Abstract
β-Adrenergic receptor (β-AR) stimulation of cardiac muscle has been proposed to enhance Ca2+ release from the sarcoplasmic reticulum (SR) through ryanodine receptors (RyRs). However, the anticipated increase in RyR Ca2+ sensitivity has proven difficult to study in intact cardiomyocytes, due to accompanying alterations in SR Ca2+ content, inward Ca2+ current (ICa) and diastolic cytosolic Ca2+ concentration ([Ca2+]i). Here, we studied whole-cell Ca2+ release and spontaneous Ca2+ leak (Ca2+ sparks) in guinea-pig ventricular myocytes with confocal Ca2+ imaging before and during β-AR stimulation by isoproterenol (Iso), but under otherwise nearly identical experimental conditions. The extent of SR Ca2+ loading was controlled under whole-cell voltage-clamp conditions. UV flash-induced uncaging of Ca2+ from DM-nitrophen was employed as an invariant trigger for whole-cell Ca2+ release. At matched SR Ca2+ content, we found that Iso enhanced the spatiotemporal coherence of whole-cell Ca2+ release, evident from spatially intercorrelated release and accelerated release kinetics that resulted in moderately (∼20%) increased release amplitude. This may arise from higher RyR Ca2+ sensitivity, and was also reflected in spontaneous SR Ca2+ leak. At comparable SR Ca2+ content and cytosolic [Ca2+]i, we observed a ∼4-fold increase in Ca2+ spark frequency in Iso that also appeared in quiescent cells within 2 min without increased SR Ca2+ content. This was likely to have been mediated by Ca2+/calmodulin-dependent protein kinase (CaMKII), rather than cAMP dependent protein kinase (PKA). We conclude that Iso increases the propensity of RyRs to open, both in response to rapid elevations of [Ca2+]i and at diastolic [Ca2+]i. While this could be beneficial in enhancing and synchronizing systolic whole-cell SR Ca2+ release, the same behaviour could also be proarrhythmogenic during diastole.
Introduction
In cardiac muscle, the Ca2+ release channel, or ryanodine receptor (RyR), plays a central role in excitation–contraction (EC) coupling. Activated by the Ca2+ that enters the cardiac myocyte through voltage-gated L-type Ca2+ channels during the action potential, clusters of RyRs throughout the cell respond by releasing substantial amounts of Ca2+ from intracellular Ca2+ stores, the sarcoplasmic reticulum (SR). This amplification process, referred to as Ca2+-induced Ca2+ release (CICR) (Fabiato, 1983), elevates cytosolic Ca2+ concentration ([Ca2+]i) to the levels required to ensure cell contraction during systole.
Multiple targets involved in cardiac EC coupling are subject to regulation during exercise or stress, and respond synergistically to transient activation of the sympathetic nervous system by enhancing myocyte Ca2+ cycling and contraction (Hussain & Orchard, 1997; for review see Bers, 2002). Catecholamine-mediated activation of β-adrenergic receptors (β-AR) in the heart stimulates adenylate cyclase to produce cAMP, which increases the activation of cAMP-dependent protein kinase (PKA). PKA phosphorylation of L-type Ca2+ channels enhances Ca2+ current (ICa) (Reuter & Scholz, 1977; Kameyama et al. 1985), providing a stronger trigger for SR Ca2+ release as well as the source for an increased pool of intracellular Ca2+. Furthermore, Ca2+ uptake by the SR Ca2+-ATPase (SERCA) is favoured by PKA-mediated phosphorylation of phospholamban (PLB), which relieves its inhibition of SERCA, thus accelerating relaxation and increasing SR Ca2+ content (Lindemann et al. 1983).
The RyR is known to serve as a substrate for phosphorylation early upon β-AR stimulation of cardiac myocytes (Yoshida et al. 1992). The physiological relevance of RyR phosphorylation, however, has been intensely debated during the past years. In particular, the possibly higher propensity of clusters of RyRs to spontaneously release (or leak) Ca2+ from the SR during diastole during β-AR stimulation has received extensive attention, for its potential implication in arrhythmogenesis and in the progression of contractile dysfunction under conditions of sustained β-AR stimulation, such as during heart failure (Marx et al. 2000; reviewed in Wehrens et al. 2005 and George, 2008). The fact that the contribution of the phosphorylated RyR to the normal, physiological response to sympathetic activation in the healthy myocyte still remains poorly understood has rendered the reconciliation of findings on its role in cardiac disease particularly difficult.
A significant number of studies carried out on isolated systems have provided invaluable information in revealing a multitude of potential changes in RyR behaviour upon phosphorylation (Takasago et al. 1991; Witcher et al. 1991; Hain et al. 1995; Lokuta et al. 1995; Valdivia et al. 1995; Uehara et al. 2002; Carter et al. 2006). However, the divergence of existing findings raises questions about the extrapolation to its role in modulating EC coupling and diastolic SR Ca2+ leak during β-AR stimulation of intact cells. Thus, the importance of studying RyR function during β-AR stimulation in its native environment is becoming increasingly evident, where accessory proteins and coupled gating between RyRs within a cluster may be essential in conferring physiological Ca2+ sensitivity to SR Ca2+ release (Marx et al. 2001; Gyorke et al. 2004). However, the altered Ca2+ sensitivity of the phosphorylated RyR as anticipated from observations in isolated systems has proven difficult to study in intact cardiomyocytes, mainly due to the aforementioned accompanying alterations in SR Ca2+ content and ICa, as well as in diastolic [Ca2+]i, with RyR open probability (Po) being particularly sensitive to the Ca2+ concentration inside the SR ([Ca2+]SR) (Gyorke & Gyorke, 1998).
Previous studies in whole-cell voltage-clamped ventricular myocytes have shown that β-AR stimulation may synchronize triggered release of Ca2+ from the SR, increase SR Ca2+ release flux and thus the rate of whole-cell SR Ca2+ release (Song et al. 2001; Ginsburg & Bers, 2004). Furthermore, we have recently reported on stimulation of subcellular, local CICR in quiescent ventricular myocytes during β-AR stimulation in response to highly localized elevations of cytosolic [Ca2+]i after two-photon excitation-induced Ca2+ liberation from caged Ca2+ (Lindegger & Niggli, 2005). Findings from studies on SR Ca2+ leak, manifested as local elevations of [Ca2+]i resulting from the spontaneous release of Ca2+ from the SR through a single cluster of RyRs (Cheng et al. 1993), are conflicting, however. To date, observations on either increased amplitude or increased frequency of these elementary Ca2+ signalling events (Ca2+ sparks) during β-AR stimulation of intact ventricular myocytes or addition of cAMP to permeabilized cells, have been reported (Gomez et al. 1996; Tanaka et al. 1997; Li et al. 2002).
To our knowledge, no previous study performed on intact cells addressed the modulation of spontaneous SR Ca2+ release during β-AR stimulation under conditions of matched SR Ca2+ content and comparable diastolic [Ca2+]i, and how it translates to whole-cell CICR under similar experimental conditions. Therefore, we tested the hypothesis that if acute β-AR stimulation alters the Ca2+ sensitivity of the RyR, this should be reflected in the behaviour of whole-cell CICR and in spontaneous, Ca2+ spark-mediated SR Ca2+ leak. We studied SR Ca2+ release before and during β-AR stimulation by isoproterenol (Iso) under matched SR Ca2+ loading both in response to a rapid, artificial elevation of [Ca2+]i that is invariant to β-AR stimulation, as well as at diastolic [Ca2+]i under nearly identical experimental conditions. We found that clusters of RyRs exhibited a significantly higher propensity to open spontaneously in Iso, manifested as an elevation in SR Ca2+ leak that was likely to have been mediated by Ca2+/calmodulin-dependent protein kinase, CaMKII, rather than PKA. This apparent increase in Ca2+ sensitivity of the RyR was also reflected in higher spatiotemporal synchronization of whole-cell CICR during β-AR stimulation, resulting in accelerated release kinetics and moderate increase in the amplitude of SR Ca2+ release. These results have been presented in preliminary form to the Biophysical Society (Ogrodnik & Niggli, 2009).
Methods
Isolation of guinea-pig ventricular myocytes
Single cardiac ventricular myocytes were isolated from adult male guinea-pigs according to established enzymatic procedures (Mitra & Morad, 1985). All animal handling procedures were performed with the permission of the State Veterinary Administration and according to Swiss Federal Animal protection law. All animal experimentation in the present study complies with journal policies and regulations (Drummond, 2009). Guinea-pigs were killed by cervical dislocation. The hearts were rapidly excised and mounted on a Langendorff column by cannulation of the aorta for retrograde perfusion at 37°C for ∼5 min with a Ca2+-free solution containing (in mm): 135 NaCl, 5.4 KCl, 1 MgCl2, 0.33 NaH2PO4, 5 Hepes, 11 glucose, pH 7.3 (NaOH adjusted). Hearts were digested by subsequent addition of collagenase type II (0.5 mg ml−1, Worthington, Switzerland) and protease type XIV (0.1 mg ml−1, Sigma, Switzerland) to the perfusion solution for another 5–7 min. After digestion, the atria were removed and the ventricles transferred to a solution containing additionally 200 μm Ca2+, where they were minced into small pieces. Single cardiac myocytes were liberated by gentle trituration of the digested ventricular tissue. Cells were washed and the Ca2+ concentration was progressively raised to 1 mm within ∼30 min. The cell suspension was placed on a gently rotating shaker, and stored at room temperature (21–22°C) until use, within 8 h after isolation.
Experimental solutions
A sample of cells was transferred to an experimental chamber mounted on the stage of an inverted microscope (Diaphot TMD, Nikon, Küsnacht, Switzerland). Individual cells were continuously superfused with an extracellular solution containing (in mm): 140 NaCl, 5 KCl, 1.8 CaCl2, 1 CsCl, 0.5 BaCl2, 10 Hepes, 10 glucose, pH 7.4 (NaOH adjusted). In some experiments, a Na+- and Ca2+-free solution (0 Na+, 0 Ca2+) with similar composition was used, in which Li+ was substituted for Na+, and Ca2+ was omitted in exchange for 1 mm EGTA. In this solution, pH was adjusted with LiOH. A custom-made, gravity-driven superfusion system allowed for rapid switching (half-time of solution exchange, t1/2 < 500 ms) to or between superfusates containing additionally 1 μm isoproterenol ((–)-N-iso-propyl-l-noradrenaline hydrochloride, Iso) for β-AR stimulation, 10 mm caffeine for emptying of the SR, 5 μm H-89 for PKA inhibition or 5 μm KN-93 for CaMKII inhibition. Solutions containing Iso were prepared each experimental day from a fresh aliquot of a 10 mm stock in water, and solutions containing H-89 and KN-93 from 10 mm stocks in DMSO. Iso, caffeine, H-89 and KN-93 were from Sigma, Switzerland. Voltage-clamped cells were dialysed with a pipette solution containing (in mm): 120 caesium glutamate, 20 TEA-Cl, 20 Hepes, 5 K2-ATP, 2 Na4-DM-nitrophen (Calbiochem, La Jolla, CA, USA), 2 reduced glutathione (GSH), 0.5 CaCl2, 0.1 K5-fluo-3 (Biotium, Hayward, CA, USA), pH 7.20 (CsOH adjusted). All experiments were carried out at room temperature (21–22°C).
Electrophysiological recordings
Electrodes were pulled from filamented borosilicate glass capillaries (BF150-86-7.5, Sutter Instrument Company, Novato, CA, USA) on a horizontal puller (DMZ, Zeitz Instrumente, Augsburg, Germany) to a final inner tip diameter of 1–2 μm (series resistance 2–3 MΩ). Guinea-pig ventricular myocytes were voltage-clamped in the whole-cell configuration of the patch-clamp technique and held at a resting potential of −80 mV using an Axopatch 200 amplifier (Axon Instruments, Union City, CA, USA). The liquid junction potential for the described solution compositions was estimated to be around −15 mV (calculated using Patcher's Power Tools, Dr F. Mendez and F. Würriehausen, Max Planck Institute for Biophysical Chemistry, Göttingen, Germany), and was not corrected for. After establishment of the whole-cell configuration, cells were allowed at least 5 min for dialysis to ensure equilibration with the intracellular solution. Following initial emptying of the SR by rapid application of 10 mm caffeine, the extent of SR Ca2+ reloading was controlled with a train of varying number of depolarizing steps of 200 ms duration to 0 mV at 0.5 Hz, to activate inward ICa carried by plasmalemmal voltage-gated L-type Ca2+ channels. SR Ca2+ content and changes therein were assessed from the integrated Na+–Ca2+ exchange current (INCX), in addition to the Ca2+ transient amplitude accompanying rapid caffeine exposure. Currents were recorded with the use of custom-written data acquisition software developed in-house under Labview (National Instruments, Ennetbaden, Switzerland), running on an Apple Macintosh G3 computer. Recordings were digitized at a sampling frequency of 1.5–3 kHz using an A/D converter, and electrophysiological data were stored on hard disk for off-line analysis using IgorPro software (WaveMetrics, Lake Oswego, OR, USA).
Confocal Ca2+ imaging and whole-cell Ca2+ uncaging
Cells were loaded with the Ca2+-sensitive fluorescent indicator fluo-3 by dialysis through the recording pipette and imaged with a 40× oil-immersion objective lens (Fluor, N.A. 1.3, Nikon). Fluo-3 was excited at λ= 488 nm with an optically pumped semiconductor laser (Sapphire 488-10, Coherent, Santa Clara, CA, USA) attenuated to 50 μW measured at the back plane of the objective. Fluorescence was detected at λ > 515 nm with a laser-scanning confocal microscope (MRC 1000, Bio-Rad, Glattbrugg, Switzerland) operating in the imaging (x,y) or the line-scan (x,t) mode (6 ms per line). Line-scans of 1024 lines were collected in two equal, sequential segments (each image 384 × 512 pixels). In Ca2+ uncaging experiments, UV flashes from a xenon short-arc flash lamp (total energy discharge up to 230 J in ∼400 μs) were delivered through the objective in an epi-illumination arrangement. UV flashes were synchronized with the pixel clock of the line-scan image acquisition system, and applied during the laser-scan retrace to elicit rapid, artificial, and spatially homogeneous elevations in [Ca2+]i by photolysis of DM-nitrophen to induce whole-cell Ca2+ release from the SR. UV flash energy was set to 100–110 J in order to reliably trigger moderate whole-cell SR Ca2+ release while at the same time minimize photoconsumption of DM-nitrophen. Acquired fluorescence line-scan images were processed and transformed into pseudo-ratiometric images off-line using a customized version of Image SXM software (Barrett, 2002), from which temporal Ca2+ transient profiles were extracted. Amplitudes and time courses of cytosolic Ca2+ transients were analysed after normalization to resting fluorescence levels (F/F0) in IgorPro software. Time constants (τ) were extracted from monoexponential fits to UV flash-induced Ca2+ transient decays. Maximal rate of SR Ca2+ release (d[Ca2+]max dt−1) and time to peak were calculated starting from the 3rd scan line of the upstroke of UV flash-induced Ca2+ transient profiles. In some Ca2+ uncaging experiments, the UV flash-induced Ca2+ transients were clearly characterized by a biphasic upstroke in [Ca2+]i elevation, the initial (fast) phase corresponding to the very rapid uncaging of Ca2+ from DM-nitrophen (2 scan lines), and the subsequent (slow) phase to SR Ca2+ release. In these experiments, the amplitude of the initial (fast) phase was used to quantitatively verify the stability of the elevation in [Ca2+]i induced by photolysis of DM-nitrophen (see Fig. 3Fa and G). In addition, in some Ca2+ uncaging experiments, the stability of the photolytic trigger was quantitatively assessed from the corresponding Ca2+ transients recorded in the constant presence of 10 mm caffeine, acquired in the beginning as well as at the end of the experimental protocol (see Fig. 3Fb). SR Ca2+ content and changes therein were assessed from peak Ca2+ transient amplitude accompanying rapid caffeine exposure, in addition to the integrated INCX as mentioned previously. Spontaneous SR Ca2+ release was analysed using ImageJ software (W. Rasband, National Institute of Mental Health, Bethesda, MD, USA, http://rsb.info.nih.gov/ij), in the SparkMaster plug-in (Picht et al. 2007). Under our experimental conditions, SR Ca2+ release events composed of multiple Ca2+ sparks (i.e. macrosparks or wavelets), as identified by their spatiotemporal characteristics, were frequent during β-AR stimulation with Iso. For simplicity, these SR Ca2+ release events were not separated into individual Ca2+ sparks, but were quantified as one single SR Ca2+ release event, as detected by the SparkMaster plug-in. Ca2+ spark amplitudes were not compared due to (1) the very rare occurrence of Ca2+ sparks in control, (2) frequent macrosparks in Iso and (3) minor, albeit existent differences in diastolic [Ca2+]i under whole-cell voltage-clamp conditions after rest and after SR Ca2+ reloading protocols.
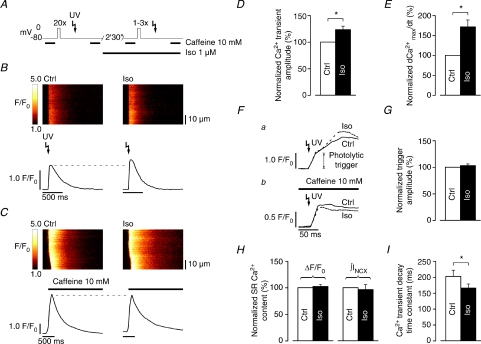
Figure 3. UV flash-induced SR Ca2+ release is increased in Iso at matched SR Ca2+ content.
A, whole-cell SR Ca2+ release induced by UV flash-photolysis of DM-nitrophen in control (SR loaded to steady-state), and again following an adapted preloading protocol (1–3 preloading steps) in Iso. Rapid application of 10 mm caffeine 2 s after the UV flash in both control and Iso was used to verify comparable SR Ca2+ content. B, whole-cell SR Ca2+ release was higher in Iso (right) compared to control (left), despite similar SR Ca2+ content (C). On average, whole-cell SR Ca2+ release was significantly stimulated to ΔF/F0: 123.4 ± 6.3% of control (n= 8 cells, D). E, Iso accelerated whole-cell SR Ca2+ release kinetics (d[Ca2+]max dt−1: 171.6 ± 17.0% of control, n= 8 cells). F, the amplitude of the initial (rapid) phase of UV flash-induced Ca2+ transients (a) confirmed that the photolytical trigger for whole-cell SR Ca2+ release was comparable in control and Iso, as did UV flash-induced Ca2+ transients recorded in the constant presence of 10 mm caffeine (b, data from another cell). G, average amplitude of the photolytical trigger extracted from the biphasic upstroke of UV flash-induced Ca2+ transients (ΔF/F0: 102.9 ± 2.9% of control, n= 4 cells). H, SR Ca2+ content was reliably matched in these experiments (caffeine-induced ΔF/F0: 102.1 ± 3.6% and ∫INCX: 96.4 ± 9.1% of control, n= 8 cells). I, Iso also accelerated whole-cell Ca2+ transient decay kinetics (τ: 202.4 ± 19.0 ms in control to 165.8 ± 12.4 ms in Iso, n= 8 cells) (*P < 0.01).
Expression of results and statistical analysis
Statistical analysis was performed with IgorPro software. All results, absolute values or relative to corresponding control values in percent, are expressed as means ± standard error of the mean (s.e.m.). Values were compared for significance using Student's t test. Statistical significance (*) was assumed for P values less than 0.01.
Results
β-AR stimulation increases Ca2+ transients by enhancing ICa and increasing SR Ca2+ content
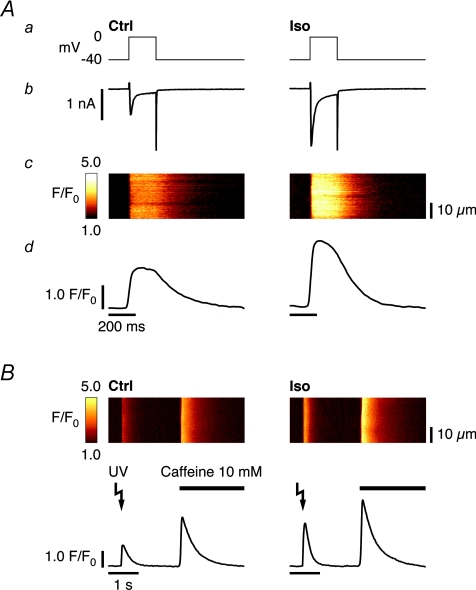
It is well established that β-AR stimulation of ventricular myocytes acutely enhances ICa through voltage-gated L-type Ca2+ channels, thus increasing the main trigger for physiological SR Ca2+ release (Callewaert et al. 1988; Hussain & Orchard, 1997). Following a given (identical) preconditioning protocol, a depolarizing step of 200 ms duration (from −40 mV to 0 mV) will activate larger ICa, which reaches steady amplitude after 2 min 30 s of β-AR stimulation with Iso. This leads to an increase in CICR and, in consequence, a larger cytosolic Ca2+ transient (Fig. 1A). Stimulation of ICa also provides the source for an increased pool of intracellularly cycling Ca2+. Together with the accompanying stimulation of SERCA activity, progressive accumulation of Ca2+ within the SR following each depolarization during β-AR stimulation is favoured, until steady-state is reached. Thus, SR Ca2+ release triggered by rapid, UV flash-induced Ca2+ uncaging from DM-nitrophen, a trigger that is invariant to β-AR stimulation, will also result in a larger cytosolic Ca2+ transient in Iso (Fig. 1B). The elevated SR Ca2+ content, as confirmed with rapid application of 10 mm caffeine, will increase CICR independently of any functional modification of the RyR resulting from β-AR stimulation. This is due to the direct dependence of RyR Po on [Ca2+]SR (Gyorke & Gyorke, 1998), as well as the larger driving force resulting from the higher gradient in [Ca2+] between the SR and the cytosol (Kettlun et al. 2003). A pronounced modulation of SR Ca2+ release during acute β-AR stimulation induced by other targets could therefore mask a subtle change resulting from altered RyR Ca2+ sensitivity.
Figure 1. Iso increases Ca2+ transients by enhancing ICa and elevating SR Ca2+ content.
A, depolarizing step (200 ms, from −40 mV to 0 mV, a), recorded current (b), acquired line-scan image (c) and average temporal Ca2+ transient profile (d). Compared to control (left), 2 min 30 s of β-AR stimulation with 1 μm Iso activates larger ICa and increases CICR, resulting in a larger cytosolic Ca2+ transient (right). B, whole-cell SR Ca2+ release in response to UV flash-induced Ca2+ uncaging from DM-nitrophen, following identical preconditioning in control (left) and Iso (right), recorded in a different cell. Stimulation of SERCA activity in Iso leads to more SR Ca2+ loading (confirmed with rapid application of 10 mm caffeine), and a larger cytosolic UV flash-induced Ca2+ transient, and may mask direct functional modulation of the RyR resulting from β-AR stimulation.
SR Ca2+ content can be matched during β-AR stimulation by adaptation of the preconditioning protocol
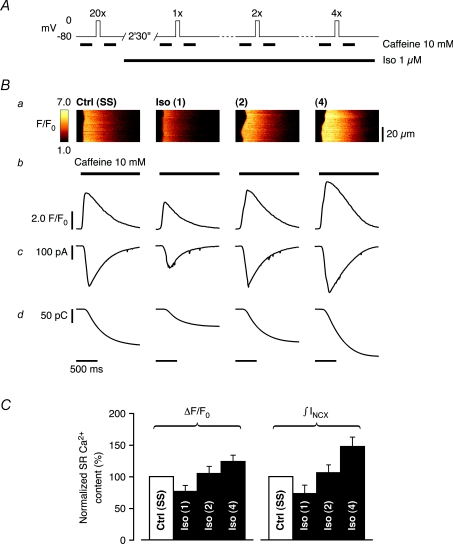
In order to discern a difference in RyR Ca2+ sensitivity during β-AR stimulation, SR Ca2+ release would thus not only have to be studied in response to an invariant cytosolic trigger or similar diastolic [Ca2+]i, but also under conditions of strictly matched SR Ca2+ content. Therefore, we investigated how SR Ca2+ content can be controlled under our experimental conditions. Rapid application of 10 mm caffeine was used to estimate SR Ca2+ content, but also to reset SR Ca2+ content from previous history. Subsequent activation of ICa was used to control the extent of SR Ca2+ reloading with a variable train of depolarizing steps. Initially, we estimated steady-state SR Ca2+ content in control conditions immediately after reloading with a train of 20 depolarizing steps of 200 ms duration to 0 mV at 0.5 Hz, following initial emptying. During β-AR stimulation with Iso, the SR was successively emptied and its content reassessed after reloading with one, two and four identical depolarizing steps (Fig. 2A). Figure 2B shows the acquired line-scan image of the caffeine-induced Ca2+ release (Fig. 2Ba), the Ca2+ transient profile (Fig. 2Bb), the accompanying INCX (Fig. 2Bc) and its integral, ∫INCX (Fig. 2Bd), respectively. From each cell, the Ca2+ transient amplitude (ΔF/F0) and the integral of the INCX accompanying rapid caffeine exposure were normalized to control steady-state SR Ca2+ content, and the relative loading conditions in Iso are shown in Fig. 2C. On average, SR reloading with 2 depolarizing steps in Iso best matched steady-state SR Ca2+ content in control (caffeine-induced ΔF/F0: 104.8 ± 11.0% and ∫INCX: 106.0 ± 12.1% of control, respectively). In some cells, however, one or even four depolarizing steps resulted in a closer match. Therefore, in subsequent experiments, whole-cell CICR and spontaneous SR Ca2+ leak were studied following an adapted preloading protocol with 1–3 depolarizing steps in Iso to match steady-state SR Ca2+ content in control. Comparable SR Ca2+ content in Iso was confirmed in each experiment, and only the recording in Iso with the closest match was compared to control from each cell.
Figure 2. SR Ca2+ content can be matched in Iso by adaptation of the preconditioning protocol.
A, adjusting SR Ca2+ loading under our experimental conditions. Following initial emptying with caffeine, SR Ca2+ content was assessed in control after reloading to steady-state (SS) with 20 depolarizing steps (200 ms, from −80 mV to 0 mV, 0.5 Hz). In Iso, the SR was successively emptied and its content reassessed with caffeine after reloading with 1, 2 and 4 depolarizing steps. B, acquired line-scan images of caffeine-induced Ca2+ release after the number of depolarizing steps indicated in A (a), corresponding Ca2+ transient profiles (b), accompanying INCX (c) and their integrals (d). As expected, SR Ca2+ content increased with the number of preloading steps in Iso. C, relative SR loading conditions normalized to control, as estimated from the amplitude of the Ca2+ transient (ΔF/F0) and integral of the INCX (∫INCX) accompanying rapid caffeine exposure after 1, 2 and 4 loading steps. On average, SR reloading with 2 depolarizing steps in Iso best matched SR Ca2+ content in steady-state in control (ΔF/F0: 104.8 ± 11.0% and ∫INCX: 106.0 ± 12.1% of control, n= 5 cells), although in some cells 1 or even 4 depolarizing steps yielded a closer match.
UV flash-induced SR Ca2+ release is increased during β-AR stimulation at matched SR Ca2+ content
Whole-cell SR Ca2+ release was induced by UV flash-photolysis of DM-nitrophen following loading of the SR to steady-state in control, and following an adapted preloading protocol during β-AR stimulation with Iso. Rapid application of 10 mm caffeine 2 s after the UV flash in both control and Iso was used to verify comparable SR Ca2+ content (Fig. 3A). SR Ca2+ release was small to moderate in all cells, as judged by the relative amplitude of the UV flash-induced to the caffeine-induced Ca2+ transient amplitude. Figure 3B shows that whole-cell SR Ca2+ release was higher in Iso compared to control, despite similar SR Ca2+ content, as confirmed by the amplitude of the caffeine-induced Ca2+ transient (Fig. 3C). Statistical analysis reveals a significant stimulation of whole-cell SR Ca2+ release in Iso (ΔF/F0: 123.4 ± 6.3% of control, Fig. 3D). Moreover, whole-cell release kinetics were also significantly accelerated in Iso (d[Ca2+]max dt−1: 171.6 ± 17.0% of control, Fig. 3E). The amplitude of the initial (rapid) phase of UV flash-induced Ca2+ transients confirmed that the photolytical trigger for whole-cell SR Ca2+ release was comparable in control and Iso (ΔF/F0: 102.9 ± 2.9% of control, Fig. 3Fa and G), as did UV flash-induced Ca2+ transients recorded in the constant presence of 10 mm caffeine (Fig. 3Fb). Furthermore, Fig. 3H shows that SR Ca2+ content was reliably matched in these experiments (caffeine-induced ΔF/F0: 102.1 ± 3.6% and ∫INCX: 96.4 ± 9.1% of control, respectively). These results support the notion of increased SR Ca2+ release flux and/or improved temporal synchronization of triggered elementary SR Ca2+ release events, and also confirm the expected stimulation of SERCA activity, as whole-cell decay kinetics of the Ca2+ transient were significantly accelerated in Iso as well (τ: 202.4 ± 19.0 ms in control to 165.8 ± 12.4 ms in Iso, Fig. 3I, visible in Fig. 3B).
Increased spatial synchronization of whole-cell SR Ca2+ release during β-AR stimulation is revealed at near-threshold triggers
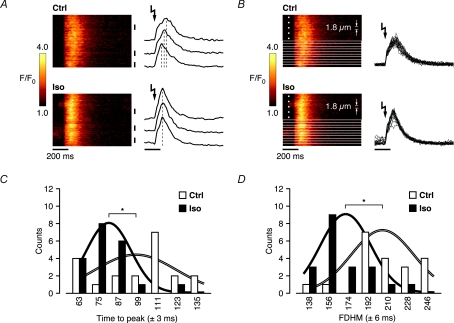
Enhanced whole-cell release kinetics suggest a higher RyR Po, possibly as a result of increased Ca2+ sensitivity that could underlie synchronized opening of more RyRs or clusters of RyRs. Furthermore, increased single-channel conductance and/or gating properties of the RyR or clusters of RyRs would in principle also result in accelerated whole-cell release kinetics. In some Ca2+ uncaging experiments described in Fig. 3, the UV flash-induced Ca2+ transients were clearly characterized by a biphasic upstroke in [Ca2+]i elevation, and spatial inhomogeneities in the Ca2+ transients were also evident in these cells. The initial (fast) phase corresponds to the very rapid, spatially homogeneous uncaging of Ca2+ from DM-nitrophen, and the subsequent (slower) phase to SR Ca2+ release, which in these cells is thought to occur near threshold of CICR (Lipp & Niggli, 1996). To further investigate the behaviour of this near-threshold CICR, we performed a detailed analysis of the release kinetics on the subcellular level in 1 of 4 cells where the Ca2+ transients in control and Iso were characterized by a clear biphasic upstroke and spatial inhomogeneities. Figure 4A shows that release kinetics markedly differ throughout the cell in control, as indicated by the temporal Ca2+ transient profiles from three different (subcellular) regions. SR Ca2+ release in Iso in the same cell, however, exhibited an obvious spatial synchronization, as reflected by the peaks of the corresponding three subcellular Ca2+ transient profiles. Therefore, we divided the line-scan images in control and Iso into 20 equal parts (each 1.8 μm wide), of which the temporal characteristics were analysed (Fig. 4B). Figure 4C shows the distributions of the time to peak of the Ca2+ transients from the 20 subcellular regions in control and Iso. Subcellular Ca2+ transients with time to peak in successive groups of two consecutive pixels were binned together. The distributions reveal that the average time to peak throughout the cell was significantly shorter in Iso (time to peak: 99.3 ± 5.4 ms in control to 78.9 ± 3.0 ms in Iso), which could explain the higher maximal rate of whole-cell SR Ca2+ release described above. The time to peak throughout the cell was less spread in time in Iso, suggesting increased spatial synchronization of SR Ca2+ release over larger distances. This is also highlighted by the relative amplitudes of the representative (integral normalized) Gaussians superimposing the distributions in Fig. 4C. Moreover, the distribution in Iso is notably better represented by its corresponding Gaussian in contrast to control, where the subcellular distribution of time to peak appears spatially uncorrelated, indicating a possibly increased coupling between SR Ca2+ release from neighbouring RyR clusters during β-AR stimulation. The accelerated release and decay kinetics in Iso also resulted in shorter duration of the Ca2+ transient, as reflected in the significantly shorter average full duration at half-maximum (FDHM) amplitude throughout the cell (207.6 ± 6.3 ms in control to 168.0 ± 5.0 ms in Iso, Fig. 4D). The distributions of the FDHM of the Ca2+ transients from the 20 subcellular regions in control and Iso are shown in Fig. 4D, together with (integral normalized) Gaussians representing the distributions (subcellular Ca2+ transients with FDHM in successive groups of three consecutive pixels were binned together). These observations suggest that spatial synchronization throughout the cell contributes to an increased rate of whole-cell SR Ca2+ release and shorter time to peak of the cellular Ca2+ transient. β-AR stimulation thus reduces the duration of the Ca2+ transient as a consequence of both enhanced spatiotemporal summation of elementary SR Ca2+ release events and stimulation of SERCA activity.
Figure 4. Increased spatial synchronization of whole-cell SR Ca2+ release in Iso is revealed at near-threshold triggers.
A, near-threshold SR Ca2+ release kinetics exhibited clear spatial inhomogeneities in control, as indicated by the temporal Ca2+ transient profiles from 3 different (subcellular) regions. Iso synchronized SR Ca2+ release throughout the cell, as reflected by the coordinated peaks of the corresponding subcellular Ca2+ transient profiles. B, the line-scan images in control and Iso in A were divided into 20 equal parts (each 1.8 μm wide), on which a detailed analysis of the temporal characteristics was performed. C, the distribution of the time to peak in the subcellular regions revealed a significantly shorter average time to peak in Iso (99.3 ± 5.4 ms in control to 78.9 ± 3.0 ms in Iso), and was less spread in time throughout the cell (reflected by the relative widths and amplitudes of the representative, integral-normalized Gaussians). D, accelerated release and decay kinetics in Iso are also reflected in shorter average full duration at half-maximum (FDHM) amplitude throughout the cell (207.6 ± 6.3 ms in control to 168.0 ± 5.0 ms in Iso) (*P < 0.01).
Ca2+ sparks in guinea-pig ventricular myocytes
Accelerated SR Ca2+ release kinetics during β-AR stimulation resulting from increased spatiotemporal synchronization would reflect a higher propensity of clusters of RyRs to open and contribute to the triggered opening of neighbouring clusters. The higher Ca2+ sensitivity of RyRs that would underlie this behaviour could thus also modulate the occurrence of elementary SR Ca2+ release events, Ca2+ sparks, during resting conditions. Although spontaneous Ca2+ sparks have been observed in myocytes from most mammalian hearts, few investigators have reported on Ca2+ sparks in guinea-pig ventricular myocytes, where their appearance is relatively rare. We studied the behaviour of Ca2+ sparks in guinea-pig ventricular myocytes, and how Ca2+ spark-mediated SR Ca2+ leak is altered upon β-AR stimulation with Iso, under conditions nearly identical to those in the experiments described in previous sections.
Ca2+ sparks are more frequent in quiescent guinea-pig ventricular myocytes during β-AR stimulation
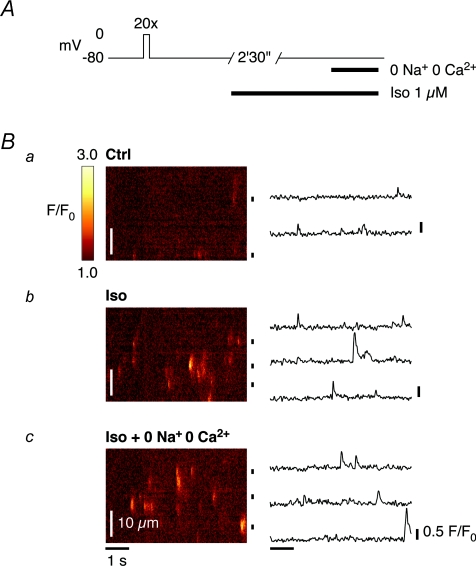
Figure 5 shows the occurrence of Ca2+ sparks in a voltage-clamped ventricular myocyte. Line-scan images were acquired from a quiescent cell after loading of the SR with Ca2+ to steady-state (Fig. 5A). In our experiments, Ca2+ sparks were relatively rare under control conditions (Fig. 5Ba). However, superfusion of quiescent cells with Iso (1 μm for 2 min 30 s) markedly increased the number of readily visible Ca2+ sparks (Fig. 5Bb). The occurrence of macrosparks (or wavelets), with spatiotemporal characteristics typical of SR Ca2+ release events composed of multiple Ca2+ sparks, was also frequent during β-AR stimulation with Iso. Moreover, the more frequent appearance of Ca2+ sparks in Iso persisted when the extracellular solution was exchanged for a solution without Na+ or Ca2+ (0 Na+, 0 Ca2+) (Fig. 5Bc), indicating that the SR Ca2+ leak is spontaneous in the sense that the Ca2+ sparks are not triggered by Ca2+ influx through L-type Ca2+ channels, the NCX or any other pathway dependent on extracellular Ca2+ (or Na+).
Figure 5. Ca2+ sparks are more frequent in quiescent guinea-pig ventricular myocytes in Iso.
A, the occurrence of Ca2+ sparks in voltage-clamped ventricular myocytes was studied in quiescent cells, after loading of the SR with Ca2+ to steady-state in control conditions. B, Ca2+ sparks were relatively rare in control (a), but the number of readily visible Ca2+ sparks markedly increased during β-AR stimulation with 1 μm Iso for 2 min 30 s (b). Their appearance persisted when the extracellular solution was exchanged for a solution without Na+ or Ca2+ (0 Na+, 0 Ca2+) (c), indicating that the SR Ca2+ leak is spontaneous and independent of extracellular Ca2+ (i.e. not triggered by any Ca2+ influx).
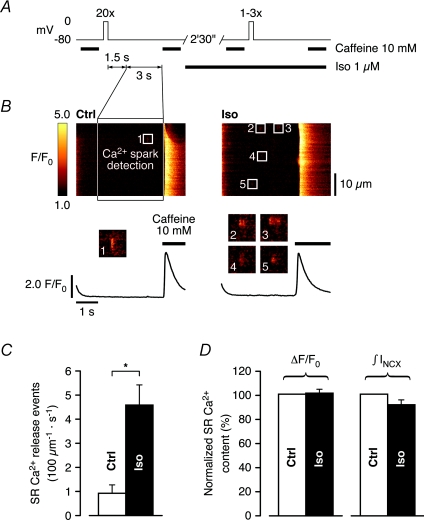
Spontaneous SR Ca2+ release is increased at matched SR Ca2+ content and comparable diastolic [Ca2+]i during β-AR stimulation
Intracellular Ca2+ retention is favoured upon β-AR stimulation of quiescent, intact ventricular myocytes, due to the aforementioned accompanying stimulation of SERCA activity. Therefore, the marked increase in spontaneous Ca2+ spark frequency shown in Fig. 5 could be a result of elevated SR Ca2+ content during rest. To compare spontaneous SR Ca2+ release in control and during β-AR stimulation at matched SR Ca2+ content, we recorded Ca2+ sparks in control cells immediately after reloading of the SR with Ca2+ to steady-state, and again in Iso following a preloading protocol identical to the one described in Fig. 3A. Analysis was performed on Ca2+ sparks during a 3 s long period starting 1.5 s after the final repolarization to −80 mV. Subsequent rapid application of 10 mm caffeine was used to verify comparable SR Ca2+ content (Fig. 6A). Figure 6B shows the occurrence of Ca2+ sparks after repolarization to the holding potential and complete decay of [Ca2+]i to diastolic levels, and suggests that sparks were generally more frequent in Iso (a single spark in control to 4 in Iso in Fig. 6B). Statistical analysis revealed an ∼5-fold higher Ca2+ spark frequency in Iso (0.92 ± 0.34 s−1 (100 μm)−1 in control to 4.58 ± 0.83 s−1 (100 μm)−1 in Iso, Fig. 6C), in recordings where the SR Ca2+ content was matched (caffeine-induced ΔF/F0: 100.8 ± 3.0% and ∫INCX: 91.3 ± 3.8% of control, respectively, Fig. 6D). Furthermore, diastolic [Ca2+]i was not significantly elevated in Iso in these experiments (110.0 ± 4.7% of control, not shown). The higher propensity for spontaneous SR Ca2+ release observed here could represent a significant SR Ca2+ leak during diastolic intervals.
Figure 6. Spontaneous SR Ca2+ release is increased at matched SR Ca2+ content and comparable diastolic [Ca2+]i in Iso.
A, spontaneous SR Ca2+ release was compared in control and in Iso at matched SR Ca2+ content (cf. experimental protocol described in Fig. 3). Ca2+ sparks were recorded in control (SR loaded to steady-state), and again following an adapted preloading protocol (1–3 preloading steps) in Iso. Analysis was performed on Ca2+ sparks during a 3 s long period starting 1.5 s after the final repolarization to −80 mV. Subsequent rapid application of 10 mm caffeine was used to verify comparable SR Ca2+ content. B, Ca2+ sparks after repolarization and complete decay of [Ca2+]i to diastolic levels were generally more frequent in Iso (a single spark in control, left, to 4 in Iso, right). C, on average, Ca2+ spark frequency increased ∼5-fold in Iso (0.92 ± 0.34 s−1 (100 μm)−1 in control to 4.58 ± 0.83 s−1 (100 μm)−1 in Iso), in recordings where the SR Ca2+ content was matched to D, SR Ca2+ content was matched in these recordings (caffeine-induced ΔF/F0: 100.8 ± 3.0% and ∫INCX: 91.3 ± 3.8% of control) (n= 9 cells, *P < 0.01).
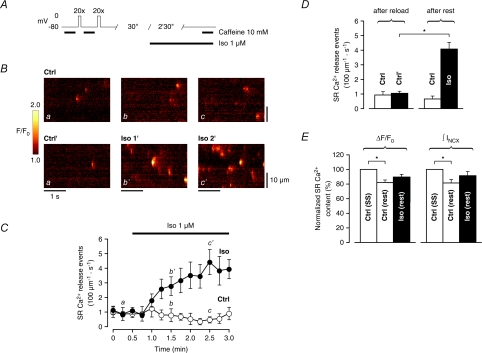
Ca2+ spark frequency increases rapidly in quiescent cells during β-AR stimulation without significantly altering SR Ca2+ content
Increased SR Ca2+ leak during β-AR stimulation could also result in progressive net loss of Ca2+ from the cell during diastolic intervals through extrusion by the Na+–Ca2+ exchanger (NCX). To address this issue, we designed an experimental protocol to investigate to what extent the observed increase in SR Ca2+ leak affects global SR Ca2+ content during rest, while simultaneously monitoring the time course of spontaneous SR Ca2+ release upon β-AR stimulation with Iso. The SR Ca2+ content at steady-state was initially assessed with caffeine under control conditions. Following reloading of the SR to steady-state, the cells were left to rest for 3 min, either for 30 s in control followed by 2 min 30 s in Iso, or 3 min in control (Fig. 7A). SR Ca2+ content was again assessed in both groups after the total resting period of 3 min, and compared to the one recorded initially. Line-scan images of quiescent cells were acquired every 15 s throughout the resting period. In Fig. 7B, a progressive, significant increase in Ca2+ spark frequency is clearly visible during superfusion with Iso (a, b′, c′), whereas the cells left to rest in control did not exhibit any obvious change in Ca2+ spark frequency during rest (a, b, c). The time course in Fig. 7C shows that the increase in Ca2+ spark frequency started after ∼30 s in Iso, and reached a ∼4-fold higher level after 2 min. In contrast, Ca2+ spark frequency did not increase in control cells during 3 min rest, but rather exhibited a tendency to decrease over time. Statistical analysis was performed on data pooled from the initial three points in time (in Fig. 7C) after reloading of the SR to steady-state (0–30 s in control), as well as on data pooled from the final three points from both groups after rest (2 min 30 s–3 min in control and 2 min–2 min 30 s in Iso). Ca2+ spark frequency was not significantly lower after rest in control (from 0.93 ± 0.21 s−1 (100 μm)−1 to 0.66 ± 0.19 s−1 (100 μm)−1). However, after rest in Iso, Ca2+ spark frequency increased from 1.05 ± 0.13 s−1 (100 μm)−1 to 4.08 ± 0.47 s−1 (100 μm)−1 (Fig. 7D). Figure 7E shows that SR Ca2+ content decreased significantly after rest in control (caffeine-induced ΔF/F0: 82.0 ± 3.3% and ∫INCX: 81.4 ± 4.7% of initial SR Ca2+ content, respectively), whereas this loss of Ca2+ was more limited after rest in Iso (caffeine-induced ΔF/F0: 89.5 ± 3.5% and ∫INCX: 91.4 ± 5.5% of initial SR Ca2+ content, respectively). Moreover, diastolic [Ca2+]i had a tendency to decrease in control after rest and slightly more in Iso, although the decrease was not significant in any group (94.0 ± 2.3% of initial diastolic [Ca2+]i after rest in control to 91.5 ± 3.2% in Iso, not shown). Taken together, these results reveal that in control, guinea-pig ventricular myocytes behave as expected, exhibiting a rest decay of SR Ca2+ content with a tendency to parallel reduction of SR Ca2+ leak. However, despite the rapid, significant increase in Ca2+ spark frequency during rest in Iso, loss of Ca2+ from the cell through the NCX is limited, probably due to stimulation of SERCA activity. Higher SR Ca2+ content cannot explain the observed rapid increase in Ca2+ spark frequency in Iso, the time course of which is well in accordance with published data on the time course of RyR phosphorylation (Takasago et al. 1991; Yoshida et al. 1992), and could thus reflect a modulation of RyR Po through phosphorylation.
Figure 7. Ca2+ spark frequency increases rapidly in quiescent cells in Iso without significantly altering SR Ca2+ content.
A, experimental protocol to study the time course of spontaneous SR Ca2+ release and how it affects SR Ca2+ content during rest. SR Ca2+ content was assessed with caffeine after loading of the SR with Ca2+ to steady-state in control. Following reloading of the SR to steady-state, cells were left to rest for 3 min (30 s in control + 2 min 30 s in Iso, or 3 min in control), after which SR Ca2+ content was again assessed with caffeine. B and C, a progressive increase in Ca2+ spark frequency appeared after ∼30 s superfusion with Iso, reaching an ∼4-fold higher level within 2 min (a, b′, c′). Cells in control did not exhibit any obvious change in Ca2+ spark frequency during rest (a, b, c). D, Ca2+ spark frequency was not significantly lower after rest in control (0.93 ± 0.21 s−1 (100 μm)−1 to 0.66 ± 0.19 s−1 (100 μm)−1). After rest in Iso, Ca2+ spark frequency increased from 1.05 ± 0.13 s−1 (100 μm)−1 to 4.08 ± 0.47 s−1 (100 μm)−1; data were pooled from the initial 3 points and the final 3 points from both groups). E, SR Ca2+ content decreased significantly after rest in control (caffeine-induced ΔF/F0: 82.0 ± 3.3% and ∫INCX: 81.4 ± 4.7% of initial SR Ca2+ content), whereas this loss of Ca2+ was more limited after rest in Iso (caffeine-induced ΔF/F0: 89.5 ± 3.5% and ∫INCX: 91.4 ± 5.5% of initial SR Ca2+ content) (control: n= 10 cells, Iso: n= 9 cells, *P < 0.01).
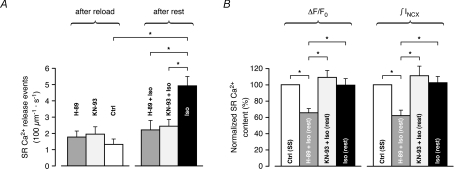
Increased spontaneous SR Ca2+ release during β-AR stimulation is mediated by CaMKII rather than PKA
β-AR stimulation of cardiomyocytes activates dual signalling pathways, mediated by cAMP and Ca2+/calmodulin-dependent protein kinases, PKA and CaMKII, respectively. While the cAMP/PKA pathway is known to undergo significant activation upon acute β-AR stimulation, it is unclear whether important activation of CaMKII occurs during the relatively short time frame of the experiments in the present study (Wang et al. 2004). Recent observations, however, suggest that CaMKII could potentially undergo significant activation during acute β-AR stimulation of quiescent cardiomyocytes, independently of the primary increase in Ca2+ cycling mediated by PKA (Curran et al. 2007). Therefore, we investigated whether the elevated Ca2+ spark frequency in Iso could be attributed to PKA or CaMKII. Using an experimental protocol similar to the one described in Fig. 7, we monitored the time course of spontaneous SR Ca2+ release during rest. Immediately after reloading of the SR to steady-state, the extracellular solution was exchanged for a solution containing additionally 5 μm of the widely used membrane-permeant inhibitors of PKA and CaMKII, H-89 or KN-93, respectively. The cells were left to rest for 3 min, initially for 30 s in H-89 or KN-93, followed by 2 min 30 s in H-89 or KN-93 together with 1 μm Iso. SR Ca2+ content was assessed with caffeine in both groups after the total resting period of 3 min, and compared to the steady-state SR Ca2+ content recorded initially. Interestingly, treatment with either H-89 or KN-93 almost completely suppressed the increase in Ca2+ spark frequency observed during rest in Iso (from 1.77 ± 0.36 s−1 (100 μm)−1 in H-89 to 2.21 ± 0.57 s−1 (100 μm)−1 in H-89 + Iso, n.s., and from 1.95 ± 0.44 s−1 (100 μm)−1 in KN-93 to 2.44 ± 0.40 s−1 (100 μm)−1 in KN-93 + Iso, n.s., Fig. 8A), supporting the hypothesis that downstream activation of these kinases could underlie the ∼4-fold increase again observed in Iso (from 1.32 ± 0.32 s−1 (100 μm)−1 to 4.92 ± 0.56 s−1 (100 μm)−1). However, during rest in H-89 treated cells, the sustained SR Ca2+ leak was accompanied by a dramatic loss of Ca2+ from the SR (caffeine-induced ΔF/F0: 65.4 ± 4.9% and ∫INCX: 62.0 ± 6.5% of initial SR Ca2+ content). In contrast, in cells treated with KN-93, SR Ca2+ content rather exhibited a tendency to increase during rest, although this increase was not significant (caffeine-induced ΔF/F0: 108.8 ± 8.6% and ∫INCX: 110.9 ± 11.9% of initial SR Ca2+ content). Similarly to the results presented in Fig. 7E, SR Ca2+ content was again unaffected after rest in Iso (caffeine-induced ΔF/F0: 99.6 ± 7.8% and ∫INCX: 102.7 ± 7.4% of initial SR Ca2+ content, respectively). The comparable Ca2+ spark frequency despite much lower SR Ca2+ content in H-89 treated cells suggests a more likely implication of CaMKII, rather than PKA, in the stimulation of spontaneous SR Ca2+ release in Iso, which was normalized by KN-93 without significantly altering SR Ca2+ content.
Figure 8. CaMKII rather than PKA mediates the increased spontaneous SR Ca2+ release in Iso.
The time course of spontaneous SR Ca2+ release during rest was monitored following incubation with either the PKA inhibitor H-89 (5 μm) or the CaMKII inhibitor KN-93 (5 μm). H-89 or KN-93 was added to the extracellular solution immediately after reloading of the SR to steady-state. A, compared to Iso, which increased Ca2+ spark frequency from 1.32 ± 0.32 s−1 (100 μm)−1 to 4.92 ± 0.56 s−1 (100 μm)−1, treatment with either inhibitor almost completely suppressed the increase in Ca2+ spark frequency (from 1.77 ± 0.36 s−1 (100 μm)−1 in H-89 to 2.21 ± 0.57 s−1 (100 μm)−1 in H-89 + Iso, n.s., and from 1.95 ± 0.44 s−1 (100 μm)−1 in KN-93 to 2.44 ± 0.40 s−1 (100 μm)−1 in KN-93 + Iso, n.s.). B, during rest in KN-93 + Iso, SR Ca2+ content exhibited a tendency to increase in parallel, although this increase was not significant (caffeine-induced ΔF/F0: 108.8 ± 8.6% and ∫INCX: 110.9 ± 11.9% of initial SR Ca2+ content). During rest in H-89 + Iso, however, the loss of Ca2+ from the SR was dramatic (caffeine-induced ΔF/F0: 65.4 ± 4.9% and ∫INCX: 62.0 ± 6.5% of initial SR Ca2+ content). SR Ca2+ content was again unaffected after rest in Iso (caffeine-induced ΔF/F0: 99.6 ± 7.8% and ∫INCX: 102.7 ± 7.4% of initial SR Ca2+ content) (H-89 ± Iso: n= 8 cells, KN-93 ± Iso: n= 7 cells, Iso: n= 5 cells, *P < 0.01).
Discussion
Cardiac hypertrophy and failure have been associated with abnormal cardiomyocyte Ca2+ handling (Gwathmey & Morgan, 1985; Beuckelmann et al. 1992; Gomez et al. 1997) that can be induced by chronic activation of the β-AR pathway (Engelhardt et al. 2004). Marx et al. provided a plausible mechanistic link between the hyperadrenergic state observed in the failing heart and spontaneously active RyRs, relating excessive PKA phosphorylation of the RyR to abnormal channel gating through altered interactions between the channel and accessory proteins (Marx et al. 2000). This postulate has recruited an ever increasing number of studies, many of which have severely challenged the proposed molecular and biochemical nature of RyR phosphorylation (target sites, kinase candidates, interactions with accessory proteins) and its functional impact in cardiac disease (for example Jiang et al. 2002; Ai et al. 2005; Xiao et al. 2005; Benkusky et al. 2007).
The intact cardiomyocyte: a complex Ca2+ signalling system
The understanding of the role played by RyR phosphorylation in pathogenesis has been considerably hindered by the fact that its physiological relevance during acute β-AR stimulation in the healthy myocyte still remains to be elucidated. Extensive attention has been devoted to resolving the physiological role of RyR phosphorylation in a multitude of studies carried out on isolated systems, cumulatively providing a framework of potential changes that RyR behaviour could undergo upon phosphorylation. As previously mentioned, when addressed in intact ventricular myocytes, clear manifestation of alterations in the SR Ca2+ release mechanism of RyRs clustered in their native environment has proven difficult to discern from accompanying increases in SR Ca2+ content and ICa. Both exert a pronounced stimulation of SR Ca2+ release during β-AR stimulation that could mask a subtle change resulting from altered RyR Ca2+ sensitivity.
In the present study, we observed a significant stimulation of whole-cell SR Ca2+ release in Iso, induced by UV flash-photolysis of DM-nitrophen in voltage-clamped guinea-pig ventricular myocytes. Rapid Ca2+ uncaging from DM-nitrophen has the advantage of providing a stable trigger for CICR, while at the same time remaining absolutely invariant to β-AR stimulation. Thus, as SR Ca2+ content was strictly matched in these experiments, our observation suggests a modulation of the SR Ca2+ release mechanism triggered by rapid elevations in [Ca2+]i. Stimulatory regulation of single-channel or cluster gating properties, such as increased Po (Ca2+ sensitivity), longer openings or higher single-channel conductance could all, in principle, underlie increased cellular SR Ca2+ release and higher rate of whole-cell SR Ca2+ release. Increased RyR Po after PKA phosphorylation is supported by findings from several studies carried out in isolated systems, such as [3H]ryanodine binding in cardiac microsomes (Takasago et al. 1989) and recordings of single channel activity of RyRs incorporated into planar lipid bilayers (Hain et al. 1995; Uehara et al. 2002; Carter et al. 2006). Interestingly, Valdivia et al. (1995) reported on more complex single-channel behaviour, where PKA phosphorylation induced a transiently higher RyR Po with fast adaptation to lower steady-state Po when activated by UV flash-photolysis of caged Ca2+, implying a different gating mode of the phosphorylated RyR. In addition, there is evidence that the RyR can serve as a substrate for kinases other than PKA, particularly Ca2+/calmodulin-dependent protein kinase (CaMKII). Sustained β-AR stimulation is believed to progressively increase CaMKII activity, and RyR phosphorylation by CaMKII has shown similar potentiation of RyR Po (Takasago et al. 1991; Witcher et al. 1991; Wehrens et al. 2004). However, whether significant activation of this kinase occurs during acute β-AR stimulation is still unclear (Wang et al. 2004; but see Curran et al. 2007).
Macroscopic and microscopic changes of triggers for SR Ca2+ release
Similar to our findings, Ginsburg & Bers (2004) found that β-AR stimulation increased the maximal rate of whole-cell SR Ca2+ release after careful matching of SR Ca2+ content and triggering ICa in voltage-clamped rabbit and mouse ventricular myocytes. Interestingly, these experiments did not reveal an alteration of SR Ca2+ release amplitude itself. By reducing channel availability to compensate for increased Po of L-type Ca2+ channels during β-AR stimulation, comparable macroscopic ICa was activated to trigger SR Ca2+ release. However, as the macroscopic ICa does not exhibit absolute microscopic invariance to β-AR stimulation, it is possible that this operation enhanced the coupling fidelity by reducing the number of L-type Ca2+ channels required to trigger the same amount of SR Ca2+ release during β-AR stimulation, hence increasing the number of redundant channel openings (Altamirano & Bers, 2007). In other words, changes of microscopic trigger features may alter coupling sufficiently to mask subtle changes of SR Ca2+ release itself.
Physiological role of RyR sensitization
Nonetheless, ICa remains the physiological trigger for SR Ca2+ release. Redundant Ca2+ supply from influx through L-type Ca2+ channels together with the tight ultrastructural organization of these channels with respect to clusters of RyRs in the SR membrane most likely serve to ensure EC coupling fidelity. The stimulation of whole-cell SR Ca2+ release in Iso observed in the present study may thus be minor in comparison to the more pronounced stimulation exerted by increases in SR Ca2+ content and ICa during physiological β-AR stimulation. However, under conditions where the tight coupling between L-type Ca2+ channels and clusters of RyRs is disrupted, the significance of our observations could be more relevant. For example, metabolic inhibition was found to fracture the pattern of Ca2+ transients as a result of reduced ability of ICa to recruit Ca2+ release locally (Fukumoto et al. 2005). Moreover, Louch et al. (2004) related dispersion of T-tubules, a network of plasmalemmal invaginations that allow the coupling of L-type Ca2+ channels to clusters of RyRs throughout the cell interior, to reduced synchrony of whole-cell SR Ca2+ release. Similarly, T-tubule disorganization is thought to contribute to dyssynchronous SR Ca2+ release in the failing heart (Song et al. 2006). Such loss of ultrastructural organization could compromise the tight coupling between L-type Ca2+ channels and clusters of RyRs by rendering the physiological trigger for SR Ca2+ release, ICa, more spatially homogeneous and by consequence less efficient, but at the same time more similar to the trigger used in the present study.
Consistent with this hypothesis, we found that SR Ca2+ release appeared spatially uncorrelated throughout the cell in control near the threshold for CICR when triggered by rapid, spatially homogeneous uncaging of Ca2+ from DM-nitrophen, and that this was particularly reflected in the subcellular distribution of time to peak of the Ca2+ transient. Moreover, we found that Iso synchronized whole-cell SR Ca2+ release over larger distances at strictly matched SR Ca2+ content. This observation further supports the notion of increased Ca2+ sensitivity of the RyR in Iso, as it is presumably not only reflected in the (primary) opening of clusters in response to the rapid elevation in [Ca2+]i, but also in the (secondary) coupling between neighbouring clusters, as suggested by the narrow, correlated distribution of time to peak of the Ca2+ transient throughout the cell. Song et al. (2001) first proposed that β-AR stimulation may synchronize triggered release of Ca2+ from the SR and increase SR Ca2+ release flux at comparable SR Ca2+ content in whole-cell voltage-clamped ventricular myocytes, although they did not compensate for the stimulation of ICa. Iso has also been shown to reduce the dyssynchrony of Ca2+ transients in myocytes isolated from infarcted hearts (Litwin et al. 2000). On a cellular level, increased RyR Ca2+ sensitivity during β-AR stimulation may thus play an important compensatory role in the shaping of Ca2+ transients under certain pathological conditions, where the coupling between L-type Ca2+ channels and clusters of RyRs is compromised and SR Ca2+ content is reduced (e.g. during heart failure).
SR Ca2+ leak and arrhythmias
Spontaneous release of Ca2+ through clusters of RyRs (or single RyRs) accounts for the leak of Ca2+ from the SR during diastole. Ca2+ spark-mediated SR Ca2+ leak can initiate propagating Ca2+ waves under certain conditions (Cheng et al. 1993) that give rise to arrhythmogenic, transient inward currents (Berlin et al. 1989). Recently, transient inward currents carried by the NCX were shown to directly evoke triggered arrhythmias in the heart during β-AR stimulation (Fujiwara et al. 2008). Thus, thorough understanding of Ca2+ spark-mediated SR Ca2+ leak and its regulation during β-AR stimulation is essential, as it may play an important role not only in SR Ca2+ load but also in arrhythmogenesis. Tanaka et al. (1997) studied Ca2+ sparks in intact rat ventricular myocytes, and found an increased Ca2+ spark amplitude during β-AR stimulation, but did not take possible alterations in SR Ca2+ content into account. Conversely, Gomez et al. (1996) did not observe an increased Ca2+ spark amplitude during β-AR stimulation at comparable SR Ca2+ content, but noted a 3-fold increase in Ca2+ spark frequency under these conditions in whole-cell voltage clamped rat ventricular myocytes, suggesting significantly higher RyR Ca2+ sensitivity at diastolic [Ca2+]i.
In agreement with the findings by Gomez et al. (1996) we found an ∼4-fold increase in Ca2+ spark frequency at comparable SR Ca2+ content in Iso, and that this significantly higher diastolic SR Ca2+ leak appeared within 2 min of β-AR stimulation with Iso, consistent with reports on the time course of RyR phosphorylation (Takasago et al. 1991; Yoshida et al. 1992). An increase in Ca2+ sensitivity of the RyR is also supported by the frequent occurrence of SR Ca2+ release events composed of multiple Ca2+ sparks (macrosparks) in Iso, and reflects an increased propensity of Ca2+ sparks from one cluster to trigger release from neighbouring clusters, similar to the coupling between neighbouring clusters that contributes to synchronization of whole-cell SR Ca2+ release revealed at near threshold triggers. The significant increase in SR Ca2+ leak observed after repolarization and complete decay of [Ca2+]i to diastolic levels together with the apparent loss of local control could, in principle, when enhanced by parallel increase in SR Ca2+ content during β-AR stimulation, produce the intense, synchronous Ca2+ waves required to trigger delayed afterdepolarizations (Fujiwara et al. 2008).
Li et al. (2002) attributed increased Ca2+ spark frequency after addition of cAMP to permeabilized cells entirely to higher SR Ca2+ content. However, the strong rest potentiation of SR Ca2+ content they observed in mouse myocytes could have masked a more subtle stimulation of RyR Po, which in the present study could be revealed in guinea-pig ventricular myocytes exhibiting rest decay of SR Ca2+ content. Similarly, the maximally Ca2+ loaded SR in mice lacking PLB used in the study by Li et al. could have overridden any functional modulation of RyR Ca2+ sensitivity resulting from phosphorylation. Carter et al. (2006) found that the functional manifestation of PKA phosphorylation of the RyR may critically depend on differential, possibly species-dependent basal phosphorylation levels, as well as the extent of phosphorylation, underscoring the sensitivity of the RyR to the reigning balance between kinase and phosphatase activity. Li et al. also addressed this issue, and noted that addition of cAMP only resulted in less than 50% of maximal phosphorylation. It is thus possible that RyR phosphorylation by PKA did not reach the threshold required for functional manifestation in these experiments on myocytes from wild-type mice and from mice lacking phosphorylatable PLB.
The dramatic increase in Ca2+ spark frequency in our experiments (from very rare Ca2+ sparks in control to 4- to 5-fold higher in Iso) during acute β-AR stimulation of guinea-pig ventricular myocytes could result from RyR phosphorylation by CaMKII. In fact, Curran et al. (2007) recently reported on increased diastolic SR Ca2+ leak in intact, quiescent rabbit ventricular myocytes in Iso that could be attributed to CaMKII. Interestingly, activation of CaMKII in the experiments by these investigators appeared to be independent of elevated Ca2+ cycling mediated by PKA. Rather, it required nitric oxide synthase activation (Curran et al. 2009), and other oxidation-dependent mechanisms have also been reported to activate CaMKII (Erickson et al. 2008). In good agreement with the results by Curran et al. we found that the dramatic increase in Ca2+ spark frequency in Iso observed in the present study could be normalized with the widely used CaMKII inhibitor KN-93, without altering SR Ca2+ content. Conversely, although increased Ca2+ spark frequency in Iso could not be observed in cells treated with the PKA inhibitor H-89, SR Ca2+ leak was accompanied by a strong depression of SR Ca2+ content. Increased SR Ca2+ leak resulting from sensitization of RyRs by CaMKII phosphorylation could underlie the progressive depletion of the SR, in the absence of a counterbalancing stimulation of SERCA activity. Inhibition of PKA phosphorylation of PLB by H-89 would thus explain the inability of the SR to sustain the elevated Ca2+ spark frequency observed in Iso. In line with these arguments, Curran et al. also reported that the SR Ca2+ content required to maintain comparable Ca2+ spark frequency in Iso in cells treated with H-89 was significantly lower, similarly indicating a failure of H-89 to prevent the increased SR Ca2+ leak in Iso. Although we cannot exclude a contribution of PKA to the increased SR Ca2+ leak observed in the present study, the results presented here suggest a predominant role of CaMKII, rather than PKA, in the stimulation of spontaneous SR Ca2+ release in Iso.
In addition to being arrhythmogenic, increased diastolic SR Ca2+ leak is thought to contribute to progressive impairment of cardiac function by reducing SR Ca2+ content during chronic activation of the β-AR pathway (Wehrens et al. 2006). We found that despite the significant increase in SR Ca2+ leak, loss of Ca2+ from the cell through the NCX is limited, probably due to stimulation of SERCA activity, which rather tended to sustain the elevated spontaneous SR Ca2+ release. This is also supported by the finding that inhibition of SERCA stimulation results in a dramatic reduction of SR Ca2+ content. Thus, the higher Ca2+ sensitivity of the RyR observed here does not appear sufficient to alone depress SR Ca2+ content during acute β-AR stimulation of resting guinea-pig ventricular myocytes. However, it could potentially exacerbate the reduction of SR Ca2+ content in heart failure where SERCA activity is reduced and extrusion of Ca2+ from the cell by the NCX is increased (Schmidt et al. 1998; Pieske et al. 1999; Gomez et al. 2002). Finally, we showed that SR Ca2+ leak can be significantly higher during β-AR stimulation at levels where SR Ca2+ content is comparable to control. This finding could provide a potential explanation of the occurence of exercise- or stress-induced arrhythmias in conditions such as heart failure where SR Ca2+ content is reduced and stimulation of SERCA activity during β-AR stimulation is limited, particularly if oxidative stress further elevates SR Ca2+ leak (Yano et al. 2005), or in the presence of mutations in the RyR or closely associated proteins (Liu & Priori, 2008).
Conclusions
Taken together, our results suggest that β-AR stimulation increases the Ca2+ sensitivity of the RyR, resulting in a higher probability of clusters to open and trigger release from neighbouring clusters. Modulation of the SR Ca2+ release mechanism is reflected in slightly larger Ca2+ transients as a consequence of enhanced spatiotemporal synchronization of whole-cell SR Ca2+ release. Although the stimulation may be modest when EC coupling fidelity is normal, it could play a compensatory role when coupling is compromised and SR Ca2+ content is reduced. Higher Ca2+ sensitivity of the RyR during β-AR stimulation is also manifested in an elevated diastolic SR Ca2+ leak that is predominantly mediated by CaMKII rather than PKA, and an increased propensity of Ca2+ sparks to trigger neighbours. The higher spontaneous SR Ca2+ leak is sustained by stimulation of SERCA activity, limiting the loss of Ca2+ from the SR. Thus, the same mechanism that could be beneficial in enhancing and synchronizing whole-cell SR Ca2+ release could also be responsible for the generation of delayed afterdepolarizations.
Acknowledgments
We would like to thank the Swiss National Science Foundation (grant 31-109693 to E.N.), the Swiss Cardiovascular Research and Training Network (SCRTN) and the Swiss Foundation for Research on Muscle Diseases for support. We thank Drs Nicolas Lindegger and Nina Ullrich for helpful comments on the manuscript, and Daniel Lüthi for technical assistance.
Glossary
Abbreviations
- β-AR
β-adrenergic receptor
- [Ca2+]SR
intra-SR Ca2+ concentration
- CaMKII
Ca2+/calmodulin-dependent protein kinase
- CICR
Ca2+-induced Ca2+ release
- EC
excitation–contraction
- FDHM
full duration at half-maximum
- ICa
L-type Ca2+ current
- INCX
Na+–Ca2+ exchange current
- Iso
isoproterenol
- Po
open probability
- PKA
cAMP-dependent protein kinase
- PLB
phospholamban
- NCX
Na+–Ca2+ exchanger
- RyR
ryanodine receptor
- SERCA
sarcoplasmic reticulum Ca2+-ATPase
Author contributions
All authors contributed to the conception and design of the study, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, and final approval of the version to be published.
References
- Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- Altamirano J, Bers DM. Voltage dependence of cardiac excitation-contraction coupling: unitary Ca2+ current amplitude and open channel probability. Circ Res. 2007;101:590–597. doi: 10.1161/CIRCRESAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- Barrett SD. Software for scanning microscopy. Proc Roy Microsc Soc. 2002;37:164–174. [Google Scholar]
- Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Intact β-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101:819–829. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- Berlin JR, Cannell MB, Lederer WJ. Cellular origins of the transient inward current in cardiac myocytes. Role of fluctuations and waves of elevated intracellular calcium. Circ Res. 1989;65:115–126. doi: 10.1161/01.res.65.1.115. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992;85:1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- Callewaert G, Cleemann L, Morad M. Epinephrine enhances Ca2+ current-regulated Ca2+ release and Ca2+ reuptake in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988;85:2009–2013. doi: 10.1073/pnas.85.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S, Colyer J, Sitsapesan R. Maximum phosphorylation of the cardiac ryanodine receptor at serine-2809 by protein kinase A produces unique modifications to channel gating and conductance not observed at lower levels of phosphorylation. Circ Res. 2006;98:1506–1513. doi: 10.1161/01.RES.0000227506.43292.df. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Curran J, Ahmed U, Bers DM, Ziolo M, Shannon TR. Isoproterenol-enhanced diastolic sarcoplasmic reticulum Ca leak in ventricular myocytes requires activation of nitric oxide synthase. Biophys J. 2009;96:120–121a. [Google Scholar]
- Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. β-Adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt S, Hein L, Dyachenkow V, Kranias EG, Isenberg G, Lohse MJ. Altered calcium handling is critically involved in the cardiotoxic effects of chronic β-adrenergic stimulation. Circulation. 2004;109:1154–1160. doi: 10.1161/01.CIR.0000117254.68497.39. [DOI] [PubMed] [Google Scholar]
- Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol Cell Physiol. 1983;245:C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Tanaka H, Mani H, Nakagami T, Takamatsu T. Burst emergence of intracellular Ca2+ waves evokes arrhythmogenic oscillatory depolarization via the Na+-Ca2+ exchanger: simultaneous confocal recording of membrane potential and intracellular Ca2+ in the heart. Circ Res. 2008;103:509–518. doi: 10.1161/CIRCRESAHA.108.176677. [DOI] [PubMed] [Google Scholar]
- Fukumoto GH, Lamp ST, Motter C, Bridge JH, Garfinkel A, Goldhaber JI. Metabolic inhibition alters subcellular calcium release patterns in rat ventricular myocytes: implications for defective excitation-contraction coupling during cardiac ischemia and failure. Circ Res. 2005;96:551–557. doi: 10.1161/01.RES.0000159388.61313.47. [DOI] [PubMed] [Google Scholar]
- George CH. Sarcoplasmic reticulum Ca2+ leak in heart failure: mere observation or functional relevance? Cardiovasc Res. 2008;77:302–314. doi: 10.1093/cvr/cvm006. [DOI] [PubMed] [Google Scholar]
- Ginsburg KS, Bers DM. Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J Physiol. 2004;556:463–480. doi: 10.1113/jphysiol.2003.055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez AM, Cheng H, Lederer WJ, Bers DM. Ca2+ diffusion and sarcoplasmic reticulum transport both contribute to [Ca2+]i decline during Ca2+ sparks in rat ventricular myocytes. J Physiol. 1996;496:575–581. doi: 10.1113/jphysiol.1996.sp021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez AM, Schwaller B, Porzig H, Vassort G, Niggli E, Egger M. Increased exchange current but normal Ca2+ transport via Na+-Ca2+ exchange during cardiac hypertrophy after myocardial infarction. Circ Res. 2002;91:323–330. doi: 10.1161/01.res.0000031384.55006.db. [DOI] [PubMed] [Google Scholar]
- Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- Gwathmey JK, Morgan JP. Altered calcium handling in experimental pressure-overload hypertrophy in the ferret. Circ Res. 1985;57:836–843. doi: 10.1161/01.res.57.6.836. [DOI] [PubMed] [Google Scholar]
- Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hain J, Onoue H, Mayrleitner M, Fleischer S, Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. J Biol Chem. 1995;270:2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]
- Hussain M, Orchard CH. Sarcoplasmic reticulum Ca2+ content, L-type Ca2+ current and the Ca2+ transient in rat myocytes during β-adrenergic stimulation. J Physiol. 1997;505:385–402. doi: 10.1111/j.1469-7793.1997.385bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- Kameyama M, Hofmann F, Trautwein W. On the mechanism of β-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985;405:285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Kettlun C, Gonzalez A, Rios E, Fill M. Unitary Ca2+ current through mammalian cardiac and amphibian skeletal muscle ryanodine receptor channels under near-physiological ionic conditions. J Gen Physiol. 2003;122:407–417. doi: 10.1085/jgp.200308843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- Lindegger N, Niggli E. Paradoxical SR Ca2+ release in guinea-pig cardiac myocytes after β-adrenergic stimulation revealed by two-photon photolysis of caged Ca2+ J Physiol. 2005;565:801–813. doi: 10.1113/jphysiol.2005.084376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann JP, Jones LR, Hathaway DR, Henry BG, Watanabe AM. β-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983;258:464–471. [PubMed] [Google Scholar]
- Lipp P, Niggli E. Submicroscopic calcium signals as fundamental events of excitation–contraction coupling in guinea-pig cardiac myocytes. J Physiol. 1996;492:31–38. doi: 10.1113/jphysiol.1996.sp021286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin SE, Zhang D, Bridge JH. Dyssynchronous Ca2+ sparks in myocytes from infarcted hearts. Circ Res. 2000;87:1040–1047. doi: 10.1161/01.res.87.11.1040. [DOI] [PubMed] [Google Scholar]
- Liu N, Priori SG. Disruption of calcium homeostasis and arrhythmogenesis induced by mutations in the cardiac ryanodine receptor and calsequestrin. Cardiovasc Res. 2008;77:293–301. doi: 10.1093/cvr/cvm004. [DOI] [PubMed] [Google Scholar]
- Lokuta AJ, Rogers TB, Lederer WJ, Valdivia HH. Modulation of cardiac ryanodine receptors of swine and rabbit by a phosphorylation–dephosphorylation mechanism. J Physiol. 1995;487:609–622. doi: 10.1113/jphysiol.1995.sp020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules: A comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res. 2004;62:63–73. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR. Coupled gating between cardiac calcium release channels (ryanodine receptors) Circ Res. 2001;88:1151–1158. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Mitra R, Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol Heart Circ Physiol. 1985;249:H1056–1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Ogrodnik J, Niggli E. Altered ryanodine receptor sensitivity after β-adrenergic stimulation of guinea-pig ventricular myocytes. Biophys J. 2009;96:276a. [Google Scholar]
- Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol. 2007;293:C1073–1081. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- Reuter H, Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977;264:49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U, Hajjar RJ, Helm PA, Kim CS, Doye AA, Gwathmey JK. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol. 1998;30:1929–1937. doi: 10.1006/jmcc.1998.0748. [DOI] [PubMed] [Google Scholar]
- Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci U S A. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LS, Wang SQ, Xiao RP, Spurgeon H, Lakatta EG, Cheng H. β-Adrenergic stimulation synchronizes intracellular Ca2+ release during excitation-contraction coupling in cardiac myocytes. Circ Res. 2001;88:794–801. doi: 10.1161/hh0801.090461. [DOI] [PubMed] [Google Scholar]
- Takasago T, Imagawa T, Furukawa K, Ogurusu T, Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem. 1991;109:163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- Takasago T, Imagawa T, Shigekawa M. Phosphorylation of the cardiac ryanodine receptor by cAMP-dependent protein kinase. J Biochem. 1989;106:872–877. doi: 10.1093/oxfordjournals.jbchem.a122945. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Nishimaru K, Sekine T, Kawanishi T, Nakamura R, Yamagaki K, Shigenobu K. Two-dimensional millisecond analysis of intracellular Ca2+ sparks in cardiac myocytes by rapid scanning confocal microscopy: increase in amplitude by isoproterenol. Biochem Biophys Res Commun. 1997;233:413–418. doi: 10.1006/bbrc.1997.6470. [DOI] [PubMed] [Google Scholar]
- Uehara A, Yasukochi M, Mejia-Alvarez R, Fill M, Imanaga I. Gating kinetics and ligand sensitivity modified by phosphorylation of cardiac ryanodine receptors. Pflugers Arch. 2002;444:202–212. doi: 10.1007/s00424-002-0791-3. [DOI] [PubMed] [Google Scholar]
- Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhu W, Wang S, Yang D, Crow MT, Xiao RP, Cheng H. Sustained β1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signalling pathway. Circ Res. 2004;95:798–806. doi: 10.1161/01.RES.0000145361.50017.aa. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, Walsh MP, Warltier DC, Cheng H, Chen SR. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- Yano M, Okuda S, Oda T, Tokuhisa T, Tateishi H, Mochizuki M, Noma T, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Correction of defective interdomain interaction within ryanodine receptor by antioxidant is a new therapeutic strategy against heart failure. Circulation. 2005;112:3633–3643. doi: 10.1161/CIRCULATIONAHA.105.555623. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Takahashi M, Imagawa T, Shigekawa M, Takisawa H, Nakamura T. Phosphorylation of ryanodine receptors in rat myocytes during β-adrenergic stimulation. J Biochem. 1992;111:186–190. doi: 10.1093/oxfordjournals.jbchem.a123735. [DOI] [PubMed] [Google Scholar]