Abstract
It has been suggested that primary afferent C-fibres that respond to innocuous tactile stimuli are important in the sensation of pleasurable touch. Although it is known that C-tactile fibres terminate in the substantia gelatinosa (lamina II) of the spinal cord, virtually all of the neurons in this region are interneurons, and currently it is not known how impulses in C-mechanoreceptors are transmitted to higher centres. In the current study, I have tested the quantitative response properties of ‘wide dynamic range’ projection neurons in lamina I of the spinal cord to graded velocity brushing stimuli to identify whether low-threshold mechanoreceptor input to these neurons arises from myelinated or umyelinated nerve fibres. Graded velocity brushing stimuli (6.6–126 cm s−1) were used to characterize the mechanoreceptor inputs to ‘wide dynamic range’ neurons in lamina I of the dorsal horn that had axons that projected to the contralateral parabrachial nucleus. The most effective tactile stimuli for activation of ‘wide dynamic range’ lamina I spinoparabrachial neurons were low velocity brush strokes: peak discharge occurred at a mean velocity of 9.2 cm s−1 (range 6.6–20.4 cm s−1, s.d. 5.0 cm s−1), and declined exponentially as brush velocity increased. The data indicate that C-fibres, but not A-fibres, conveyed low-threshold mechanoreceptor inputs to lamina I projection neurons.
Introduction
It is well recognized that peripheral nerve fibres with unmyelinated (C) axons are associated with nociception and thermoreception, as well as the concomitant sensations of pain and temperature (Ochoa & Torebjörk, 1989). Emerging evidence (Craig, 2002) suggests that sensory information transmitted by C-fibres has a critical role in a diverse range of sensations that all have a homeostatic component in common, e.g. pain, temperature, itch and exercise. A role for a specific class of unmyelinated low-threshold mechanoreceptors in the sensation of pleasurable touch has recently been described in two patients, (Olausson et al. 2002, 2008) who had rare polyneuropathies causing complete loss of myelinated peripheral nerve fibres. Low-threshold C-fibre mechanoreceptors are well documented in experimental animals (Iggo & Kornhuber, 1977; Kumazawa & Perl, 1977; Lynn & Carpenter, 1982), and in certain nerves in some species they can comprise up to 40% of the population of C-fibre afferents (Bessou & Perl, 1969). However, there is little available information regarding the identity of the central neurons activated by C-fibre mechanoreceptors, and the ascending pathway(s) activated by C-fibre mechanoreceptors are largely unknown.
It is known from in vivo intra-axonal labelling studies (Sugiura, 1996) that the central terminals of low-threshold C-fibres occupy a characteristic location in inner lamina II of the spinal dorsal horn. Similarly, Light et al. (1979) have shown examples of spinal neurons excited exclusively by slowly moving brushing stimuli, and receiving inputs solely from unmyelinated afferents, that had their cell bodies in inner lamina II. Morphologically, two of the neurons described by Light et al. (1979) appeared to be vertical neurons (Grudt & Perl, 2002, also termed stalked cells by Gobel, 1975), and the third was a central neuron. Both of these morphological types can be excitatory (Maxwell et al. 2007), and the vertical neurons have axons that arborize in lamina I (Maxwell et al. 2007), where they can contact projection neurons (Lu & Perl, 2005). Thus the available anatomical and physiological data support the hypothesis that sensory information that is carried by C-fibre mechanoreceptors is transmitted to higher centres by lamina I projection neurons; however, there is no direct evidence to support this. Thus, the aim of the current study was to test the hypothesis that projection neurons in lamina I of the spinal cord transmit tactile information carried by C-fibre mechanoreceptors to the brain. Although the majority of mechanoreceptors have myelinated axons, C-fibre mechanoreceptors are preferentially activated by slowly moving mechanical stimuli, a feature that distinguishes them from A-fibre (myelinated) mechanoreceptors (Greenspan, 1992; Vallbo et al. 1999; Löken et al. 2009). Thus preferential sensitivity to slowly moving versus rapidly moving stimuli was used to characterize the low-threshold response properties of lamina I projection neurons, and therefore infer the identity (myelinated or unmyelinated) of the primary afferent fibres that were activated by the brushing stimuli.
Methods
Ethical approval
All experiments were approved by the Ethical Review Panel at Sheffield University, and were licensed under the UK Animals (Scientific Procedures) Act 1986.
Animal preparation for single-unit recording
The data reported here were obtained during the course of other experiments (Andrew, 2009) that were performed on anaesthetized rats. The animals were anaesthetized with urethane (1.2 g kg−1) injected intra-peritoneally and neuromuscular blockade was induced with d-tubocurarine (150 μg) injected intravenously. Monitoring during neuromuscular blockade (stable blood pressure and heart rate during noxious stimulation) ensured that anaesthetic depth was sufficient. At the end of the experiment, the animals were killed with an overdose of barbiturates.
Full details of the preparation and maintenance of the animals and the recording procedures are given in a recent publication (Andrew, 2009). Briefly, the lumbar spinal cord was exposed by laminectomy, and a craniotomy performed to enable the insertion of an array of three bipolar stimulation electrodes into the right parabrachial nucleus. The activity of single spinal dorsal horn lamina I spinoparabrachial neurons with receptive fields on the left hindlimb was recorded with tungsten microelectrodes. Units were confirmed as spinoparabrachial neurons if they followed a train of six antidromic stimuli delivered at 250 Hz from an array of electrodes in the contralateral parabrachial nucleus, and if collision between antidromic and orthodromic impulses was observed. The critical collision interval was not measured directly because the ongoing activity of all of the units studied quantitatively was too low to permit it. The recording sites of neurones were marked with electrolytic lesions, and the recording and stimulating sites were identified in 50 μm cryostat sections that were stained with thionin.
Unit characterization
Single, antidromically identified lamina I spinoparabrachial neurons were selected for study if they received inputs from low-threshold mechanoreceptors (determined qualitatively using a camel's hair paintbrush) and if their cutaneous receptive fields were accessible to quantitative stimulation. For quantitative characterization, graded velocity brushing stimuli were applied using a custom-made stimulator that was based on the design of Edin et al. (1995). A stepper motor was used to rotate a soft, camel's hair paintbrush (bristle length: 15 mm, brush width: 7 mm, traverse length 3 cm) with velocities of 6.6, 8.9, 12.0, 20.4, 27.5, 37.1, 50.1, 67.2, 91.2 or 126 cm s−1. The brush exerted a force of 10–20 mN and it was positioned over the receptive field so that each brush stroke was in a proximal to distal direction along the central long axis of the receptive field. Two series of counter-balanced stimuli were applied: first, 6.6–126 cm s−1 and then again in reverse order, 126–6.6 cm s−1. At each velocity, 10 stimuli were applied, and responses were averaged for quantitative analysis. A photocell that was activated by a vane on the rotating spindle of the stimulator provided an indication of stimulus timing as well as brush velocity. Units activated by brushing stimuli were also tested with innocuous and noxious thermal stimuli (graded cooling and graded heating) applied with a feedback-controlled thermoelectric (Peltier) element, as well as with noxious mechanical stimuli (pinching with fine-tipped forceps). After unit characterization was completed, the conduction velocities of the afferent fibres supplying a neuron were determined by intracutaneous electrical stimulation. A pair of needle electrodes was inserted into the cutaneous receptive field, and graded electrical stimuli (1 ms stimulus duration, 0.25 Hz) applied. The latencies of different components of the afferent inputs were recorded from oscilloscope traces, and the conduction distance estimated with a suture thread.
Results
General properties
Recordings were made from 95 antidromically-identified lamina I spinoparabrachial neurons, of which 10 were activated by low-threshold mechanical stimulation. All of these 10 neurons were, in addition to being activated by brushing, also activated by noxious mechanical (pinching with smooth-tipped forceps) and noxious heat stimuli, but none was excited by graded intensity cooling/cold (4–31°C) stimuli. The discharge of all 10 units increased as the stimulus intensity increased from innocuous to noxious, and they were therefore classified as ‘wide dynamic range’ neurons (Mendell, 1966; Fig. 1). No neurons were encountered that were exclusively activated by tactile stimuli, similar to other studies of lamina I spinoparabrachial neurons (Bester et al. 2000; Keller et al. 2007). The central conduction velocities of these ‘wide dynamic range’ lamina I spinoparabrachial neurons were in the range 5.9–25.0 m s−1 (mean 13.0 m s−1, s.d. 5.8 m s−1), which is significantly faster than the conduction velocities of nociceptive-specific lamina I spinoparabrachial neurons (P < 0.02, unpaired t test; Andrew, 2009). ‘Wide dynamic range’ neurons had low levels of ongoing (background) activity, with the average being 0.05 impulses s−1 (range 0–0.14, s.d. 0.1) over a 1 min recording period at room temperature, prior to quantitative characterization. All of the receptive fields of the neurons reported here included both hairy and glabrous skin, but low-threshold responses were only evoked from hairy skin. Receptive field sizes covered a broad range with the smallest covering just a single digit and the largest extending across the whole ventral and lateral surface of the hindpaw. However, the mean receptive field size of ‘wide dynamic range’ lamina I spinoparabrachial neurons (160 mm2, range 42–327 mm2, s.d. 124 mm2) tended to be larger than those of nociceptive-specific lamina I spinoparabrachial neurons (mean 83 mm2, range 16–197 mm2, s.d. 72 mm2). Receptive field organization was comparable to other studies of lamina I projection neurons (e.g. Ferrington et al 1987): a small, high sensitivity zone where both low- and high-threshold stimuli were effective, surrounded by a larger region of lower sensitivity where only noxious stimuli evoked responses. The anatomical sites of terminations of the axons of wide dynamic range lamina I spinoparabrachial neurons were comparable to the population of lamina I spinoparabrachial neurons as a whole (Andrew, 2009): 90% of neurons were activated from the internal lateral subnucleus, 80% from the external lateral subnucleus and 70% from the Kölliker–Fuse nucleus.
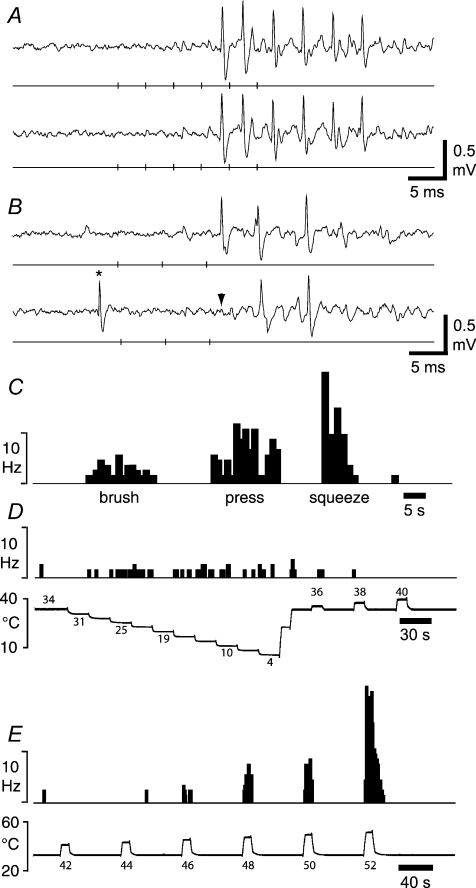
Figure 1. Identification of ‘wide dynamic range’ lamina I spinoparabrachial neurons in vivo.
A, pair of traces showing 1-for-1 following of a train of 6 antidromic electrical stimuli (40 μA, 1 ms, 250 Hz; vertical ticks) delivered from a stimulating electrode in the contralateral parabrachial nucleus. The conduction distance was 87 mm. B, collision of the first antidromic impulse in a train of 3 (150 Hz, upper trace) when an orthodromic impulse (asterisk, lower trace) occurred within the critical interval. The arrowhead indicates the point at which the first antidromic response should have occurred. Vertical ticks indicate the timing of the antidromic stimuli. C, peri-stimulus time histogram showing the response of the neuron shown above to innocuous (brushing with a hand-held brush; velocity ∼1 cm s−1) and noxious mechanical stimuli. D, histogram showing the response of the same neuron to graded cooling stimuli, applied with a feedback-controlled Peltier element. E, response of the same unit to graded heat.
Quantitative characterization
Graded velocity brushing was used to activate low-threshold mechanoreceptors that conveyed tactile information to lamina I spinoparabrachial neurons. An example of the response of a single neuron to repeated brushing stimuli is shown in Fig. 2A. As can be clearly seen, the response to repeated stimuli, delivered at the same velocity, gradually declined over the series of 10 brush strokes; such fatigue is a characteristic feature of C-fibre mechanoreceptors in both experimental animals and humans (Bessou et al. 1971; Nordin, 1990; Vallbo et al. 1999). The responses of the same unit to brushing at increasing velocities is shown in Fig. 2B. As the velocity of the brush increased, the response of the neuron diminished. Quantitative responses to the full range of brush velocities are shown in Fig. 3A. As can be seen from these stimulus–response functions, all of the neurons were preferentially sensitive to slowly moving stimuli. Maximal discharge rates (based on inter-spike intervals) were in the range 8–73 impulses s−1 (mean 40 impulses s−1, s.d. 26 impulses s−1) and occurred at a mean brush velocity of 9.2 cm s−1 (range 6.6–20.4 cm s−1, s.d. 5.0 cm s−1). In contrast, only two neurons were activated at the highest velocity tested (126 cm s−1), and their maximal discharge rates were only 1.2 and 0.1 impulses s−1. The population stimulus–response function could be fitted with a first-order exponential decay function of the form y=ae−bx (a= 48.7, b= 0.06; r2= 0.93). Plotting the data on a logarithmic scale showed that the decline in neuronal response to increasing velocity was linear (Fig. 3B), and it could be fitted with a straight line of the form y=mx+c (m=−1.87, c= 3.33; r2= 0.93).
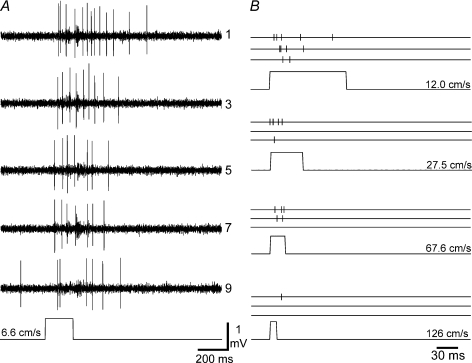
Figure 2. Responses to brushing stimuli.
A, raw responses from a typical ‘wide dynamic range’ lamina I spinoparabrachial neuron to repeated brushing at the lowest velocity tested (6.6 cm s−1). The first, then every other response to a series of 10 stimulus repetitions are shown. A marker trace (bottom) from a photocell indicates stimulus timing. As can be seen, there is a gradual reduction in response as the stimulus is repeated, similar to primary afferent C-fibre mechanoreceptors. B, responses of the same cell to 4 other of the 10 different brush velocities that were tested. Responses to the 1st, 5th and 10th stimuli are shown, with each action potential represented by a vertical tick mark. As can be seen, increasing stimulus velocity caused a progressive reduction in response.
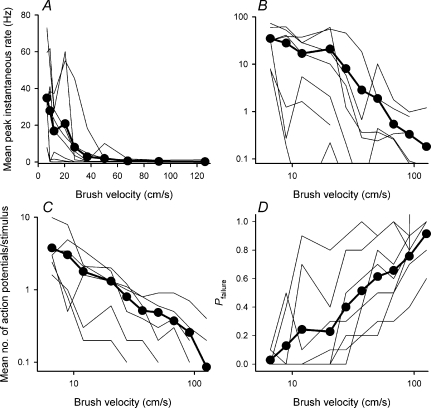
Figure 3. Quantitative velocity encoding by ‘wide dynamic range’ lamina I spinoparabrachial neurons.
A, simulus–response curves of velocity encoding for the individual neurons, as well as the population mean (•, thick line). The population response was well fitted by a first-order exponential decay function of the form y= 48.7e−0.06x (r2= 0.93). B, the same data as A, but transformed logarithmically. The population response was well fitted by a straight line function of the form y=−1.87x+ 3.33 (r2= 0.93). C, stimulus–response curves of the velocity dependence of the mean number of action potentials evoked per stimulus, as well as the population mean (•, thick line). As can be seen, higher velocity stimuli evoked fewer action potentials, due to shorter stimulus duration. D, stimulus–response curves of response probability as a function of velocity for the individual neurons, as well as the population mean (•, thick line). For each neuron at each velocity the proportion of stimuli that evoked any action potentials was determined, and the failure probability, i.e. the proportion of trials that evoked no response calculated. As can be seen, as velocity increased, the probability of response failure increased.
The velocity-dependent reduction in evoked discharge was due in part to the fact that stimulus duration was shorter at higher velocities, and therefore less action potentials were evoked per stimulus (Fig. 3C). However, there was also a concomitant decrease in the number of stimulus applications that evoked action potential discharge, i.e. an increase in the probability of failure (Fig. 3D).
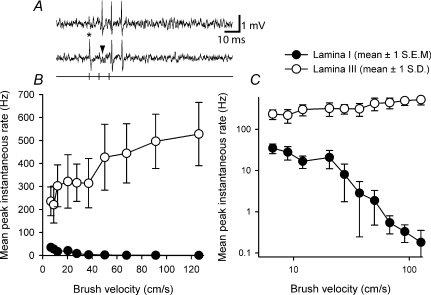
Serendipitously, recordings were also made from two spinoparabrachial neurons located in lamina III, one of which was a ‘wide dynamic range’ neuron, as it responded to both low-threshold brushing stimuli and to noxious stimuli; the other neuron was a nociceptive-specific cell. The response of the lamina III ‘wide dynamic range’ neuron to graded brushing is compared to that of the lamina I spinoparabrachial neuron population in Fig. 4. As can be clearly seen, in both linear and logarithmic plots, the firing rate of the lamina III cell increases smoothly as brush velocity increases, whereas the firing of lamina I neurons shows the inverse behaviour. This comparison confirms that the diminishing response of lamina I neurons to increasing velocity brushing is not simply due to the method of stimulus delivery.
Figure 4. Differentiation of the low-threshold inputs to lamina I neurons from low-threshold inputs to lamina III neurons.
A, raw responses from a single lamina III spinoparabrachial neuron showing collision of the first antidromic impulse in a train of 3 (150 Hz, upper trace) when an orthodromic impulse (asterisk, lower trace) occurred within the critical interval. The arrowhead indicates the point at which the first antidromic response should have occurred. B, stimulus–response curves from the population of lamina I neurons and the single ‘wide dynamic range’ lamina III neuron to graded velocity brushing. Note how evoked discharge increases for the lamina III neuron. C, same data as in B but transformed logarithmically.
Afferent inputs
All of the neurons tested received inputs from fibres with both myelinated and unmyelinated axons. Inputs from myelinated fibres were time locked (monosynaptic), and the conduction velocities of the most rapidly conducting A-fibres were in the range 8.8–15.7 m s−1 (mean 12.0, s.d. 2.7, n= 9). This value is very close to the average conduction velocity of Aδ-nociceptors in the rat foot (12.5 ± 4.5 m s−1) reported by Leem et al. (1993), but substantially slower than the conduction velocities of myelinated mechanoreceptors (mode 25–30 m s−1, Lynn & Carpenter, 1982; mean 32.6–46.6 m s−1 for various classes of mechanoreceptors, Leem et al. 1993). Unmyelinated fibre inputs were polysynaptic in all cases. Responses to electrical stimulation using intra-cutaneous needle electrodes confirmed that ‘wide dynamic range’ neurons received inputs from fibres with conduction velocities in the range 0.6–0.9 m s−1 and slower. However, these inputs were not time locked, and varied temporally by up to 28 ms (mean 16 ms, s.d. 5 ms). In addition, in 8 of 10 neurons studied, repeated electrical stimulation at C-fibre strength evoked a sustained increase in ongoing (background) activity, reminiscent of ‘wind-up’ (Mendell, 1966).
Discussion
The main finding in the current study was that low-threshold mechanoreceptor inputs to lamina I spinoparabrachial neurons appear to be transmitted by C-fibre primary afferents, acting via at least one interneuron. This is the first study to identify a neural circuit that conveys information from C-tactile fibres to higher centres in the central nervous system, where further processing may occur en route to the insular cortex.
Comparison with studies of C-fibre mechanoreceptors
Prior studies of C-fibre mechanoreceptors focused on basic receptive properties, and also contrasted their responses to those of either A-fibre (myelinated) mechanoreceptors or C-fibre nociceptors (Bessou et al. 1971; Iggo & Kornhuber, 1977; Nordin, 1990; Vallbo et al. 1999). Preferential activation by slowly moving stimuli was emphasized (Bessou et al. 1971), but quantitative stimulus–response curves of velocity encoding by C-tactile fibres have only been described recently (Löken et al. 2009). Using brush velocities in the range 0.1–30 cm s−1, the stimulus–response curves derived from human C-fibre mechanoreceptors by Löken et al. had an inverted U-shape, with maximal discharge occurring over the range 1–10 cm s−1. As the brushing stimulator used in the current experiments was based on a stepper motor, it was not possible to use brush velocities of less than 6.6 cm s−1 because of brush vibration due to the individual steps. Notwithstanding this technical limitation, over the range of brushing velocities common to both the present study and that of Löken et al. (2009), the velocity dependence of ‘wide dynamic range’ lamina I spinoparabrachial neurons is remarkably similar to that of human C-fibre mechanoreceptors; that is, as brush velocity increased, peak firing rate decreased. The fatigue to repeated brushing that was observed in the current study is also a property of C-tactile fibres (Nordin, 1990), and strengthens the case that mechanoreceptors with unmyelinated axons provide inputs to lamina I spinoparabrachial neurons; however, it is possible that habituation at synapses within the dorsal horn could also have contributed to the fatigue evoked by repeated stimulation. The observations from the single lamina III spinoparabrachial neuron that was studied, which showed the velocity dependence expected from myelinated mechanoreceptors (Greenspan, 1992; Löken et al. 2009), further supports the hypothesis that unmyelinated primary afferent nerve fibres transmitted tactile information to lamina I spinoparabrachial neurons.
Several previous studies have noted that C-fibre mechanoreceptors are also excited phasically by cooling stimuli, particularly when the rate of temperature change is rapid (>2°C s−1, Bessou et al. 1971; Kumazawa & Perl, 1977; Nordin, 1990). However, this cold sensitivity does not seem to be present in rodents, as in their study of primary afferent fibres innervating the rat hindlimb, Lynn & Carpenter (1982) found that none of the 15 C-fibre mechanoreceptors studied responded when a metal rod cooled in ice was applied to the receptive field. This latter observation is consistent with the current study, where temperature steps in the range 31–4°C applied with a 1 cm2 Peltier element activated none of the 10 units studied.
Comparative aspects of supraspinal pathways
Functional imaging studies in humans have shown that selective C-mechanoreceptor stimulation activates the left anterior insula (Olausson et al. 2002, 2008), a cortical region thought to be important in processing positive emotions (Craig, 2002). In humans and monkeys it is probable that C-tactile information is transmitted to insular cortex by the lamina I spinothalamic–Vmpo (posterior part of the ventromedial nucleus) pathway, which terminates in the fundus of the superior limiting sulcus of the dorsal margin of the insula (Craig, 2002). In comparison, most ascending lamina I activity in the rat is relayed by the spinoparabrachial pathway, as in a typical rat lumbar spinal segment there are about 400 lamina I spinoparabrachial neurons but around only 15 lamina I spinothalamic neurons (Al-Khater & Todd, 2009). Somatosensory projections from the parabrachial nucleus mainly target the amygdala, hypothalamus and bed nucleus of the stria terminalis (Bernard et al. 1993; Alden et al. 1994; Bester et al. 1997), but a projection to the parvicellular part of the ventral posterior thalamic nucleus which subsequently projects to the dorsal insula has also been identified, although this probably has a viscerosensory component (Cechetto & Saper, 1987; Iwata et al. 1992). Also, collaterals from lamina I spinoparabrachial neurons project to the posterior triangular nucleus of the thalamus (Al-Khater & Todd, 2009). Interestingly, about half of the somatosensory neurons in the posterior triangular thalamic nucleus can be excited by innocuous brushing stimuli, and axonal tracing studies have shown that posterior triangular nucleus neurons that are exclusively activated by mechanoreceptors project to the insula (Gauriau & Bernard, 2004). Thus, in the rat there are two possible routes for somatosensory information to be conveyed to insular cortex: both derived from the lamina I spinoparabrachial pathway, but one transmitted via the parvicellular part of the ventroposterior medial thalamic nucleus and the other via the posterior triangular nucleus.
Functional implications
Ascending somatosensory projections from lamina I of the spinal cord are often thought of as ‘labelled lines’ because they are anatomically and physiologically distinct, and their activity corresponds with distinct sensations (for review see Craig, 2003). However, wider consideration of the anatomy and physiology of ‘pain pathways’ has lead to the view that pain results from the integrated activity of ‘labelled lines’ and convergent pathways, that together constitute a hierarchical system that subserves homeostasis (Craig, 2002, 2003). In this view, pain is an aspect of the physiological condition of the body (interoception), and it is, like other sensations that have a motivational component, a homeostatic emotion. The current findings provide explicit support for this hypothesis as C-tactile fibres are thought to underlie pleasurable touch and emotional well-being (Löken et al. 2009). In animals, pleasurable touch such as skin-to-skin contact is thought to be important for maternal bonding with offspring, and it is interesting to note that suckling rat pups produce an oxytocin-independent activation of maternal insular cortex (Febo et al. 2005); this activation may be transmitted via C-tactile primary afferent fibres and lamina I ‘wide dynamic range’ spinoparabrachial neurons.
At first inspection, the convergence of low-threshold afferents and nociceptive afferents onto ‘wide dynamic range’ neurons in the current study appears to violate the ‘labelled lines’ concept that is a well-recognized feature of lamina I projection neurons. However, both C-fibre mechanoreceptors and nociceptors have a homeostatic functional component in common. Therefore the concept of ‘labelled lines’ could encompass function as well as modality; however, it is important to differentiate C-fibre mechanoreceptors from the much larger population of A-fibre mechanoreceptors, which are exteroceptive rather than interoceptive. Nonetheless, although ‘wide dynamic range’ lamina I spinoparabrachial neurons were activated by slowly moving brushing stimuli, noxious stimuli evoked a much greater discharge than innocuous stimuli, indicating a role in pain perception. Keller et al. (2007) have shown that the amplitude of the response to a brushing stimulus increases significantly in animals that have mechanical allodynia as a consequence of nerve injury. Thus a role for these neurons in the allodynia of neuropathic pain is also suggested.
Acknowledgments
All of the experiments described in this paper were performed at the University of Sheffield. This work was supported by the Wellcome Trust.
References
- Alden M, Besson JM, Bernard JF. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: a PHA-L study in the rat. J Comp Neurol. 1994;341:289–314. doi: 10.1002/cne.903410302. [DOI] [PubMed] [Google Scholar]
- Al-Khater KM, Todd AJ. Collateral projections of neurons in laminae I, III and IV of rat spinal cord to thalamus, periaqueductal grey matter, and lateral parabrachial area. J Comp Neurol. 2009;515:629–646. doi: 10.1002/cne.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D. Sensitization of lamina I spinoparabrachial neurons parallels heat hyperalgesia in the chronic constriction injury model of neuropathic pain. J Physiol. 2009;587:2005–2018. doi: 10.1113/jphysiol.2009.170290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Bessou P, Burgess PR, Perl ER, Taylor CB. Dynamic properties of mechanoreceptors with unmyelinated (C) fibres. J Neurophysiol. 1971;34:116–131. doi: 10.1152/jn.1971.34.1.116. [DOI] [PubMed] [Google Scholar]
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibres to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Bester H, Besson JM, Bernard JF. Organization of efferent projections from the parabrachial area to the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1997;383:245–281. doi: 10.1002/(sici)1096-9861(19970707)383:3<245::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bester H, Champman V, Besson J-M, Bernard J-F. Physiological properties of the lamina I spinoparabrachial neurons in the rat. J Neurophysiol. 2000;83:2239–2259. doi: 10.1152/jn.2000.83.4.2239. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel ? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labelled lines versus convergence in central processing. Ann Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- Edin BB, Essick GK, Trulsson M, Olsson KÅ. Receptor encoding of moving tactile stimuli in humans. I. Temporal pattern of discharge of individual low-threshold mechanoreceptors. J Neurosci. 1995;15:830–847. doi: 10.1523/JNEUROSCI.15-01-00830.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother–pup bonding during suckling. J Neurosci. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DG, Sorkin LS, Willis WD. Responses of spinothalamic tract cells in the superficial dorsal horn of the primate lumbar spinal cord. J Physiol. 1987;388:681–703. doi: 10.1113/jphysiol.1987.sp016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci. 2004;24:752–761. doi: 10.1523/JNEUROSCI.3272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel S. Golgi studies of the substantia gelatinosa neurons in the spinal trigeminal nucleus. J Comp Neurol. 1975;162:397–416. doi: 10.1002/cne.901620308. [DOI] [PubMed] [Google Scholar]
- Greenspan JD. Influence of velocity and direction of surface-parallel cutaneous stimuli on responses of mechanoreceptors in feline hairy skin. J Neurophysiol. 1992;68:876–889. doi: 10.1152/jn.1992.68.3.876. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Kornhuber HH. A quantitative study of C-mechanoreceptors in hairy skin of the cat. J Physiol. 1977;271:549–565. doi: 10.1113/jphysiol.1977.sp012014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata K, Kenshalo DR, Dubner R, Nahin RL. Diencephalic projections from the superficial and deep laminae of the medullary dorsal horn in the rat. J Comp Neurol. 1992;321:404–420. doi: 10.1002/cne.903210308. [DOI] [PubMed] [Google Scholar]
- Keller AF, Beggs S, Salter MW, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T, Perl ER. Primate cutaneous sensory units with unmyelinated (C) afferent fibres. J Neurophysiol. 1977;40:1325–1338. doi: 10.1152/jn.1977.40.6.1325. [DOI] [PubMed] [Google Scholar]
- Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- Light AR, Trevino DL, Perl ER. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979;186:151–172. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II) J Neurosci. 2005;25:3900–3907. doi: 10.1523/JNEUROSCI.0102-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B, Carpenter SE. Primary afferent units from the hairy skin of the rat hindlimb. Brain Res. 1982;238:29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Belle MD, Cheunsuang O, Stewart A, Morris R. Morphology of inhibitory and excitatory interneurons in the superficial laminae of the rat dorsal horn. J Physiol. 2007;584:521–533. doi: 10.1113/jphysiol.2007.140996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM. Physiological properties of unmyelinated fibre projection to the spinal cord. Exp Neurol. 1966;16:316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Nordin M. Low-threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibres in the human supraorbital nerve. J Physiol. 1990;426:229–240. doi: 10.1113/jphysiol.1990.sp018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa J, Torebjörk E. Sensations evoked by intraneural microstimulation of C nociceptor fibres in human skin nerves. J Physiol. 1989;415:583–599. doi: 10.1113/jphysiol.1989.sp017737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Cole J, Vallbo Å, McGlone F, Elam M, Krämer HH, Rylander K, Wessberg J, Bushnell MC. Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neurosci Lett. 2008;436:128–132. doi: 10.1016/j.neulet.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo ÅB, Bushnell MC. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;9:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Sugiura Y. Spinal organization of C-fibre afferents related with nociception or non-nociception. In: Kumazawa T, Kruger L, Mizumura K, editors. Progress in Brain Research. Vol. 113. New York: Elsevier Science; 1996. pp. 319–339. [PubMed] [Google Scholar]
- Vallbo ÅB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J Neurophysiol. 1999;81:2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]