proteins, is subject to phosphorylation, and as such is involved in the regulation of receptor trafficking and function (Salter & Kalia, 2004; Lau & Zukin, 2007; Groc et al. 2009). Glutamate binds to the NR2 subunits while the co-agonist glycine binds to the NR1 subunit. The N-terminal domain of NR2 subunits is an important determinant for the functional properties of NMDARs. It is involved in the sensitivity to allosteric inhibitors like ifenprodil and zinc, as well as in the modulation of the open probability (Perin-Dureau et al. 2002; Hatton & Paoletti, 2005; Gielen et al. 2008). NR2 subunits are also critical for determining the high affinity for glutamate, modulation by glycine, sensitivity to voltage-dependent block by Mg2+, fractional Ca2+ current and channel kinetics (Cull-Candy & Leszkiewicz, 2004; Paoletti & Neyton, 2007).
Synaptic NMDARs are localized in the post-synaptic density where they are structurally organized in a large macromolecular complex composed of scaffolding proteins and adaptors that physically link NMDARs to downstream signalling molecules, kinases and phosphatases, and to other transmembrane proteins such as adhesion proteins and mGluRs (Husi et al. 2000). Trafficking of NMDARs from the intracellular compartments to the synaptic and non-synaptic membrane has been extensively studied and reviewed recently (Chen & Roche, 2007; Lau & Zukin, 2007; Groc et al. 2009). Membrane export and synaptic insertion of NMDARs involves intrinsic trafficking signals specific for each subunit and splice variant, and complex interactions between NMDARs and a variety of interacting proteins, including PDZ-domain proteins such as PSD-95 and SAP102. Membrane insertion and regulated endocytosis of NMDARs is also tightly controlled by phosphorylation (Chen & Roche, 2007; Lau & Zukin, 2007). Synaptic activity regulates the number and subunit composition of synaptic membrane receptors (Lau & Zukin, 2007). Despite the extensive literature on the regulation of cellular trafficking of NMDARs, our understanding of the molecular mechanisms underlying the insertion and retrieval of receptors in LTP or LTD of NMDARs lags well behind our current knowledge on the synaptic plasticity of AMPARs.
Modulation of NMDA receptor function
Because changes in the number, composition and/or function of NMDARs are expected to have important physiological and pathological consequences, there has been a wide interest in describing cell-signalling molecules capable of modulating synaptic NMDARs. Extracellularly, NMDARs can be modulated by glycine and d-serine, which act as co-agonists to potentiate NMDAR function (Johnson & Ascher, 1987; Oliet & Mothet, 2009). Extracellular zinc inhibits NMDAR function by binding to the N-terminal domain and by increasing proton inhibition (Paoletti et al. 2009). Polyamines and redox modulators have also been reported to influence NMDA receptor functions (Cull-Candy & Leszkiewicz, 2004).
The C-terminal domain of NMDAR subunits contains many serine/threonine phosphorylation sites, which are substrates for cAMP-dependent protein kinase A (PKA), protein kinase C (PKC), protein kinase B (PKB), CaMKII, cyclin-dependent kinase-5 (Cdk5) and casein kinase II (CKII) (Chen & Roche, 2007). For example, PKC and PKA activation increase NMDAR-mediated currents and Ca2+ permeability (Chen & Huang, 1992; Raman et al. 1996; Lu et al. 1999; Lan et al. 2001; Skeberdis et al. 2006). Phosphorylation by the Src family of protein tyrosine kinases (SFKs) upregulates NMDAR function. Activation of numerous G protein-coupled receptors, including M1 muscarinic receptors, LPA receptors, mGluR1 and mGluR5 and PACAP1 receptors, enhances NMDA receptor function via phosphorylation of the NMDA receptor channel (Salter & Kalia, 2004). Interestingly, the different G protein-coupled receptors seem to converge onto Src-family kinases which constitute a molecular hub for signalling pathways that enhance NMDAR activity (Salter & Kalia, 2004). All these studies have relied on the exogenous activation of NMDAR modulatory pathways. Therefore, the physiological conditions under which such modulations occur and their consequences on activity-dependent synaptic plasticity have mostly remained elusive.
LTP of synaptic NMDA receptors
There has been considerable debate whether LTP of AMPAR-mediated responses was accompanied by a long-lasting potentiation of synaptic NMDARs. Pharmacologically isolated NMDA receptor-mediated responses in CA1 were reported to undergo synapse-specific LTP in response to high frequency stimulation of Schaffer collaterals (Sch) (Bashir et al. 1991; Berretta et al. 1991) in parallel to LTP of AMPAR-mediated responses, although LTP of NMDAR-mediated responses requires a higher threshold for induction (Berretta et al. 1991; Aniksztejn & Ben-Ari, 1995). In CA1, LTP was initially seen as a predominant potentiation of the AMPA receptor-mediated component and followed by a delayed proportional potentiation of the NMDA component of the field EPSP, such that LTP did not affect the relative contribution of the two types of receptors (Xiao et al. 1995; see also Watt et al. 2004) at neocortical synapses. LTP of isolated NMDA-EPSCs in CA1 pyramidal cells was later observed and proposed to be of presynaptic origin (Kullmann et al. 1996). However, several other studies have found little or no LTP of NMDA-EPSCs under similar experimental conditions (Kauer et al. 1988; Muller & Lynch, 1988; Perkel & Nicoll, 1993; Liao et al. 1995; Durand et al. 1996; Heynen et al. 2000; Montgomery & Madison, 2002). The reason for these discrepancies is unclear, but likely to rely on the experimental conditions, especially intracellular Ca2+ buffering (see Harney et al. 2006), and possibly on the developmental stages of the animals.
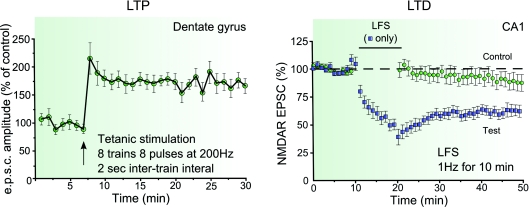
LTP of NMDA-EPSCs also occurs in the dentate gyrus (O’Connor et al. 1994, 1995) (Fig. 1), at hippocampal mossy fibre synapses onto CA3 pyramidal cells (mf-CA3 synapses) (Kwon & Castillo, 2008; Rebola et al. 2008), and in ventral midbrain dopamine neurons (Harnett et al. 2009). Most of the earlier studies providing evidence for LTP of NMDA-mediated synaptic responses used protocols that also triggered LTP of the synaptic AMPA component. Recently, we and others have observed that a robust LTP of NMDA-EPSCs but not of AMPA-EPSCs was induced at mf-CA3 synapses with short bursts of stimuli (Kwon & Castillo, 2008; Rebola et al. 2008). This selective LTP of NMDA-EPSCs, induced with physiologically relevant patterns of stimulation, provides clear evidence that synaptic NMDARs can undergo activity-dependent long-term modifications with mechanisms different from the ones responsible for LTP of AMPARs.
Figure 1. Examples of LTP and LTD of NMDARs.
Tetanic stimulation consisting of eight trains each of eight pulses at 200 Hz, inter-train interval 2 s, applied under current clamp configuration at resting membrane potential during tetanus induces LTP of NMDA-EPSCs in dentate gyrus granule cells (reproduced from O’Connor et al. 1994 with permission from Nature Publishing Group). On the other hand, a 1 Hz stimulation for 10 min while holding the cells at −40 mV induces LTD of NMDA-EPSCs in CA1 area of the hippocampus (reproduced from Selig et al. 1995 with permission from Elsevier).
Our understanding of the mechanisms of LTP of NMDA-EPSCs has recently progressed (Fig. 2) (Grosshans et al. 2002; Harney et al. 2006; Harney et al. 2008; Kwon & Castillo, 2008; Rebola et al. 2008; Harnett et al. 2009). These studies have converged on the notion that LTP of NMDA-EPSCs is expressed postsynaptically and requires an influx of postsynaptic Ca2+ consecutive to the activation of type I mGluRs and NMDARs (O’Connor et al. 1995; Harney et al. 2006; Kwon & Castillo, 2008; Rebola et al. 2008; Peng et al. 2009). In DA neurons, LTP requires release of Ca2+ from internal stores, whereas PKA activity gates LTP induction by regulating the magnitude of Ca2+ signal amplification (Harnett et al. 2009). At mf-CA3 synapses postsynaptic adenosine A2A receptors are essential for inducing LTP of NMDA-EPSCs (Rebola et al. 2008). A2A receptors may play a role in the amplification of Ca2+ signals, although their precise function in the induction LTP of NMDA-EPSCs is unknown. The expression mechanism is likely to involve insertion of NMDARs in the membrane in a PKC- and Src-dependent process (Grosshans et al. 2002; Kwon & Castillo, 2008; Rebola et al. 2008), although long-lasting changes in the properties of existing synaptic NMDARs cannot be excluded. In the dentate gyrus, there is evidence for the recruitment of extrasynaptic NMDARs, in particular NR2D-containing NMDARs, during LTP of NMDA-EPSCs (Harney et al. 2008). In contrast, in DA neurons, LTP of NMDA-EPSCs is unlikely to be associated with changes in subunit composition (Harnett et al. 2009). Interestingly, in CA1 pyramidal cells, the subunit composition of synaptic NMDARs can quickly change in an activity-dependent manner at neonatal, but not mature, synapses (Bellone & Nicoll, 2007). The incorporation of NR2B subunit in synaptic NMDARs changes both the charge transfer and kinetics of synaptic NMDA EPSCs but not their amplitude (Bellone & Nicoll, 2007). However, it was recently reported that in 2- to 3-week-old rats, LTP of NMDA-EPSCs in CA1 pyramidal cells resulted from the membrane insertion of NR2A-containing receptors (Peng et al. 2009). Much further work is needed to understand the molecular mechanisms of LTP of NMDA-EPSCs.
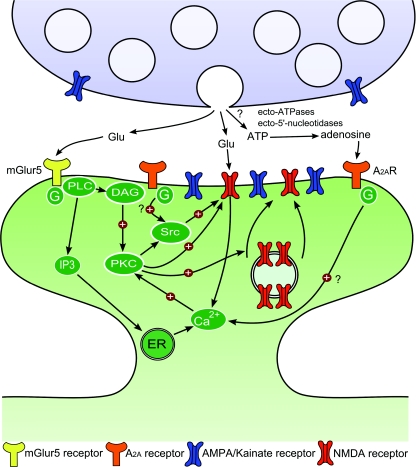
Figure 2. Schematic illustration of LTP of NMDARs at hippocampal mossy fibre synapses.
Short bursts of stimulation of mossy fibres lead to the activation of NMDA, mGluR5 and adenosine A2A receptors that jointly induce LTP of NMDA-EPSCs in CA3 pyramidal neurons. mGluR5 couples to phospholipase C (PLC) via a Gq protein, which promotes the formation of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers Ca2+ release from intracellular stores, and both Ca2+ and DAG activate protein kinase C. Once activated, PKC can also activate Src kinases. PKC or Src may trigger the SNARE-dependent insertion of new NMDARs into the postsynaptic membrane. PKC or Src kinases can also enhance open probability of postsynaptic NMDARs, increasing postsynaptic Ca2+ rise and thus favouring LTP induction. The role of adenosine A2A receptors in LTP of NMDAR induction is not clear but could rely on an increase in postsynaptic Ca2+ rise through direct coupling of A2A receptors to the PLC pathway or on a potentiation of mGluR5 function. Additionally, activation of Src kinase by A2A receptors might also facilitate the induction of LTP of NMDA-EPSCs.
LTD of synaptic NMDA receptors
In contrast to LTP, LTD of NMDAR-mediated synaptic responses has been consistently observed in response to induction protocols that elicit NMDAR-dependent LTD of AMPAR responses (Xie et al. 1992; Gean & Lin, 1993; Xiao et al. 1994; Selig et al. 1995; Montgomery & Madison, 2002) (Fig. 1). In the dentate gyrus the direction of plasticity of NMDAR following either high of low frequency stimulation appears to depend on intracellular free Ca2+ concentration in granule cells, with LTD being induced in conditions of high buffer capacity (Harney et al. 2006). LTD of NMDAR-mediated synaptic responses can be induced by specific patterns of afferent activity in CA1 pyramidal cells, through mechanisms apparently distinct from that underlying LTD of AMPAR-mediated responses (Selig et al. 1995; Morishita et al. 2005).
Unlike LTD of AMPA-EPSCs, which depends on dynamin-mediated receptor endocytosis, LTD of NMDA-EPSCs in CA1 pyramidal cells is unaffected by inhibitors of internalization (Morishita et al. 2005). Instead, the actin stabilizer phalloidin and a cofilin inhibitory peptide each block LTD of NMDAR-EPSCs, supporting a role for Ca2+-dependent depolymerization of the actin cytoskeleton (Morishita et al. 2005). These studies thus propose a change in the function of synaptic NMDARs, consistent with studies reporting an activity-dependent inactivation of NMDAR channels (Rosenmund et al. 1995) by modulation of the actin cytoskeleton (Krupp et al. 1999). However, at synapses between CA3 connected pairs, LTD of NMDA-EPSCs is dynamin dependent and appears to involve NMDAR endocytosis (Montgomery & Madison, 2002; Montgomery et al. 2005). The role of mGluRs in LTD of NMDA-synaptic responses is also debated, with reports of either independence (Morishita et al. 2005) or dependence on group I mGluRs (Yi et al. 1995; Harney et al. 2006; Peng et al. 2009). The dependency of LTD on mGluRs is possibly associated with the endocytic events involved in the depression of NMDAR-mediated synaptic responses. Indeed, application of the group I mGluR agonist (RS)-3,5-dihydroxyphenylglycine (DHPG) induces a long lasting depression of NMDA-EPSCs at CA1 synapses as well as internalization of NMDARs in cultured hippocampal neurons (Snyder et al. 2001). In addition, LTD triggered in the hippocampus in vivo correlates with a decrease of both AMPARs and NMDARs in synaptoneurosomal biochemical fractions (Heynen et al. 2000).
Future directions
Like synaptic AMPARs, synaptic NMDARs can be bidirectionally modified by different patterns of synaptic activity. There is in addition a large wealth of evidence, not addressed here, that NMDARs are tightly regulated by experience during development. Our understanding of the mechanisms of activity-dependent synaptic plasticity of NMDA receptors is only starting to emerge. Historically, the topic has faced contradictory results and might have been overlooked in part due to the apparently modest extent of synaptic plasticity of NMDARs and the relatively higher induction threshold required for LTP. A series of recent studies have provided evidence that robust forms of LTP of synaptic NMDARs can be elicited by physiologically relevant patterns of presynaptic stimulation. It is expected that this should stimulate the field to investigate the mechanisms contributing to synaptic plasticity, and to provide links with our extensive knowledge on NMDAR protein complexes and molecular mechanisms of trafficking events to and from synaptic sites. Future studies should for instance explore the role of membrane-associated guanylate kinases (MAGUKs) and the possibility of a switch in subunit composition of synaptic NMDARs, as well as changes in important functional properties such as Ca2+ permeability. A pioneering study using two-photon uncaging of glutamate observed that NMDARs can indeed undergo activity-dependent modulation of Ca2+ permeability (Sobczyk & Svoboda, 2007). The possibility that LTD corresponds to increased lateral mobility of NMDARs and LTP to immobilization and stabilization of existing receptors at synaptic sites should also be investigated (Groc et al. 2009).
Synaptic plasticity of NMDARs is particularly important because these receptors play a pivotal role in synaptic plasticity, such that changes in the strength of synaptic NMDARs are expected to critically influence the threshold for induction of AMPAR-mediated synaptic plasticity, as well as experience-dependent structural plasticity during the course of development. Given that NMDARs mediate a rise in postsynaptic Ca2+, future studies are warranted to explore the consequences of synaptic plasticity of NMDA receptors for the activity of Ca2+-dependent processes, ranging from the regulation of intrinsic excitability to gene expression, in physiological and pathological conditions.
Acknowledgments
This study was supported by grants from the Centre National de la Recherche Scientifique, the Conseil Régional d’Aquitaine and the European Commission (EUSynapse Project, contract no. LSHM-CT-2005-019055), and an EMBO fellowship to N.R.
References
- Aniksztejn L, Ben-Ari Y. Expression of LTP by AMPA and/or NMDA receptors is determined by the extent of NMDA receptors activation during the tetanus. J Neurophysiol. 1995;74:2349–2357. doi: 10.1152/jn.1995.74.6.2349. [DOI] [PubMed] [Google Scholar]
- Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Berretta N, Berton F, Bianchi R, Brunelli M, Capogna M, Francesconi W. Long-term potentiation of NMDA receptor-mediated EPSP in guinea-pig hippocampal slices. Eur J Neurosci. 1991;3:850–854. doi: 10.1111/j.1460-9568.1991.tb00096.x. [DOI] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Gean PW, Lin JH. D-2-Amino-5-phosphonovaleate blocks induction of long-term depression of the NMDA receptor-mediated synaptic component in rat hippocampus. Neurosci Lett. 1993;158:170–172. doi: 10.1016/0304-3940(93)90256-k. [DOI] [PubMed] [Google Scholar]
- Gielen M, Le Goff A, Stroebel D, Johnson JW, Neyton J, Paoletti P. Structural rearrangements of NR1/NR2A NMDA receptors during allosteric inhibition. Neuron. 2008;57:80–93. doi: 10.1016/j.neuron.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Bard L, Choquet D. Surface trafficking of N-methyl-D-aspartate receptors: physiological and pathological perspectives. Neuroscience. 2009;158:4–18. doi: 10.1016/j.neuroscience.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- Harnett MT, Bernier BE, Ahn KC, Morikawa H. Burst-timing-dependent plasticity of NMDA receptor-mediated transmission in midbrain dopamine neurons. Neuron. 2009;62:826–838. doi: 10.1016/j.neuron.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney SC, Jane DE, Anwyl R. Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J Neurosci. 2008;28:11685–11694. doi: 10.1523/JNEUROSCI.3035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney SC, Rowan M, Anwyl R. Long-term depression of NMDA receptor-mediated synaptic transmission is dependent on activation of metabotropic glutamate receptors and is altered to long-term potentiation by low intracellular calcium buffering. J Neurosci. 2006;26:1128–1132. doi: 10.1523/JNEUROSCI.2753-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46:261–274. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Quinlan EM, Bae DC, Bear MF. Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron. 2000;28:527–536. doi: 10.1016/s0896-6273(00)00130-6. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signalling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC, Nicoll RA. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988;1:911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Interactions of calmodulin and α-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J Neurosci. 1999;19:1165–1178. doi: 10.1523/JNEUROSCI.19-04-01165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Erdemli G, Asztely F. LTP of AMPA and NMDA receptor-mediated signals: evidence for presynaptic expression and extrasynaptic glutamate spill-over. Neuron. 1996;17:461–474. doi: 10.1016/s0896-6273(00)80178-6. [DOI] [PubMed] [Google Scholar]
- Kwon HB, Castillo PE. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fibre synapses. Neuron. 2008;57:108–120. doi: 10.1016/j.neuron.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Glutamate receptor ion channels. Curr Opin Neurobiol. 2005;15:282–288. doi: 10.1016/j.conb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Madison DV. State-dependent heterogeneity in synaptic depression between pyramidal cell pairs. Neuron. 2002;33:765–777. doi: 10.1016/s0896-6273(02)00606-2. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Selcher JC, Hanson JE, Madison DV. Dynamin-dependent NMDAR endocytosis during LTD and its dependence on synaptic state. BMC Neurosci. 2005;6:48. doi: 10.1186/1471-2202-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita W, Marie H, Malenka RC. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat Neurosci. 2005;8:1043–1050. doi: 10.1038/nn1506. [DOI] [PubMed] [Google Scholar]
- Muller D, Lynch G. Long-term potentiation differentially affects two components of synaptic responses in hippocampus. Proc Natl Acad Sci U S A. 1988;85:9346–9350. doi: 10.1073/pnas.85.23.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Spine microdomains for postsynaptic signalling and plasticity. Trends Cell Biol. 2009;19:218–227. doi: 10.1016/j.tcb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- O’Connor JJ, Rowan MJ, Anwyl R. Long-lasting enhancement of NMDA receptor-mediated synaptic transmission by metabotropic glutamate receptor activation. Nature. 1994;367:557–559. doi: 10.1038/367557a0. [DOI] [PubMed] [Google Scholar]
- O’Connor JJ, Rowan MJ, Anwyl R. Tetanically induced LTP involves a similar increase in the AMPA and NMDA receptor components of the excitatory postsynaptic current: investigations of the involvement of mGlu receptors. J Neurosci. 1995;15:2013–2020. doi: 10.1523/JNEUROSCI.15-03-02013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Mothet JP. Regulation of N-methyl-D-aspartate receptors by astrocytic D-serine. Neuroscience. 2009;158:275–283. doi: 10.1016/j.neuroscience.2008.01.071. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158:126–136. doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Peng Y, Zhao J, Gu QH, Chen RQ, Xu Z, Yan JZ, Wang SH, Liu SY, Chen Z, Lu W. Distinct trafficking and expression mechanisms underlie LTP and LTD of NMDA receptor-mediated synaptic responses. Hippocampus. 2009 doi: 10.1002/hipo.20654. in press. [DOI] [PubMed] [Google Scholar]
- Perin-Dureau F, Rachline J, Neyton J, Paoletti P. Mapping the binding site of the neuroprotectant ifenprodil on NMDA receptors. J Neurosci. 2002;22:5955–5965. doi: 10.1523/JNEUROSCI.22-14-05955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel DJ, Nicoll RA. Evidence for all-or-none regulation of neurotransmitter release: implications for long-term potentiation. J Physiol. 1993;471:481–500. doi: 10.1113/jphysiol.1993.sp019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Tong G, Jahr CE. β-Adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron. 1996;16:415–421. doi: 10.1016/s0896-6273(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fibre synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Feltz A, Westbrook GL. Calcium-dependent inactivation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol. 1995;73:427–430. doi: 10.1152/jn.1995.73.1.427. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Selig DK, Hjelmstad GO, Herron C, Nicoll RA, Malenka RC. Independent mechanisms for long-term depression of AMPA and NMDA responses. Neuron. 1995;15:417–426. doi: 10.1016/0896-6273(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Sobczyk A, Svoboda K. Activity-dependent plasticity of the NMDA-receptor fractional Ca2+ current. Neuron. 2007;53:17–24. doi: 10.1016/j.neuron.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Ulbrich MH, Isacoff EY. Rules of engagement for NMDA receptor subunits. Proc Natl Acad Sci U S A. 2008;105:14163–14168. doi: 10.1073/pnas.0802075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, Sjostrom PJ, Hausser M, Nelson SB, Turrigiano GG. A proportional but slower NMDA potentiation follows AMPA potentiation in LTP. Nat Neurosci. 2004;7:518–524. doi: 10.1038/nn1220. [DOI] [PubMed] [Google Scholar]
- Xiao MY, Karpefors M, Niu YP, Wigstrom H. The complementary nature of long-term depression and potentiation revealed by dual component excitatory postsynaptic potentials in hippocampal slices from young rats. Neuroscience. 1995;68:625–635. doi: 10.1016/0306-4522(95)00173-g. [DOI] [PubMed] [Google Scholar]
- Xiao MY, Wigstrom H, Gustafsson B. Long-term depression in the hippocampal CA1 region is associated with equal changes in AMPA and NMDA receptor-mediated synaptic potentials. Eur J Neurosci. 1994;6:1055–1057. doi: 10.1111/j.1460-9568.1994.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Xie X, Berger TW, Barrionuevo G. Isolated NMDA receptor-mediated synaptic responses express both LTP and LTD. J Neurophysiol. 1992;67:1009–1013. doi: 10.1152/jn.1992.67.4.1009. [DOI] [PubMed] [Google Scholar]
- Yi PL, Chang FC, Tsai JJ, Hung CR, Gean PW. The involvement of metabotropic glutamate receptors in long-term depression of N-methyl-D-aspartate receptor-mediated synaptic potential in the rat hippocampus. Neurosci Lett. 1995;185:207–210. doi: 10.1016/0304-3940(95)11264-w. [DOI] [PubMed] [Google Scholar]