Abstract
Serotonin (5-HT) can potently activate and modulate spinal locomotor circuits in a variety of species. Many of these findings have been obtained by applying serotonin exogenously to the isolated spinal cord of in vitro preparations, which has the drawback of indiscriminately activating extrasynaptic receptors and neurons. To investigate the role of endogenously released serotonin in modulating locomotor networks, the selective serotonin reuptake inhibitor citalopram was used. Fictive locomotion was elicited by either electrical stimulation of the brainstem or the sacral 4 (S4) dorsal root. The addition of 20 μm of citalopram caudal to thoracic segment 5 (T5) had an overall inhibitory effect on the lumbar central pattern generator (CPG). Left–right and flexor–extensor coupling were significantly decreased, and there was also a phase shift in the flexor–extensor relationship. In addition, there was a significant decrease in burst amplitude. These effects were observed during both afferent and brainstem evoked fictive locomotion. When citalopram was added in the presence of 5-HT1A and 5-HT1B antagonists, the inhibitory effects were largely reversed. The remaining excitatory effects were mediated by 5-HT7 and 5-HT2 receptors. These results suggest that endogenous 5-HT release can modulate locomotor-like activity early in neonatal development.
Introduction
It has been realized for some time that monoamines contribute to the control of locomotion generated by the spinal cord (Jankowska et al. 1967; Schmidt & Jordan, 2000). 5-HT has attracted the most attention, largely because of its ability to evoke and modulate locomotor patterns. This appears to be especially true during development where 5-HT has been shown to be capable of evoking locomotor activity in the neonatal mouse and rat (Sqalli-Houssaini et al. 1993; Cowley & Schmidt, 1994; Nishimaru et al. 2000; Madriaga et al. 2004; Liu & Jordan, 2005). Overall, it has been concluded that 5-HT excites networks and motoneurons acting through 5-HT7 and 5-HT2 receptor subtypes (Schmidt & Jordan, 2000; Hochman et al. 2001; Madriaga et al. 2004; Liu & Jordan, 2005; Pearlstein et al. 2005; Liu et al. 2009). However, the actions of 5-HT are unlikely to be that simple. 5-HT acts through 15 known receptor subtypes, and its actions on synaptic transmission and ion channel conductances are only partly known. A useful example in this regard is work demonstrating that 5-HT1A and 5-HT2 receptors interact to inhibit and excite, respectively, spinal networks that produce locomotion (Beato & Nistri, 1998; Hochman et al. 2001).
5-HT neurons are contained within the raphe nuclei of the brainstem, which in turn project to all segments of the spinal cord. We know that 5-HT fibres are present in the mouse spinal cord at birth (Ballion et al. 2002). That said, the 5-HT descending system takes at least three weeks after birth to develop, and at birth 5-HT immuno-reactive (5-HTir) fibres are only apposed to approximately 50% of quadriceps motoneurons in the neonatal rat, to take one example (Tanaka et al. 1992). Considering this, it is important to examine whether endogenous release of 5-HT can affect locomotor circuits. In contrast to multiple studies that have investigated the effects of exogenously applied 5-HT, there have been only a couple of studies that have examined the effects of the release of endogenous 5-HT on spinal networks. In the neonatal rat, it was shown that stimulation of the parapyramidal region of the brainstem could elicit bouts of fictive locomotion that were dependent on the activation of 5-HT7 receptors. Brainstem stimulation in neonatal rats has also been shown to hyperpolarize the threshold of motoneuron Na+ spike initiation, an effect that is partly dependent on 5-HT2 receptors (Gilmore & Fedirchuk, 2004). In the adult lamprey spinal cord, increasing endogenous extracellular 5-HT largely mimics the effects of exogenously applied serotonin (Christenson et al. 1989). There is evidence that 5-HT cells in the caudal raphe are active during the performance of treadmill walking in conscious cats, and evidence for release of 5-HT in the spinal cord has been found in freely walking rats (Gerin et al. 1994).
In the present work we tested the hypothesis that increased extracellular concentrations of endogenously released 5-HT would modulate the locomotor-like rhythm produced in neonatal mice. We evoked locomotor activity by stimulation of the brainstem and then increased the concentration of 5-HT in the spinal cord by using a selective serotonin reuptake inhibitor (SSRI), citalopram. SSRIs act to increase the extracellular concentration of serotonin by inhibiting reuptake transporters which normally transport serotonin into the presynaptic terminal. Here we first examined the changes in the underlying locomotor rhythm following an increase in endogenous 5-HT, and then we identified the 5-HT receptors responsible. Our data suggest that when the endogenous extracellular concentration of 5-HT is increased it can lead to a general inhibition of the rhythm, largely by acting on the 5-HT1 receptor class. These data will be discussed in the context of a possible bidirectional effect of 5-HT. Some of these data were previously published in abstract form (Dunbar & Whelan, 2008).
Methods
Ethical approval
Experiments were performed on Swiss Webster mice (Charles River Laboratories) 0–3 days old (P0–P3, n= 46). The animals were chilled by hypothermia, decapitated and eviscerated using procedures approved by the University of Calgary Animal Care Committee. Our experiments comply with the policies and regulations of The Journal of Physiology (Drummond, 2009).
Tissue preparation
The remaining tissue was placed in a dissection chamber filled with artificial cerebrospinal fluid (aCSF; concentrations in mm: 128 NaCl, 4 KCl, 1.5 CaCl2, 1 MgSO4, 0.5 Na2HPO4, 21 NaHCO3, 30 d-glucose) and bubbled with 95% O2–5% CO2. A ventral laminectomy exposed the spinal cord sparing as much of the cauda equina as possible, and the ventral and dorsal roots were cut. The brainstem was transected at cranial nerve VII and then the brainstem–spinal cord was gently removed from the vertebral column (Gordon et al. 2008). The cerebellum was removed by gently dissecting it away. In the one set of experiments where the brainstem was not included, the transection was made at the level of cervical segment 1 (C1). The brainstem–spinal cord or isolated spinal cord preparation was then allowed to recover for at least 30 min before being transferred to the recording chamber and bubbled with 95% O2–5% CO2 aCSF. The bath solution was heated gradually from room temperature to 27°C. The temperature in the bath was continuously monitored and recorded along with the experimental data. In most experiments, the thoracolumbosacral spinal cord was separately superfused from the rest of the spinal cord and brainstem. In those experiments, the bath was divided by a piece of thin plastic and sealed with petroleum jelly at T5. In additional experiments, a triple split-bath was constructed by dividing the T10 and L6 segments and separately superfusing the three compartments. Green food colouring was added to one compartment to ensure that the seal was complete; if green colour was observed in the adjoining compartment, then the experiment was aborted.
Immunohistochemical staining
The brainstem–spinal cords were postfixed overnight in 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C. The next day, the spinal cords were stabilized in an upright position onto an agar block using 20% gelatin. Transverse sections from the brainstem (50 μm) and the spinal cord (50 μm) were cut (Leica vibrating microtome VT1000S), and the slices were collected in PBS-azide (0.02%).
The free-floating sections were washed 3 times for 10 min each in PBS with 0.1% Triton X-100 (Sigma). Non-specific binding was blocked with 5% normal donkey serum (Sigma-Aldrich) in PBS for 30 min, and the tissues were incubated overnight at 4°C with rabbit anti-5-HT primary antibody (1: 10 000, Immunostar). Sections were washed 3 times with PBS-Triton X-100 and incubated for 2 h at room temperature with a secondary antibody conjugated to Alexa 546 (1: 200 donkey anti-rabbit; Molecular Probes). After a series of PBS-Triton washes, the sections were then mounted and coverslipped with Fluoromount (Diagnostic BioSystems, Pleasanton, CA, USA) and the slides were sealed with butyl acetate. Digital images were taken using a fluorescence microscope with Apotome attachment (Axio Imager Z1, Zeiss). Negative controls were performed using slices of spinal cord where the primary antibody was omitted.
Electrophysiological recordings and activation of locomotor networks
Neurograms were recorded from the ventral lumbar roots 2 and 5 (L2, L5) using tight fitting suction electrodes. The neurograms were amplified (100–300 times), filtered (DC–1 kHz), and digitized (2.5 kHz, Axon Digidata 1322A; Molecular Devices, Sunnyvale CA, USA) for future analysis. For the neurogram pattern to be considered locomotor-like, two conditions must be met: alternation of left and right at the same segment, and ipsilateral alternation of flexors and extensors (Whelan et al. 2000). L2 neurogram burst discharge corresponds to the activation of hindlimb flexor muscles, whereas L5 neurogram bursts coincide with extensor muscle activity (Kiehn & Kjaerulff, 1996; Whelan et al. 2000), and therefore L2–L5 burst alternation is considered to be a signature of flexor–extensor alternation. Neurograms from both the right and left L2 roots were recorded to examine segmental alternation, and at least one L5 neurogram was recorded to compare to the ipsilateral L2 neurogram.
To evoke fictive locomotion by afferent stimulation, constant current stimulus trains (A360, World Precision Instruments (Sarasota, FL, USA); Master 8 pulse generator, A.M.P.I. (Jerusalem, Israel)) were delivered to an S4 dorsal root by way of a suction electrode. To determine the stimulus threshold (T), a train of pulses were delivered at increasing intensities until a polysynaptic reflex response was elicited (1–10 μA). Pulses of 1 ms duration were delivered every 3 min (4 Hz, 40 pulses, 2T range) at a constant intensity through an experiment (Whelan et al. 2000). This stimulus train elicits an augmenting neurogram discharge leading to the generation of an alternating rhythm with a frequency of approximately 1 Hz.
To evoke locomotion by brainstem stimulation, constant current stimulus trains (World Precision Instruments A360, A.M.P.I. Master 8 pulse generator) were delivered to the ventrolateral surface of the brainstem by way of a suction electrode to evoke bouts of locomotor-like activity. Stimulus intensity was determined by delivering a train of stimuli at increasing intensity until a clear, stable locomotor pattern was visible (0.1–1 mA). Pulses of 10 ms duration were delivered every 3 min (2 Hz, 120 pulses) at a constant intensity throughout an experiment (Zaporozhets et al. 2004; Gordon et al. 2008). This stimulus train produced a locomotor-like rhythm with an approximate frequency of 0.3–1 Hz.
Pharmacology
The SSRI used in the experiments was citalopram (1–100 μm, Sigma-Aldrich), dissolved in double distilled water to make single dose aliquots (20 mm). Citalopram was chosen since it is recognized as being a highly specific SSRI (Hyttel, 1977). A dose response was established for citalopram using S4 afferent trains and measuring the steady state amplitude of the response following administration of citalopram. The computed EC50 was 20 μm, which was used throughout the study. Aliquots were kept frozen until use. In one set of experiments, exogenous 5-HT was used (1–10 μm, Sigma-Aldrich). 5-HT aliquots were made fresh each day. The 5-HT1A antagonist WAY-100635 (1 μm, Sigma-Aldrich), the 5-HT1B antagonist SB-216641 (20 μm, Tocris), the 5-HT2A antagonist ketanserin (0.15 μm, Sigma-Aldrich) and the 5-HT7 antagonist SB-269970 (10 μm, Sigma-Aldrich) were used, each dissolved in double distilled water into aliquots which were then frozen until use.
Data analysis
Data were analysed using custom-written programs (Matlab; The Mathworks, Inc., Natick, MA, USA) as well as commercially available programs (SigmaStat; Systat Software Inc., San Jose, CA, USA). A spike detection algorithm was used to digitally blank the stimulus artifacts. To analyse the stability and phase of the rhythms, time series analysis techniques were used as previously described (Madriaga et al. 2004). Time series analyses were performed by taking intervals of 10–15 s of raw data, applying a low-pass digital filter (Chebyshev type I, 100 Hz), and resampling at 250 Hz. This was performed for three sweeps of each condition in brainstem stimulation experiments, and three to nine sweeps when locomotor patterns were evoked by afferent stimulation. Means were subtracted from the processed data and further smoothed using a digital smoothing polynomial filter (Savitzky–Golay 3rd order, length of segment: 250 ms). Autocorrelograms (Fig. 1) were calculated, and the quality of the rhythm was assessed in each neurogram by measuring the autocorrelation at the first peak after lag 0. The closer this value is to 1 the more regular the rhythm (Fig. 1D). A minimum autocorrelation of 0.3 (Fig. 1D) for each neurogram was required to include the data for analysis. Cross-correlograms were also calculated and the regularity of the coordinated rhythm was assessed by measuring the correlation coefficients (peak to trough correlation coefficient, PTCC) for pairs of neurograms (Fig. 1C; L2–L2, L5–L5, L2–L5). The closer the PTCC to 2 the more regular the rhythm between the pairs of neurograms. The cycle period for the L2 neurogram was calculated by measuring the number of lags from 0 to the first peak in the autocorrelogram (Fig. 1E). Burst amplitude (Fig. 1A) was calculated for the various conditions by detecting bursts for a segment of data and averaging them using custom written programs. Bursts were normalized to the maximum burst observed during control conditions. The phase between ventral root oscillations was obtained from the cross-correlogram and defined as the number of lags (Fig. 1F) from lag 0 to the next peak divided by the cycle period. Phase diagrams and burst durations (Fig. 1B) were constructed by normalizing the onset and offset of rhythmic discharges from different ventral roots to the cycle period of the discharge obtained from one ventral root or nerve (Whelan et al. 2000). Data were analysed at the furthest time point possible after the addition of citalopram, to a maximum of 1 h. The furthest time point was determined as the point at which there was a discernible rhythm meeting the inclusion criteria of a PTCC >1 and an autocorrelation coefficient >0.3. Similar to previous reports (Christenson et al. 1989), there was variability in the onset time of the citalopram effect from preparation to preparation, and this time point varied from 15 min to 1 h after citalopram application.
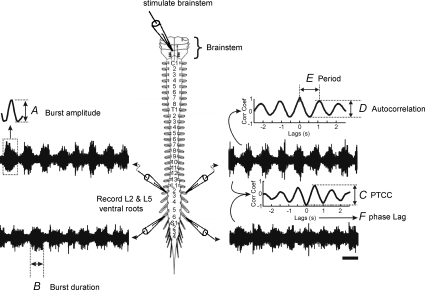
Figure 1. Explanation of parameters measured.
A, burst amplitude, which is determined by high pass filtering the trace and smoothing, rectifying and averaging the burst heights. B, burst duration was calculated by marking the beginning and end of each burst to calculate the average duration. C, the peak to trough correlation coefficient (PTCC) is calculated to compare the stability of the rhythm across different ventral root neurograms, in this case the ipsilateral L2 and L5. D, the autocorrelation coefficient was calculated to quantify the stability of the rhythm in a single root. E, period was obtained from the autocorrelation by calculating the distance between peaks. F, phase lag was determined from the cross-correlogram, with values of 0–1. Alternating rhythms (L2–L2; ipsi L2–L5) are a signature of locomotor-like activity and tend to have a phase lag close to 0.5. Scale bar = 1 s.
Statistics
Auto- and cross-correlation data were expressed as means ±s.e.m. and analysed using a repeated-measures ANOVA or Student's t test for paired data if normally distributed. In the case of multiple comparisons across independent data sets, either a 1-way or a 2-way repeated measures (RM) ANOVA was performed, with the repeated measure as the control and experimental condition after pharmacological manipulation. If significant differences were found, a post hoc Holm–Sidak test was applied to determine pairwise differences. To analyse and to illustrate phase relationships, circular plots were used in which the phase was normalized from 0 to 1 (Zar, 1999). Each experiment was represented on a circular plot with an r-value (the distance from the centre of the circle) and mean phase angle. Using second-order analysis, an average mean r and phase value were found for the set of experiments, and this is expressed on the circular plots as the length and direction of the arrow, respectively. If the length of the arrow is large, then this suggests that the rhythm has a consistent phase at the position of the arrow. Significance of data clustering was computed using Rayleigh's test. In certain cases Hotelling's parametric paired-sample F test with angles was employed (Zar, 1999). Two sample comparisons between experiments were performed using Hotelling's parametic two-sample F test with angles. The sample sizes for each experiment ranged from 3 to 8 spinal cord preparations. Significance for all tests was set at P < 0.05.
Results
Addition of citalopram caudal to T5 during brainstem-evoked fictive locomotion produced an overall inhibitory effect
Similar to our previous report, we found that stimulation of the ventrolateral surface of the brainstem produced robust bouts of fictive locomotion in the isolated brainstem–spinal cord preparation of the mouse (Gordon et al. 2008). Generally, bath application of citalopram (20 μm) caudal to T5 produced an inhibitory effect on rhythmic output (Figs 2B and 3). The burst amplitude of the L5 and L2 neurograms decreased significantly over the time course of citalopram application (n= 8, L5: P < 0.001; L2: P < 0.01; Fig. 3B and C). The normalized burst duration was significantly decreased for the L2 neurograms (n= 7; P < 0.01; Fig. 3D). Autocorrelation coefficients were calculated to examine the regularity of the rhythm produced from each ventral root neurogram, and neither the L5 nor the L2 autocorrelation coefficients were changed by citalopram (n= 7, P > 0.1). The PTCC was calculated to measure the coupling strength between pairs of neurograms. The PTCC for the L5–L5 (extensor pair) was significantly decreased (n= 7, P < 0.05), as was the L5–L2 PTCC (extensor–flexor pair) (n= 7, P < 0.05) following citalopram application. On the other hand, the PTCC for the segmental L2 neurograms showed a trend for a decrease, but this was not significant (n= 7, P > 0.05). Exogenous serotonin has been shown to slow locomotor-like rhythms (Beato & Nistri, 1998; Madriaga et al. 2004), and in our current work the L2 cycle period significantly increased following citalopram application (n= 7, P < 0.01, Fig. 3E).
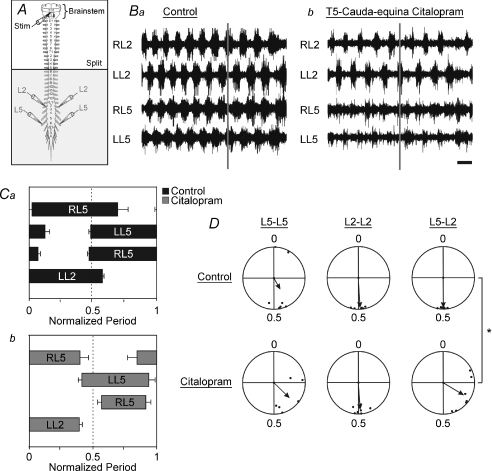
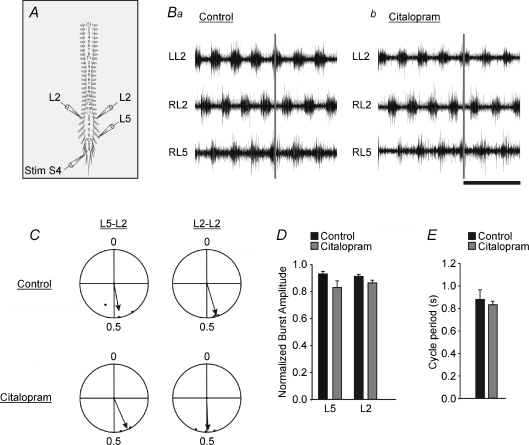
Figure 2. Increasing endogenous extracellular serotonin causes a decrease in flexor–extensor coupling and a shift in the phase relationship.
A, schematic diagram of the isolated brainstem–spinal cord preparation showing recording and stimulation sites. The horizontal line labelled ‘split’ represents the location of the petroleum jelly barrier which isolates the rostral from the caudal portion of the cord. The shaded area represents the portion of the spinal cord exposed to citalopram. B, raw traces from the L5 and L2 ventral root neurograms under control conditions (a) and 45 min after citalopram was added to the caudal bath (b). Time scale bar = 2 s. C, phase diagram of the timing of ventral neurogram discharge under control (a) and citalopram (b) conditions. Phase diagrams and burst durations were constructed by normalizing the onset and offset of rhythmic discharges from the different ventral roots to the cycle period of the discharge obtained from the LL2 ventral root. Error bars represent the standard error. D, circular plots comparing the phase lag changes from control (top row) to citalopram (bottom row conditions). The length of the arrows is a reflection of variability and direction of the arrows represents the mean phase. The L5–L2 phase lag relationship was significantly shifted due to citalopram as measured by the Hotelling second order paired angles analysis (P < 0.0025). The addition of citalopram did not significantly affect the L2–L2 or the L5–L5 phase lag relationships compared to control (P > 0.1). The Asterisk indicates significant differences (P < 0.05; Hotelling analysis).
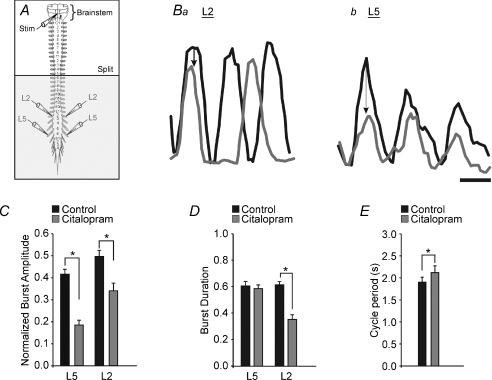
Figure 3. Increasing endogenous extracellular serotonin concentration decreases burst amplitude and decreases flexor burst duration and rhythm speed.
A, schematic diagram of the isolated brainstem–spinal cord preparation. The horizontal line labelled ‘split’ represents the location of the petroleum jelly barrier which isolates the rostral portion of the cord from the caudal. The shaded area represents the portion of the spinal cord exposed to citalopram. B, smoothed and rectified traces. Black indicates control, grey denotes the effect of citalopram. Scale bar = 1 s. Ba, L2 neurograms; arrow indicates decrease in burst amplitude. Bb, L5 neurograms; arrow indicates decrease in burst amplitude. C–E, graphs showing the normalized burst amplitude (C), the burst duration (D) and cycle period (E) under control and citalopram conditions. Error bars represent the standard error of the mean. Asterisks indicate significant differences (P < 0.05; paired t test).
Phase relationships between neurogram pairs are an important index of whether a rhythm is locomotor-like. Control phase lags had a locomotor signature of left–right alternation (L2–L2: r= 0.97, phase = 0.49, n= 7; L5–L5: r= 0.44, phase = 0.42, n= 7) and flexor–extensor alternation (L5–L2: r= 0.97, phase = 0.50, n= 7) (Fig. 2D). The addition of citalopram did not significantly affect the L2–L2 phase lag (r= 0.88, phase = 0.49, n= 7) or the L5–L5 phase lag relationships (r= 0.71, phase = 0.37, n= 7) when compared to controls using Hotelling second order paired angles analysis (P > 0.05). However, the L5–L2 phase lag relationship was significantly reduced and became more variable following addition of citalopram (r= 0.78, phase = 0.34, n= 7) as measured by the Hotelling second order paired angles analysis (P < 0.01). The Rayleigh test showed significant clustering of the data in all cases (P < 0.05), although the L5–L2 vector length was reduced.
To illustrate the phase shifts in another form, we plotted the phase onsets and offsets for all neurograms (Whelan et al. 2000). Under control conditions, the phase onsets and offsets are similar to what we would expect during fictive locomotor conditions (Fig. 2Ca). However, when citalopram was applied, the variability and the onset and offset patterns shifted substantially (Fig. 2Cb). The L2 relationship shows a reduction in burst duration (Figs 2B and C and 3D) and overlap (Fig. 2C), accompanied by an increase in the cycle period (Fig. 3E).
The effects of citalopram were cumulative over time, and in all experiments the L5 burst amplitude after 50 or more minutes was reduced to the point of being undetectable. In 3/8 preparations, this was also the case for at least one of the two L2 neurograms. In the remaining 5/8 preparations, there were still detectable analysable bursts present in the L2 neurograms at the endpoint of the experiment.
The magnitude of the citalopram effect is not dependent on the method of eliciting rhythmicity and is partially dependent on the site of application
So far we have shown that an SSRI can affect locomotor-like activity evoked by brainstem stimulation. These data are significant since they show that 5-HT is an important neuromodulator in the descending activation of spinal networks. We next wished to test whether an increase in serotonin extracellular concentrations could affect rhythmicity evoked by afferent stimulation. We turned to electrical stimulation of the sacral dorsal roots (Lev-Tov et al. 2000; Whelan et al. 2000) which activates the CPG by way of ventrolateral funiculus (VLF) projections (Strauss & Lev-Tov, 2003).
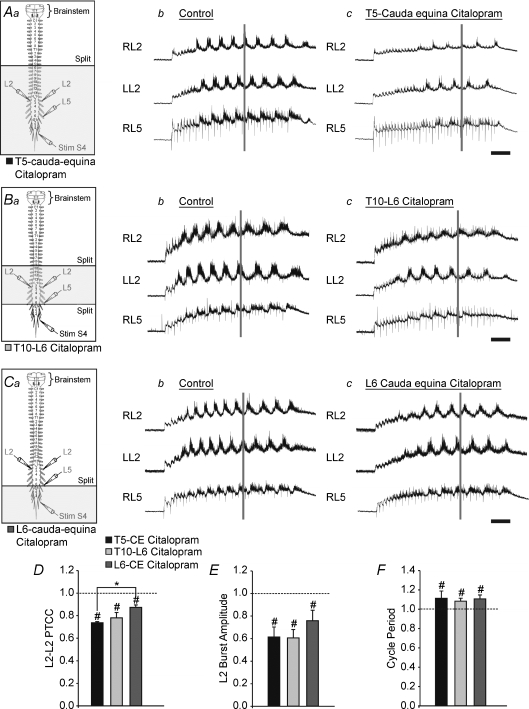
To determine whether the effects were localized to one compartment or were distributed, we delivered citalopram to three compartments in separate experiments: T5–cauda equina (Fig. 4Aa); T10–L6 (Fig. 4Ba); and L6–cauda equina (Fig. 4Ca). This also allowed us to exclude the possibility that effects were primarily due to inhibition of afferent transmission in the sacral regions (Strauss & Lev-Tov, 2003). The comparisons were performed using a two-way repeated measures ANOVA. The factors were treatment and location of application. The levels for treatment were control and citalopram, and the levels for location of application were T5–cauda equina, lumbar only, and sacral only. The repeated measure was the treatment for each preparation. For simplicity's sake, the comparison examined only the L2 neurogram parameters. The decrease in the burst amplitude due to citalopram was significant across all locations of application (P < 0.001; Fig. 4E), and although there was a trend for the effect to be larger when the lumbar segments (Fig. 4Ab and c compared to Cb and c) were exposed directly to citalopram, this was not significant. In each preparation the cross-correlation was significantly reduced by the addition of citalopram (P < 0.05, Fig. 4D). In addition, there was a significant difference in the magnitude of the effect when citalopram was applied to the sacral only compared to the T5–cauda equina segments (P < 0.01; Fig. 4D, compare Ac and Cc). This indicated that the greater the proportion of the spinal cord exposed to citalopram, the greater the effect. The decrease in the autocorrelation coefficient was significant across preparations (P < 0.001), with no effect of location of application. Across location of citalopram application, the cycle period was significantly increased by approximately 10% (Fig. 4F; P < 0.001). In summary, the effects of citalopram on the rhythm were similar when the CPG was activated by stimulating either the S4 dorsal root or the brainstem. We found that the effects of citalopram increased when bath-applied to more segments consistent with previous reports that made use of exogenously applied drugs (Kjaerulff & Kiehn, 1996; Cowley & Schmidt, 1997; Kremer & Lev-Tov, 1997; Christie & Whelan, 2005).
Figure 4. The effect of citalopram is not dependent on the mode of activation of the CPG and is partially determined by the site of application.
Aa, Ba and Ca, schematic diagrams of the isolated brainstem–spinal cord preparation. The horizontal lines labelled ‘split’ represents the locations of the petroleum jelly barrier which isolates the rostral portion of the cord from the caudal. The shaded area represents the portion of the spinal cord exposed to citalopram. The legend with the colour coded box underneath each schematic diagram corresponds with the colours of the bar graphs. a–c, raw traces with stimulus artifacts digitally attenuated. A, raw traces under control conditions (b) and 30 min after citalopram application caudal to T5 (c), with the petroleum jelly split at T5. B, raw traces under control conditions (b) and 30 min after citalopram application to the lumbar compartment (c), with the petroleum jelly split at L6. D–F, graphs comparing the decrease in the PTCC (D), the change in the L2 burst amplitude (E), and the change in the cycle period (F). Error bars represent the standard error of the mean. #Significant change from control, *significant difference across location of application (both P < 0.05) as measured by a two-way repeated measures ANOVA. Scale bar = 2 s.
Serotonergic fibres are present in both lumbar and sacral segments
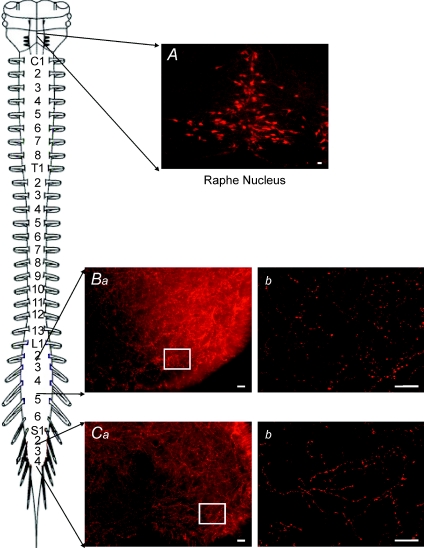
The serotonergic system is still developing in the neonatal mouse (Branchereau et al. 2002). While serotonin fibres have been observed in neonatal mice (Ballion et al. 2002), our strain was not the same and therefore it was important to replicate the findings, especially since we were attempting to manipulate the extracellular concentration of endogenous 5-HT. Immunohistochemistry detected 5-HTir positive fibres and varicosities were present at high densities throughout the rostrocaudal extent of the neonatal spinal cord, including the lumbar (Fig. 5B) and sacral segments (Fig. 5C). There was significant staining in the ventral horn region (Fig. 5B and C). Finally, a positive control showed staining of putative raphe somata in the brainstem (Fig. 5A).
Figure 5. Serotonin positive fibres are present through out the length of the spinal cord.
A, 5-HTir positive cells in the region of the raphe nucleus were used as a positive control. B, 5-HTir positive fibres within the lumbar ventral horn (transverse slice). Ba, low power view of the ventral horn showing high level of 5-HTir expression. Bb, increased magnification of ventral horn in Ba showing detail of individual 5-HTir positive fibres and varicosities. C, 5-HTir positive fibres were also present in the sacral ventral horn. Ca, low power view of the ventral horn. Cb, increased magnification of boxed area in Ca showing individual 5-HTir fibres and varicosities. Scale bar = 20 μm. Schematic diagram shows lumbar and sacral segments selected.
The brainstem was required for citalopram to affect fictive locomotion
Although the main source of serotonin in the spinal cord is descending fibres from the brainstem, there are a small number of serotonergic neurons in the sacral spinal cord which could have been partly responsible for the effects of citalopram (Ballion et al. 2002). To control for this possibility, we applied citalopram in an isolated spinal cord preparation with the brainstem removed (Fig. 6). Fictive locomotion was evoked by trains of stimuli applied to the S4 dorsal root (Fig. 6A). There was no change from control for any of the parameters measured following bath application of citalopram (Fig. 6C, D and E; P > 0.1, n= 3), demonstrating that the actions of citalopram required the brainstem to be present. The lack of effects in the isolated spinal cord preparation were not due to the mode of stimulation, since the inhibition of the locomotor pattern by citalopram following afferent-evoked stimulation in brainstem–spinal cord preparations was qualitatively similar to what we observed during brainstem-evoked locomotion (Figs 2 and 4).
Figure 6. Modulation by citalopram does not occur when the brainstem is removed.
A, schematic diagram of the isolated brainstem–spinal cord preparation. B, raw traces with stimulus artifacts removed. B, raw neurograms under control conditions (a) and 30–60 min after citalopram was added (b). Time scale bar = 2 s. Neither the phase lag (C), nor burst amplitude (D), nor cycle period (E) was changed by the addition of citalopram to the bath (P > 0.05). Error bars represent the standard error of the mean.
Modulation of fictive locomotion by exogenous serotonin is significantly different from modulation by citalopram-mediated increases in extracellular endogenous serotonin concentration
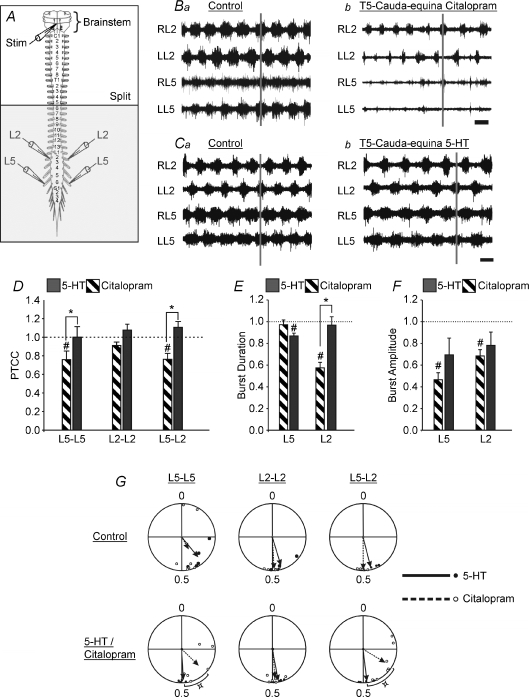
Bath application of 5-HT increases the strength of coupling between segmental neurograms (Madriaga et al. 2004; Pearlstein et al. 2005). To directly contrast the effects of bath-applying serotonin with decreasing reuptake of endogenously released serotonin, the brainstem stimulation experiments were repeated using exogenously applied serotonin which was bath applied to the thoracolumbosacral cord. With the brainstem attached and undergoing periodic stimulation, lower concentrations of 5-HT (1 μm) were bath-applied compared to previously reported values (Madriaga et al. 2004; Gordon & Whelan, 2006). If higher concentrations of 5-HT were bath-applied (2–10 μm) coordinated bursting would occur typical of what we observed using 5-HT concentrations of 20 μm in isolated spinal cord preparations (Madriaga et al. 2004). After the addition of 5-HT there was a trend for an increase in the PTCC (Fig. 7D) for two of the three neurogram pairs compared (L2–L2 and L5–L2), which was in contrast to the significant decrease observed with citalopram. The difference in the PTCC between citalopram and exogenous 5-HT was significant for the L5–L5 (P < 0.05) and the L5–L2 neurograms (P < 0.01). Another important difference between exogenous 5-HT application and citalopram was the effect on phase lag. Citalopram shifted the L5–L2 phase lag from 0.5 to 0.34 (Fig. 7G). Under control conditions, the rhythm evoked by brainstem stimulation was considered to be locomotor-like (L2–L2: r= 0.91, phase = 0.45; L5–L5: r= 0.79, phase = 0.39; n= 5; L5–L2: r= 0.94, phase = 0.46, n= 5; Rayleigh's test, P < 0.001 for all comparisons). On the other hand, after the addition of 5-HT, there was an improvement in rhythm regularity as indicated by increased r-values and phase lags closer to 0.5 (L2–L2: r= 0.97, phase = 0.47; L5–L5: r= 0.93, phase = 0.49; L5–L2: r= 0.97, phase = 0.48; n= 5; Rayleigh's test, P < 0.001 for all comparisons; Fig. 7G). The change in the L5–L5 phase lag relationship after the addition of 5-HT was significant as tested by a Hotelling test for second order paired angle analysis (P < 0.05). Although, the changes in the other phase relationships compared to the controls were not significant (P > 0.1), the differences between the effects of exogenous and endogenous serotonin on the L5–L5 and the L5–L2 phase lags were significant (P < 0.05). In addition, the decrease in the burst duration of the L2 neurograms that occurred following application of citalopram (Fig. 7Ba and b) did not occur following application of exogenous 5-HT (Fig. 7Ca and b), and this difference was significant (P < 0.001). The differences in the effects of exogenous and endogenous 5-HT contrasted with their similar effect of decreasing burst amplitude. A decrease in the polysynaptic reflex, which occurs 10 ms after the stimulus pulse, as a result of exogenous 5-HT addition has been observed (Crick & Wallis, 1991; Machacek et al. 2001; Gordon & Whelan, 2006), and we found similar effects in our work (data not shown). Both citalopram and exogenous 5-HT caused an increase in cycle period (Fig. 7Ca and b), and the magnitude of the change due to 5-HT was significantly different from control P= 0.005). The control cycle period in the citalopram experiments was 1.91 ± 0.11 s, and adding citalopram resulted in a 13% increase to 2.13 ± 0.15 s. In the experiments where exogenous 5-HT was added the cycle period increased 22% from 2.75 ± 0.21 s to 3.34 ± 0.26 s. Taken together, these results suggest a difference in the effects on burst duration of increased endogenous serotonin compared with exogenous serotonin, but similar effects on cycle period and burst amplitude.
Figure 7. There are significant differences in pattern modulation by exogenous serotonin compared to increased endogenous serotonin.
A, schematic diagram of the isolated brainstem–spinal cord preparation. The horizontal line labelled ‘split’ represents the location of the petroleum jelly barrier which isolates the rostral portion of the cord from the caudal. The shaded area represents the portion of the spinal cord exposed to citalopram or 5-HT. B–C, raw traces with stimulus artifacts removed. B, traces under control conditions (a) and 45 min after citalopram was added to the caudal bath (b). Time scale bar = 2 s. C, traces under control conditions (a) and 45 min after serotonin was added to the caudal bath (b). Time scale bar = 2 s. D–F, graphs comparing the cross correlation (D), burst duration (E), and burst amplitude (F) under citalopram and 5-HT conditions. G, comparison of the phase lag changes from control (top row) to either citalopram or 5-HT (bottom row) conditions. The length of the arrows is a reflection of variability and the direction of the arrows represents the mean phase. Dotted lines and open circles represent citalopram; continuous lines and filled circles represent bath applied 5-HT. D–G, all measurements were normalized to their own controls. Error bars represent standard error. #Significant difference from control, *significant difference between citalopram and 5-HT (both P < 0.05) as calculated by a one-way RM ANOVA. The small square symbol by the phase diagrams indicates a significant difference (P < 0.05) between the effects of citalopram and exogenous 5-HT as measured by a two-sample parametric second order Hotelling F-test.
The effects of citalopram-mediated increased endogenous serotonin can be attenuated by the application of specific 5-HT1A and 5-HT1B antagonists
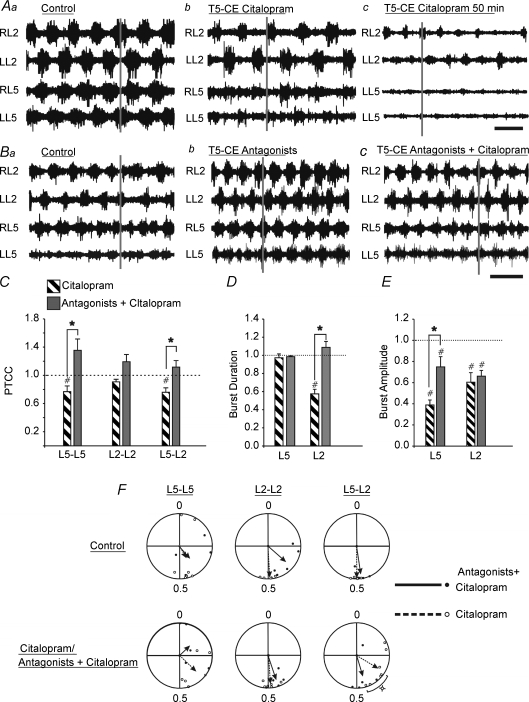
During locomotion, the inhibitory effects of serotonin have been shown to be partly mediated by 5-HT1 receptors (Beato & Nistri, 1998). To determine if 5-HT1 receptor subtypes were responsible for the inhibition observed when serotonin reuptake was blocked, a combination of WAY-100635 (5-HT1A antagonist, 1 μm) and SB-216641 (5-HT1B antagonist, 15–20 μm) were pre-incubated with the preparation before the addition of citalopram to the bath. This combination successfully attenuated some of the inhibitory effects of increased endogenous serotonin (compare Fig. 8Ac to Bc). Citalopram in the presence of WAY-100635 and SB-216641 produced a significantly greater PTCC for the L5–L5 and L5–L2 relationships compared to bath application of citalopram alone (Fig. 8C; L5–L5: P < 0.01; L5–L2: P < 0.01), but not for L2–L2 (P > 0.05). The addition of the antagonists also prevented the decrease in L2 burst duration (compare Fig. 8Aa and c to Fig. 8Ba and c; Fig. 8D; P < 0.001) and significantly attenuated the decrease in L5 burst amplitude (Fig. 8Ac, Bc and E; P < 0.001) normally observed following bath-application of citalopram. There was no change in the autocorrelation for either L2 or L5 neurograms measured over treatments. Although their was no significant change across treatments for cycle period, we note that the application of these two antagonists caused a significant decrease in L2 cycle period in 5/6 preparations (P < 0.05; Fig. 8Ba and b). The control cycle period in the antagonist experiments was 1.66 ± 0.052 s. This was decreased by the addition of the 5-HT1A/1B antagonists to 1.36 ± 0.15 s, and then increased to control values by the addition of citalopram to 1.65 ± 0.11 s. Finally, when the 5-HT1A and 5-HT1B receptors were antagonized, citalopram no longer caused a shift in the L5–L2 phase relationship (Fig. 8F; P < 0.01).
Figure 8. A combination of SB-216641 and WAY-100635 can attenuate some of the effects of citalopram-mediated increased endogenous serotonin.
A, neurograms from time points in citalopram experiments, with stimulus artifacts removed. Representative traces under control conditions (Aa), 30 min (Ab) and 50 min (Ac) after the addition of citalopram. B, representative traces from 5-HT1A/1B antagonists and citalopram experiments. Time scale bar = 2 s. Raw traces under control conditions (Ba), 30 min after the addition of SB-216641 and WAY-100635 (Bb) and 45 min after citalopram was added to the caudal bath in the presence of pre-incubated SB-216641 and WAY-100635 (Bc). Time scale bar = 2 s. C–E, graphs comparing the cross correlation (C), burst duration (D) and burst amplitude (E) for the conditions of citalopram alone and citalopram in the presence of 5-HT1A and 5-HT1B antagonists (Ab and Bc). F, comparison of the phase lag changes from control (top row) to citalopram with or without 5-HT1A/1B antagonists present (bottom row). The length of the arrows is a reflection of variability and the direction of the arrows represents the mean phase. Dotted lines and open circles represent citalopram; continuous lines and filled circles represent citalopram in the presence of SB-216641 and WAY-100635. C–G, all measurements were normalized to their own controls. Error bars represent standard error. #Significant difference from control, *significant difference between citalopram and 5-HT (both P < 0.05) as calculated by a one-way RM ANOVA. The small square symbol by the phase diagrams indicates a significant difference (P < 0.05) between the effects of citalopram and citalopram in combination with antagonists as measured by a two-sample parametric second order Hotelling F-test.
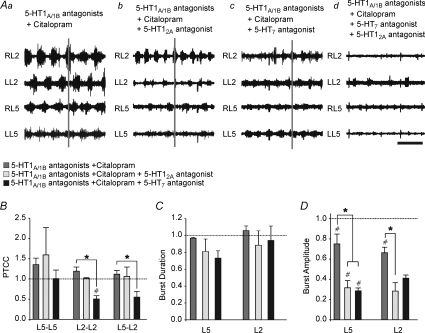
Both 5-HT2 and 5-HT7 antagonists can attenuate the 5-HT1A/1B antagonist mediated rescue of citalopram CPG effects
Both 5-HT2 and 5-HT7 receptors have been shown to mediate excitatory effects on fictive locomotor rhythms (Madriaga et al. 2004; Liu & Jordan, 2005; Gordon & Whelan, 2006; Liu et al. 2009). To determine whether these receptors contributed to CPG output in the presence of 5-HT1A/1B antagonists and citalopram, 5-HT2A (ketanserin) and 5-HT7 (SB-269970) receptor antagonists were used separately and in tandem to the 5-HT1A/1B antagonists and citalopram. Both ketanserin (Fig. 9Ab) and SB-269970 (Fig. 9Ac) decreased burst amplitude (Fig. 9D). Burst amplitude was significantly decreased by the addition of citalopram alone to the 5-HT1A/1B antagonists (Fig. 9Aa; L5: P < 0.01; L2: P < 0.001), and was further reduced following the addition of ketanserin for both the L5 (Fig. 9Ab; P < 0.05, n= 3) and L2 (P < 0.05, n= 3) neurograms. For the L5 neurograms, SB-269970 also significantly decreased burst amplitude to the same degree as ketanserin (Fig. 9Ac; P < 0.05, n= 3) (Fig. 8). Although there was not a significant difference between the effects of ketanserin and SB-269970, only SB-269970 significantly reduced both L2–L2 and L5–L2 cross-correlation compared to citalopram in the presence of the 5-HT1A/1B antagonists (Fig. 9B; P= 0.03; P= 0.049). Neither antagonist affected the L5–L5 cross-correlation. There was no significant effect on cycle period across treatments (P > 0.05). In the experiments that included the 5-HT2A antagonist, the control cycle period was 1.67 ± 0.056 s, 1.16 ± 0.27 s in the presence of the 5-HT1A/1B antagonists, 1.59 ± 0.17 s with the addition of citalopram, and finally 1.55 ± 0.22 s when the 5-HT2A antagonist was added. In the experiments that included the 5-HT7 antagonist, the control cycle period was 1.56 ± 0.018 s, 1.61 ± 0.12 s in the presence of the 5-HT1A/1B antagonists, 1.78 ± 0.22 s with the addition of citalopram, and finally 2.00 ± 0.82 s when the 5-HT7 antagonist was added. There was also no effect of either excitatory antagonist on autocorrelation as measured by a one-way RM ANOVA (P > 0.05). Burst duration was also not affected by the addition of ketanserin or SB-269970 (Fig. 9C).
Figure 9. Both 5-HT2 and 5-HT7 antagonists can prevent the 5-HT1A/1B mediated rescue of citalopram CPG effects.
A, raw representative traces of each condition with stimulus artifacts removed. In each case, the pharmacological agents listed were applied caudal to T5. Fictive locomotion was evoked by brainstem stimulation. Aa, neurograms 40 min after the application of citalopram to a preparation pre-incubated with WAY-100635 and SB-216641. Ab, neurograms 30 min after the addition of ketanserin. Ac, neurograms 30 min after the addition of SB-269970. Ad, neurograms after the addition of both ketanserin and SB-269970. Time scale bar = 2 s. B–D, graphs comparing the cross correlation (C), burst comparison (D) and burst amplitude (E) across the various conditions. C–D, all measurements were normalized to their own controls. Error bars represent standard error. #Significant difference from control, *significant difference between citalopram and 5-HT (both P < 0.05) as calculated by a one-way RM ANOVA.
When both ketanserin and SB-269970 were applied together, a cessation of rhythmic activity was observed in 1/3 preparations, and in the remaining two, rhythmic activity was present in individual neurograms but only rarely co-ordinated between neurograms (Fig. 9Ad). Although the burst amplitude was too low to be detected using our algorithm, qualitatively the burst duration was greatly reduced (compare Fig. 9Aa with Fig. 9Ad). These results indicate that without 5-HT1A/1B receptor activation, the effects of citalopram are at least partially mediated by 5-HT2A and 5-HT7 receptors.
Discussion
While 5-HT fibres are present at birth in our strain of mice and in other strains (Ballion et al. 2002), the 5-HT system is far from being developed (Schmidt & Jordan, 2000) and the efficacy of endogenously released 5-HT in eliciting locomotion has not been tested in this species. Here we demonstrate that manipulating the concentration of extracellular 5-HT using an SSRI interferes with brainstem activation of spinal networks. At first this seems counterintuitive since citalopram is known to increase the concentration of 5-HT in the synaptic cleft (Fuller & Wong, 1990). However, differential effects of 5-HT have been observed before in the spinal cord dorsal and ventral horn (Schmidt & Jordan, 2000; Millan, 2002). This is perhaps not surprising since 5-HT acts on 15 known receptor subtypes which in turn produce a multiplicity of inhibitory and excitatory effects (Hochman et al. 2001). For example, 5-HT was once thought to mainly depress nociception, but 5-HT can also potentiate nociceptive afferent transmission (Millan, 2002; Robinson et al. 2004). Relevant to our work, stimulation of the rostroventral medulla in rodents led to a facilitation of nociception at low stimulus intensities, while higher stimulus intensities were found to be inhibitory (Zhuo & Gebhart, 1997). Differential excitatory and inhibitory effects of 5-HT has also been reported for the control of locomotion and modulation of mono- and polysynaptic reflexes (Beato & Nistri, 1998; Jankowska et al. 2000; Schmidt & Jordan, 2000; Hammar et al. 2002, 2007; Gordon & Whelan, 2006). Exogenously applied 5-HT acts by way of postsynaptic 5-HT1A and 5-HT1B/D receptors to decrease the frequency of rhythmic activity (Beato & Nistri, 1998; Hochman et al. 2001), while on the other hand activating 5-HT2 and 5-HT7 receptors to boost excitability (Beato & Nistri, 1998; Madriaga et al. 2004; Liu & Jordan, 2005; Liu et al. 2009). Activation of multiple receptor subtypes is consistent with data showing that 5-HT activates multiple ionic conductances in both motoneurons (Rekling et al. 2000; Kjaerulff & Kiehn, 2001) and interneurons (Zhong et al. 2006a,b;). We have previously found that increasing exogenous concentrations of 5-HT tend to slow down the rhythm evoked from the isolated mouse spinal cord, consistent with the idea that 5-HT1 receptors are being activated (Madriaga et al. 2004). In support of this, we observed in our current work that application of the 5-HT1 antagonists WAY-100635 and SB-216641 could almost completely reverse the inhibitory effects of citalopram. It is likely that the increased concentration of 5-HT is activating 5-HT1A/B receptors located postsynaptically. However, 5-HT1B receptors have been shown to have an autoreceptor mediated inhibitory role (Murphy & Zemlan, 1988; Matsumoto et al. 1992) which could partly contribute to the effects we have observed. Recent work in the cat suggests that neurons in lamina VII and VIII that are activated during locomotion colocalize 5-HT1A, 5-HT7 and 5-HT2A receptors (Noga et al. 2009). This suggests that the divergent effects of 5-HT noted in our current work may be due to differential modulation of 2nd messenger pathways within the same neuron.
We found that citalopram application to thoracolumbar segments was more effective than sacral application but not as effective as application to the thoracolumbosacral segments. Work in the mouse and rat show that 5-HT has greater effects on network function when applied to thoracolumbar segments compared to caudal lumbar segments (Cazalets et al. 1995; Cowley & Schmidt, 1997; Kremer & Lev-Tov, 1997; Kiehn & Kjaerulff, 1998; Ribotta et al. 2000; Christie & Whelan, 2005). For example, 5-HT7 receptors are highly expressed in thoracolumbar regions of the spinal cord associated with high rhythmogenic capability (Hochman et al. 2001). On the other hand, 5-HT2 receptors do not appear to show a rostrocaudal gradient of expression (Maeshima et al. 1998), while evidence for a rostrocaudal gradient of expression for 5-HT1A receptors is mixed (Marlier et al. 1991; Giroux et al. 1999). Blockade of 5-HT2A receptors with ketanserin was effective when applied caudal to the L3 segments but ineffective when applied to T10–L2 segments, but the opposite was the case when 5-HT7 receptors were tested (Schmidt & Jordan, 2000; Liu & Jordan, 2005). Also relevant is that the release of 5-HT is probably not uniform in the mouse, since microdialysis data from the neonatal rat suggest that 5-HT release is 10-fold higher in the caudal thoracic compared to lumbar segments (Jordan & Schmidt, 2002). Taken together it is likely that some of these differences in the effectiveness of citalopram are a consequence of the 5-HT site of release and receptor distribution.
Previous work suggests that 5-HT2 and 5-HT7 receptors are important for 5-HT evoked rhythmicity in the isolated spinal cord preparation of the mouse (Madriaga et al. 2004; Liu et al. 2009) and rat (Schmidt & Jordan, 2000; Hochman et al. 2001; Liu & Jordan, 2005; Pearlstein et al. 2005). We do not believe that our work contradicts these studies. Our work supports a similar dependence on 5-HT7 as well as 5-HT2 receptors in producing the excitatory locomotor drive, since application of SB-269970 and ketanserin could block fictive locomotion. Our findings support those of Jordan and colleagues who used brainstem stimulation of the parapyramidal region of the neonatal rat (Liu & Jordan, 2005). Even without citalopram present, blocking 5-HT1A/1B receptors also produced a speeding up of the rhythm suggesting that both excitatory and inhibitory 5-HT modulatory effects contribute. Interestingly, the speeding up of the rhythm and improvement in regularity when 5-HT1A/1B receptors are blocked are similar to what we observed when the brainstem was cooled during afferent evoked stimulation (Gordon & Whelan, 2008).
While our data support a role for endogenously released 5-HT in modulating fictive locomotion in the mouse, we do not wish to suggest that it is the only modulator to contribute to the control of the CPG. There is substantial evidence that numerous neurotransmitters and peptides function as neuromodulators of spinal CPGs (Kiehn & Kjaerulff, 1996; Kiehn et al. 1999; Sqalli-Houssaini & Cazalets, 2000; Pearson et al. 2003; Barriere et al. 2005; Han et al. 2007), and it is likely that there are many more that remain to be discovered. Dopamine (DA), in particular, when combined with 5-HT can dramatically stabilize rhythms in the mouse (Jiang et al. 1999; Whelan et al. 2000). Both DA and 5-HT, because of their receptor distribution, set up rostrocaudal gradients of excitability, which may partly explain why they act synergistically to stabilize locomotor-like patterns. Since noradrenergic descending pathways originate in the brainstem, it is likely that release of noradrenalin is occurring in our brainstem–spinal cord model, although this was not directly tested in this work. As for DA, the only known descending fibres originate in the hypothalamus, and therefore release of DA is not likely to be occurring in our preparation (Clemens et al. 2006).
Functional relevance
Traditionally 5-HT has been thought to suppress sensory input and boost motor output (Jacobs & Fornal, 1993). However, increasingly the clean line between the two modulatory effects has become blurred, and the consensus is that 5-HT has much more complex effects on sensorimotor systems (Schmidt & Jordan, 2000; Hochman et al. 2001; Millan, 2002). It is well known that 5-HT evokes and modulates CPG output (Sqalli-Houssaini et al. 1993). On the other hand, the inhibitory effects of 5-HT on pattern generation are less well understood. Here we show that endogenously released 5-HT can modulate CPGs through 5-HT7, 5-HT2A and 5-HT1A/1B receptors. At birth, descending monoaminergic systems are not fully developed, yet even so our work suggests that endogenous release of 5-HT contributes to the control of locomotion. This is important since all studies in the developing mouse to this point have used exogenous application of 5-HT to modulate locomotion. While these studies reveal that 5-HT receptors are present and functional, it does not address the issue of whether endogenous 5-HT is released from descending fibres.
Our work leads us to pose the hypothesis that 5-HT has different functional effects depending on the concentration of 5-HT in the synaptic cleft. At low levels 5-HT acts to facilitate locomotion, while at higher levels, it has more of an inhibitory influence. This bidirectional effect of 5-HT has been observed in the control of pain where it has both a pro- and antinociceptive effect on neurons in the spinal cord (Millan, 2002). Since our work shows that endogenous 5-HT contributes to locomotor activity in the mouse, it provides a basis for future studies examining connectivity between the raphe nuclei and interneurons that comprise the spinal cord network in the mouse, and for testing the hypothesis of bidirectional 5-HT effects.
Acknowledgments
This work was supported by operating grants from the Christopher and Dana Reeve Foundation, and the Canadian Institutes of Health Research. P.W. is a Senior Scholar of the Alberta Heritage Foundation for Medical Research. M.D. was supported by studentships from the University of Calgary and the Natural Sciences and Engineering Foundation of Canada.
Glossary
Abbreviations
- CCINs
commissural interneurons
- CPG
central pattern generator
- DC
direct current
- 5-HTir
serotonin immuno-reactive
- P
postnatal
- PTCC
peak-to-trough correlation coefficient
- RM
repeated measures
- SSRI
selective serotonin reuptake inhibitor
Author contributions
M.D. performed the electrophysiological experiments, analysed the data, and co-wrote and revised portions of the manuscript. P.J.W. conceived the experiments, wrote custom programs to analyse the data, and co-wrote and revised the paper. M.A.T. performed the immunohistochemical experiments and revised the paper. All authors approved the final version of the manuscript.
References
- Ballion B, Branchereau P, Chapron J, Viala D. Ontogeny of descending serotonergic innervation and evidence for intraspinal 5-HT neurons in the mouse spinal cord. Brain Res Dev Brain Res. 2002;137:81–88. doi: 10.1016/s0165-3806(02)00414-5. [DOI] [PubMed] [Google Scholar]
- Barriere G, Bertrand S, Cazalets JR. Peptidergic neuromodulation of the lumbar locomotor network in the neonatal rat spinal cord. Peptides. 2005;26:277–286. doi: 10.1016/j.peptides.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Beato M, Nistri A. Serotonin-induced inhibition of locomotor rhythm of the rat isolated spinal cord is mediated by the 5-HT1 receptor class. Proc Biol Sci. 1998;265:2073–2080. doi: 10.1098/rspb.1998.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchereau P, Chapron J, Meyrand P. Descending 5-hydroxytryptamine raphe inputs repress the expression of serotonergic neurons and slow the maturation of inhibitory systems in mouse embryonic spinal cord. J Neurosci. 2002;22:2598–2606. doi: 10.1523/JNEUROSCI.22-07-02598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci. 1995;15:4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson J, Franck J, Grillner S. Increase in endogenous 5-hydroxytryptamine levels modulates the central network underlying locomotion in the lamprey spinal cord. Neurosci Lett. 1989;100:188–192. doi: 10.1016/0304-3940(89)90682-4. [DOI] [PubMed] [Google Scholar]
- Christie KJ, Whelan PJ. Monoaminergic establishment of rostrocaudal gradients of rhythmicity in the neonatal mouse spinal cord. J Neurophysiol. 2005;94:1554–1564. doi: 10.1152/jn.00299.2005. [DOI] [PubMed] [Google Scholar]
- Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–130. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. A comparison of motor patterns induced by N-methyl-D-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett. 1994;171:147–150. doi: 10.1016/0304-3940(94)90626-2. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol. 1997;77:247–259. doi: 10.1152/jn.1997.77.1.247. [DOI] [PubMed] [Google Scholar]
- Crick H, Wallis DI. Inhibition of reflex responses of neonate rat lumbar spinal cord by 5-hydroxytryptamine. Br J Pharmacol. 1991;103:1769–1775. doi: 10.1111/j.1476-5381.1991.tb09861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar MJ, Whelan PJ. 2008 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2008. Descending control of locomotion. Program No. 575.9. [Google Scholar]
- Fuller RW, Wong DT. Serotonin uptake and serotonin uptake inhibition. Ann N Y Acad Sci. 1990;600:68–78. doi: 10.1111/j.1749-6632.1990.tb16873.x. discussion 79–80. [DOI] [PubMed] [Google Scholar]
- Gerin C, Legrand A, Privat A. Study of 5-HT release with a chronically implanted microdialysis probe in the ventral horn of the spinal cord of unrestrained rats during exercise on a treadmill. J Neurosci Methods. 1994;52:129–141. doi: 10.1016/0165-0270(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Gilmore J, Fedirchuk B. The excitability of lumbar motoneurones in the neonatal rat is increased by a hyperpolarization of their voltage threshold for activation by descending serotonergic fibres. J Physiol. 2004;558:213–224. doi: 10.1113/jphysiol.2004.064717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux N, Rossignol S, Reader TA. Autoradiographic study of α1- and α2-noradrenergic and serotonin1A receptors in the spinal cord of normal and chronically transected cats. J Comp Neurol. 1999;406:402–414. [PubMed] [Google Scholar]
- Gordon IT, Dunbar MJ, Vanneste KJ, Whelan PJ. Interaction between developing spinal locomotor networks in the neonatal mouse. J Neurophysiol. 2008;100:117–128. doi: 10.1152/jn.00829.2007. [DOI] [PubMed] [Google Scholar]
- Gordon IT, Whelan PJ. Brainstem modulation of locomotion in the neonatal mouse spinal cord. J Physiol. 2008;586:2487–2497. doi: 10.1113/jphysiol.2007.148320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon IT, Whelan PJ. Monoaminergic control of cauda-equina-evoked locomotion in the neonatal mouse spinal cord. J Neurophysiol. 2006;96:3122–3129. doi: 10.1152/jn.00606.2006. [DOI] [PubMed] [Google Scholar]
- Hammar I, Chojnicka B, Jankowska E. Modulation of responses of feline ventral spinocerebellar tract neurons by monoamines. J Comp Neurol. 2002;443:298–309. doi: 10.1002/cne.10135. [DOI] [PubMed] [Google Scholar]
- Hammar I, Stecina K, Jankowska E. Differential modulation by monoamine membrane receptor agonists of reticulospinal input to lamina VIII feline spinal commissural interneurons. Eur J Neurosci. 2007;26:1205–1212. doi: 10.1111/j.1460-9568.2007.05764.x. [DOI] [PubMed] [Google Scholar]
- Han P, Nakanishi ST, Tran MA, Whelan PJ. Dopaminergic modulation of spinal neuronal excitability. J Neurosci. 2007;27:13192–13204. doi: 10.1523/JNEUROSCI.1279-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Machacek DW, Shay BL. Motor Neurobiology of the Spinal Cord. CRC Press; 2001. pp. 47–87. [Google Scholar]
- Hyttel J. Neurochemical characterization of a new potent and selective serotonin uptake inhibitor: Lu 10-171. Psychopharmacology (Berl) 1977;51:225–233. doi: 10.1007/BF00431629. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends Neurosci. 1993;16:346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Heden CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci. 2000;12:701–714. doi: 10.1046/j.1460-9568.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol Scand. 1967;70:389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res. 1999;816:493–499. doi: 10.1016/s0006-8993(98)01199-8. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Schmidt BJ. Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies? Prog Brain Res. 2002;137:125–139. doi: 10.1016/s0079-6123(02)37012-2. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Ann N Y Acad Sci. 1998;860:110–129. doi: 10.1111/j.1749-6632.1998.tb09043.x. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol. 1996;75:1472–1482. doi: 10.1152/jn.1996.75.4.1472. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Sillar KT, Kjaerulff O, McDearmid JR. Effects of noradrenaline on locomotor rhythm-generating networks in the isolated neonatal rat spinal cord. J Neurophysiol. 1999;82:741–746. doi: 10.1152/jn.1999.82.2.741. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. 5-HT modulation of multiple inward rectifiers in motoneurons in intact preparations of the neonatal rat spinal cord. J Neurophysiol. 2001;85:580–593. doi: 10.1152/jn.2001.85.2.580. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J Neurophysiol. 1997;77:1155–1170. doi: 10.1152/jn.1997.77.3.1155. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A, Delvolve I, Kremer E. Sacrocaudal afferents induce rhythmic efferent bursting in isolated spinal cords of neonatal rats. J Neurophysiol. 2000;83:888–894. doi: 10.1152/jn.2000.83.2.888. [DOI] [PubMed] [Google Scholar]
- Liu J, Akay T, Hedlund PB, Pearson KG, Jordan LM. Spinal 5-HT7 receptors are critical for alternating activity during locomotion: in vitro neonatal and in vivo adult studies using 5-HT7 receptor knockout mice. J Neurophysiol. 2009;102:337–348. doi: 10.1152/jn.91239.2008. [DOI] [PubMed] [Google Scholar]
- Liu J, Jordan LM. Stimulation of the parapyramidal region of the neonatal rat brain stem produces locomotor-like activity involving spinal 5-HT7 and 5-HT2A receptors. J Neurophysiol. 2005;94:1392–1404. doi: 10.1152/jn.00136.2005. [DOI] [PubMed] [Google Scholar]
- Machacek DW, Garraway SM, Shay BL, Hochman S. Serotonin 5-HT2 receptor activation induces a long-lasting amplification of spinal reflex actions in the rat. J Physiol. 2001;537:201–207. doi: 10.1111/j.1469-7793.2001.0201k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madriaga MA, McPhee LC, Chersa T, Christie KJ, Whelan PJ. Modulation of locomotor activity by multiple 5-HT and dopaminergic receptor subtypes in the neonatal mouse spinal cord. J Neurophysiol. 2004;92:1566–1576. doi: 10.1152/jn.01181.2003. [DOI] [PubMed] [Google Scholar]
- Maeshima T, Ito R, Hamada S, Senzaki K, Hamaguchi-Hamada K, Shutoh F, Okado N. The cellular localization of 5-HT2A receptors in the spinal cord and spinal ganglia of the adult rat. Brain Res. 1998;797:118–124. doi: 10.1016/s0006-8993(98)00360-6. [DOI] [PubMed] [Google Scholar]
- Marlier L, Teilhac JR, Cerruti C, Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- Matsumoto I, Combs MR, Jones DJ. Characterization of 5-hydroxytryptamine1B receptors in rat spinal cord via [125I]iodocyanopindolol binding and inhibition of [3H]-5-hydroxytryptamine release. J Pharmacol Exp Ther. 1992;260:614–626. [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Zemlan FP. Selective 5-HT1B agonists identify the 5-HT autoreceptor in lumbar spinal cord of rat. Neuropharmacology. 1988;27:37–42. doi: 10.1016/0028-3908(88)90198-0. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Takizawa H, Kudo N. 5-Hydroxytryptamine-induced locomotor rhythm in the neonatal mouse spinal cord in vitro. Neurosci Lett. 2000;280:187–190. doi: 10.1016/s0304-3940(00)00805-3. [DOI] [PubMed] [Google Scholar]
- Noga BR, Johnson DM, Riesgo MI, Pinzon A. Locomotor-activated neurons of the cat. I. Serotonergic innervation and co-localization of 5-HT7, 5-HT2A & 5-HT1A receptors in the thoraco-lumbar spinal cord. J Neurophysiol. 2009;102:1560–1576. doi: 10.1152/jn.91179.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein E, Mabrouk FB, Pflieger JF, Vinay L. Serotonin refines the locomotor-related alternations in the in vitro neonatal rat spinal cord. Eur J Neurosci. 2005;21:1338–1346. doi: 10.1111/j.1460-9568.2005.03971.x. [DOI] [PubMed] [Google Scholar]
- Pearson SA, Mouihate A, Pittman QJ, Whelan PJ. Peptidergic activation of locomotor pattern generators in the neonatal spinal cord. J Neurosci. 2003;23:10154–10163. doi: 10.1523/JNEUROSCI.23-31-10154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribotta MG, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J Neurosci. 2000;20:5144–5152. doi: 10.1523/JNEUROSCI.20-13-05144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA, Calejesan AA, Wei F, Gebhart GF, Zhuo M. Endogenous facilitation: from molecular mechanisms to persistent pain. Curr Neurovasc Res. 2004;1:11–20. doi: 10.2174/1567202043480189. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Sqalli-Houssaini Y, Cazalets JR. Noradrenergic control of locomotor networks in the in vitro spinal cord of the neonatal rat. Brain Res. 2000;852:100–109. doi: 10.1016/s0006-8993(99)02219-2. [DOI] [PubMed] [Google Scholar]
- Sqalli-Houssaini Y, Cazalets JR, Clarac F. Oscillatory properties of the central pattern generator for locomotion in neonatal rats. J Neurophysiol. 1993;70:803–813. doi: 10.1152/jn.1993.70.2.803. [DOI] [PubMed] [Google Scholar]
- Strauss I, Lev-Tov A. Neural pathways between sacrocaudal afferents and lumbar pattern generators in neonatal rats. J Neurophysiol. 2003;89:773–784. doi: 10.1152/jn.00716.2002. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Mori S, Kimura H. Developmental changes in the serotoninergic innervation of hindlimb extensor motoneurons in neonatal rats. Brain Res Dev Brain Res. 1992;65:1–12. doi: 10.1016/0165-3806(92)90002-e. [DOI] [PubMed] [Google Scholar]
- Whelan P, Bonnot A, O’Donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J Neurophysiol. 2000;84:2821–2833. doi: 10.1152/jn.2000.84.6.2821. [DOI] [PubMed] [Google Scholar]
- Zaporozhets E, Cowley KC, Schmidt BJ. A reliable technique for the induction of locomotor-like activity in the in vitro neonatal rat spinal cord using brainstem electrical stimulation. J Neurosci Methods. 2004;139:33–41. doi: 10.1016/j.jneumeth.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- Zhong G, Diaz-Rios M, Harris-Warrick RM. Intrinsic and functional differences among commissural interneurons during fictive locomotion and serotonergic modulation in the neonatal mouse. J Neurosci. 2006a;26:6509–6517. doi: 10.1523/JNEUROSCI.1410-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Diaz-Rios M, Harris-Warrick RM. Serotonin modulates the properties of ascending commissural interneurons in the neonatal mouse spinal cord. J Neurophysiol. 2006b;95:1545–1555. doi: 10.1152/jn.01103.2005. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J Neurophysiol. 1997;78:746–758. doi: 10.1152/jn.1997.78.2.746. [DOI] [PubMed] [Google Scholar]