Abstract
Electrically elicited Ca2+ transients reported with the fast Ca2+ dye MagFluo-4 AM and myosin heavy chain (MHC) electrophoretic patterns were obtained in intact, enzymatically dissociated fibres from adult mice extensor digitorum longus (EDL) and soleus muscles. Thirty nine fibres (23 from soleus and 16 from EDL) were analysed by both fluorescence microscopy and electrophoresis. These fibres were grouped as follows: group 1 included 13 type I and 4 type IC fibres; group 2 included 2 type IIC, 3 IIA and 1 I/IIA/IIX fibres; group 3 included 4 type IIX and 1 type IIX/IIB fibres; group 4 included 2 type IIB/IIX and 9 type IIB fibres. Ca2+ transients obtained in groups 1, 2, 3 and 4 had the following kinetic parameters (mean ±s.e.m.): amplitude (ΔF/F): 0.61 ± 0.05, 0.53 ± 0.08, 0.61 ± 0.06 and 0.61 ± 0.03; rise time (ms): 1.64 ± 0.05, 1.35 ± 0.05, 1.18 ± 0.06 and 1.14 ± 0.04; half-amplitude width (ms): 19.12 ± 1.85, 11.86 ± 3.03, 4.62 ± 0.31 and 4.23 ± 0.37; and time constants of decay (τ1 and τ2, ms): 3.33 ± 0.13 and 52.48 ± 3.93, 2.69 ± 0.22 and 41.06 ± 9.13, 1.74 ± 0.06 and 12.88 ± 1.93, and 1.56 ± 0.11 and 9.45 ± 1.03, respectively. The statistical differences between the four groups and the analysis of the distribution of the parameters of Ca2+ release and clearance show that there is a continuum from slow to fast, that parallels the MHC continuum from pure type I to pure IIB. However, type IIA fibres behave more like IIX and IIB fibres regarding Ca2+ release but closer to type I fibres regarding Ca2+ clearance. In conclusion, we show for the first time the diversity of Ca2+ transients for the whole continuum of fibre types and correlate this functional diversity with the structural and biochemical diversity of the skeletal muscle fibres.
Introduction
Earlier classification of mammalian skeletal muscle fibres into types I and II (Dubowitz & Pearse, 1960; Engel, 1962), has become more complex due to the addition of subgroups of type II fibres and of intermediate (hybrid) fibres (Brooke & Kaiser, 1970; Bär & Pette, 1988; Schiaffino et al. 1989; Staron & Pette, 1993). Furthermore, new molecular and physiological diversity criteria are being reported. Correlations between the composition of myosin heavy chain (MHC) isoforms and mechanical properties such as force, force–velocity curves, unloaded shortening velocity and power output support the idea of great variability of skeletal muscle (Bottinelli et al. 1994; Maréchal et al. 1995; Bottinelli & Reggiani, 2000).
Some molecular diversity has also been found in the molecular machinery involved in the excitation–contraction–relaxation phenomena (Bottinelli & Reggiani, 2000; Reggiani & te Kronnie, 2006), which is reflected in the conspicuous differences in Ca2+ signalling kinetics between fast and slow muscles (Eusebi et al. 1980; Carroll et al. 1997; Baylor & Hollingworth, 2003; Reggiani & te Kronnie, 2006; Calderón et al. 2009). With regard to this point, a complication arises from the fibre heterogeneity (presence of pure and hybrid fibre types) in fast and slow muscles (soleus, extensor digitorum longus (EDL) and flexor digitorum brevis (FDB)) used to compare Ca2+ transient kinetics (see Calderón et al. 2009).
Furthermore, in spite of several studies dealing with such differences, to our knowledge, no clear information exists about Ca2+ signalling parameters over the whole spectrum of fibre types in mammalian skeletal muscles and no direct evaluation has been made of the MHC isoform composition and Ca2+ transient kinetics in the same muscle fibres, either.
Ca2+ transient kinetics is the ultimate concerted expression of several mechanisms involving a great number of proteins that determine the different characteristics such as rise time, peak amplitude, half-width and time course of decay. Many of the proteins and transient characteristics are known to be different in fast and slow muscle fibres, as has been previously pointed out (Baylor & Hollingworth, 2003; Calderón et al. 2009; for reviews see Stephenson et al. 1998; Bottinelli & Reggiani, 2000; Reggiani & te Kronnie, 2006).
As suggested by Close (1965), some correlation should be expected between myofibrillar contractile properties, linked to MHC isoform composition, and contraction kinetics parameters, linked to proteins that control Ca2+ transient kinetics. The tendency to consider MHC as the main determinant of the functional properties of the different fibre types is well shown by the nomenclature used nowadays for fibre identification: type I, IIA, IIX and IIB. This also led to the intuitive idea of a master switch gene concept (Spangenburg & Booth, 2003). However, more recent evidence indicates that MHC composition may not be the unique factor determining the functional heterogeneity of skeletal muscle fibres (Bottinelli, 2001; Spangenburg & Booth, 2003). In fact, the hypothesis that there is a single master switch gene, such as that of MHC, as the only controller of fibre typing, seems to be in contrast with the multiplicity of proteins involved in Ca2+ signalling and with the variability of slow and fast muscle fibres. Published literature suggests that muscle fibre type expression is regulated by multiple signalling pathways and transcription factors rather than a single master switch gene or signalling pathway. Thus, a new paradigm for describing the variability of fibres has been proposed (Spangenburg & Booth, 2003) based on the presence of ‘modules’ of functional genes.
The evaluation of several Ca2+ transients’ parameters in pure and hybrid fibre types, and their relationship with MHC isoforms, could help us understand how relevant a specific MHC isoform is in determining differences in Ca2+ transient morphology. The shape of Ca2+ transients is the result of different mechanisms involved in Ca2+ release and reuptake processes, which are probably controlled by different ‘modules’ of genes (and proteins). The results could shed some light onto the interplay between MHC and the regulation of the different modules that define a muscle fibre phenotype.
In this work, we used fibres from enzymatically dissociated soleus and extensor digitorum longus (EDL) muscles and the fast dye MagFluo-4 AM to correlate fibre transient kinetics with fibre types. We show that functional heterogeneity accompanies molecular diversity over the whole continuum of fibre types, from type I to IIB.
Methods
Fibres preparation
The enzymatic dissociation method is basically a modification of previously published ones (Bekoff & Betz, 1977; Capote et al. 2005; Calderón et al. 2009). In brief, 42- to 49-day-old male mice (NMRI-IVIC, Navy Medicine Research Institute-Instituto Venezolano de Investigaciones Científicas) were killed by rapid cervical dislocation, according to procedures approved by the local animal care committee. Soleus or EDL muscles were dissected out, divided longitudinally and incubated in a modified Ringer solution (in mm: 2.7 KCl, 1.2 KH2PO4, 0.5 MgCl2, 138 NaCl, 0.1 Na2HPO4, 1 CaCl2, pH 7.4) containing 2.5 mg ml−1 collagenase (Worthington CLS2; 250 u mg−1) for 52–58 min at 36.6°C. After collagenase treatment, the muscles were washed twice with Tyrode solution (in mm: 5.4 KCl, 1 MgCl2, 140 NaCl, 0.33 NaH2PO4, 10 glucose, 10 Hepes (Sigma), 2 CaCl2, pH 7.3) at room temperature, and gently separated from tendons and remaining tissue with a set of fire-polished Pasteur pipettes. Fibres rendered by this procedure remained excitable and contracted briskly during experiments that lasted up to 7–8 h.
Fluorescence recordings
Dissociated fibres were transferred to the experimental chamber and incubated for 35–45 min at room temperature in a Tyrode solution containing 8–10 μm MagFluo-4 AM (Molecular Probes, Oregon, USA). This procedure allowed loading of fibres while they were adhering to the chamber bottom that consisted of a glass coverslide, previously coated with 2–4 μl of laminin (1 mg ml−1; Sigma). We have shown that this method is very useful in reducing movement artifacts in the Ca2+ records (Calderón et al. 2009). All fibres were selected at random and the use of a maximum of four per mouse was established in order to increase the number of mice used and get results as representative as possible.
The chamber was mounted on the stage of an inverted Nikon Diaphot TMD (Nikon Co., Tokyo, Japan) microscope equipped for epifluorescence, and the fibres were illuminated with a xenon lamp (100 W). Precautions were taken to diminish photodamage and photobleaching of the dye. The characteristic wavelengths (in nm) of the filter combination (excitation/dichroic/barrier) were 450–490/510/520. The light signals were collected with a photomultiplier connected to a Nikon P1 amplifier.
Intracellular Ca2+ transients were elicited by applying supra-threshold rectangular current pulses (0.6–1.4 ms) through two platinum plate electrodes placed on either side along the experimental chamber. Tetanic stimuli were of 350 ms, at 100 Hz. The amplifier output was fed into a DigiData 1200 interface (Axon Instruments, Foster City, CA, USA). The data were acquired and analysed using the pCLAMP 6 software (Axon Instruments). Acquisition frequency was 20 kHz for single twitch Ca2+ transients.
Due to uncertainties regarding the concentration and the dissociation constant (Kd) of the dye in the fibres, we present the Ca2+ transients as ΔF/F= ((Fmax−Frest)/Frest). The low affinity of MagFluo-4 for Ca2+ (Kd= 22 μm) and the fact that the peak Ca2+ concentration in mammalian muscles in a twitch has been reported to be around 20 μm, suggests that the dye is far from saturation under our experimental conditions (Caputo et al. 2004). Since MagFluo-4 AM is a fluorescent dye sensitive to both Ca2+ and Mg2+ ions, it is necessary to consider the possibility of artifacts arising from changes in the myoplasmic Mg2+ concentration due to its displacement from parvalbumin (PV) by Ca2+ ions. Considering the Kd values for Ca2+ and Mg2+ at 22°C (22 μm and 4.7 mm, respectively (although higher values were recently reported in vivo; Hollingworth et al. 2009), the amount of Ca2+ that binds to PV and the relative change of Ca2+ and Mg2+ over their basal levels during a single twitch, one would expect the dye to reliably respond to changes in myoplasmic Ca2+ concentration with little contribution from changes in Mg2+ myoplasmic concentration (Hollingworth et al. 2009). Konishi et al. (1991) used the Ca2+ and Mg2+ dye MagFura-2 at 16°C, a temperature at which the dye has a Kd for Mg2+ of 5.3 mm (very similar to that found for MagFluo4-AM at 22°C, see above), and found that the dye was not affected by changes in Mg2+ concentration during a twitch. These authors also found that interference by Mg2+ occurred at very late times (100 ms) after stimulation. If the displacement of Mg2+ from PV were to produce any effect on the Ca2+ transient, this would result in a slower relaxation phase, which would be more evident in the fastest, PV-rich, types IIX and IIB fibres. On the contrary, we found that these fibres had the fastest Ca2+ transients, suggesting that without Mg2+ interference they might actually be even faster. For the case of tetanic transients, it seems that the Mg2+ mobilization might have a larger effect, since tetanic relaxation is much more prolonged than that of a single twitch.
The decay phase of the Ca2+ transients was fitted with a double exponential function, as has been previously shown (Caputo et al. 2004, 2005; Calderón et al. 2009). All experiments were done at room temperature (21–23°C).
Ca2+ transients and movement artifacts
In 13 fibres, 25–35 μmN-benzyl-p-toluene sulphonamide (BTS, Toronto Research Chemicals, Canada) was used to diminish movement artifacts. We found a close relation between the effect and the fibre type: BTS had almost no effect on slow fibres, a great effect on fast fibres and an intermediate one on hybrid fibres containing both MHC I and IIa. The possibility of movement artifacts that might affect our results have been shown to be negligible in our preparation; moreover, we have shown that BTS cannot change the morphology of a Ca2+ transient beyond a small change associated with the reduction of the movement of the fibre, if any (Calderón et al. 2009).
Myosin heavy chain electrophoresis
Soleus and EDL muscles of 42- to 49-day-old mice were analysed for MHC isoform composition. Myofibrils were obtained following the protocol described by Sartorius et al. (1998). All work was done at 4–6°C. Pairs of muscles were excised from each mouse, washed with Ringer solution and homogenized in 1 ml of buffer A (in mm: 50 KCl, 10 K2HPO4, 2 MgCl2, 0.5 EDTA (Sigma, St Louis, MO, USA), 2 dithiothreitol (DTT, Sigma), pH 7.0). Homogenate was centrifuged at 15 800 g (Beckman L8-55) for 15 min at 4°C. The pellets were resuspended in 1 ml of buffer A plus 1% Triton X-100 (Sigma) and centrifuged again as described above. The pellets were resuspended in 0.5–1 ml of store solution: 60 mm KCl, 30 mm imidazole, 2 mm MgCl2, 1 mm DTT, pH 7.0. Proteins were measured with the Bradford method (Bradford, 1976) and samples stored at −80°C.
MHC electrophoresis under denaturing conditions (SDS-PAGE) was performed according to Talmadge & Roy (1993). Stacking gel was 4% polyacrylamide containing 30% glycerol (Sigma) and 4 mm EDTA, and the separating gel was 8% polyacrylamide containing 30% glycerol. Two to three micrograms of total proteins were loaded in each well of a Mini-Protean III electrophoresis cell (Bio-Rad) and run at 72 V for 27–28 h at 4–6°C. The interior running buffer contained 10 mm 2-mercaptoethanol (Blough et al. 1996). Following migration, the gels were stained with Coomassie blue.
For MHC isoform evaluation of single fibres, after the Ca2+ transient was recorded, the fibre was taken out of the experimental chamber with the help of small forceps and put inside a vial with 33 μl of loading buffer (62.5 mm Tris, 1% sodium dodecyl sulfate, 0.01% bromophenol blue, 5% mercaptoethanol, 15.2% glycerol), sonicated for 40–50 s (Fisher Sonic Dismembrator Model 550, Fisher Scientific Company, USA) and frozen at −80°C. MHC electrophoresis was similar to that described for whole muscles; gels were silver stained. Controls (samples from whole soleus or EDL) were loaded for every electrophoretic run and bands of the cell samples were labelled according to the distance migrated in comparison with the controls.
Pictures were taken of the gels and images were processed with ImageJ 1.33u free software (National Institutes of Health, USA) or Adobe Photoshop CS3 software. Relative MHC composition of whole muscles was calculated using the Gaussian fit function of the Gel Band Fitter free software (http://www.gelbandfitter.org).
Statistics
Statistical analysis was performed using the program Origin 7.5 (Microcal Software Inc., Northampton, MA, USA); for comparing mean values, Student's t test (using Origin 7.5 software) for two independent populations or Mann–Whitney test (using SPSS 13.0 software, SPSS Inc., Chicago, USA) were applied when appropriate. Results are given as mean ±s.e.m. Differences were considered statistically significant at P < 0.05.
Results
MHC isoform composition in whole and dissociated soleus and EDL muscles
All the muscles used in this work were dissected from animals whose weight was 34.3 ± 0.5 g, ranging from 30.6 g to 39.6 g (n= 20). There were no statistical differences between groups of mice used to perform soleus or EDL experiments.
Pure fibre types are defined by their principal MHC isoform, while hybrid fibres are defined by the fractional value of the isoforms’ content, placing the predominant isoform first. Type C fibres contain both MHC I and IIa, i.e. fibres IC have a I/IIa ratio while fibres IIC have a IIa/I ratio. Fast fibre types are designated by a capital letter, while MHC isoform are designated by a lower case letter (Staron & Pette, 1993; Hämäläinen & Pette, 1995). The relative MHC isoform composition in three pairs of soleus muscles corresponded to 70.9 ± 4.9% type I and 29.1 ± 4.9% type IIa. In four pairs of EDL muscles the relative MHC composition was: 26.5 ± 1.7% type IIx and 73.5 ± 1.7% type IIb.
A total of 54 dissociated fibres (32 from soleus and 22 from EDL muscles) were studied by SDS-PAGE. The classification of these fibres is shown in Table 1. This table also details the MHC composition of 39 fibres, out of a total of 54, which were used to obtain Ca2+ transients before MHC typing was performed. EDL and soleus fibres had the same length (4.03 ± 0.11 mm, n= 16 vs. 3.70 ± 0.16 mm, n= 14; P > 0.05) and diameter (40.75 ± 1.52 μm, n= 16 vs. 39.81 ± 1.40 μm, n= 16; P > 0.1).
Table 1.
Classification of dissociated soleus and extensor digitorum longus fibres from adult mice
| Fibre type | Evaluated by both fluorescence and electrophoresis (n) (%) | Evaluated only by electrophoresis (n) (%) | |
|---|---|---|---|
| Group 1 | I | 13 (33.33) | 18 (33.3) |
| IC | 4 (10.26) | 6 (11.1) | |
| Group 2 | IIC | 2 (5.13) | 2 (3.7) |
| IIA | 3 (7.69) | 5 (9.3) | |
| I/IIA/IIX | 1 (2.56) | 1 (1.85) | |
| Group 3 | IIX | 4 (10.26) | 5 (9.3) |
| IIX/IIB | 1 (2.56) | 1 (1.85) | |
| Group 4 | IIB/IIX | 2 (5.13) | 2 (3.7) |
| IIB | 9 (23.08) | 14 (25.9) | |
| Total | 39 (100) | 54 (100) |
To group the fibres, we performed a statistical comparison, when possible, of every group of hybrid fibres with its closest group of pure fibres, i.e. hybrid fibres IC were compared to fibres type I and so on. Given that no differences were found when comparing fibres type I vs. IC, IIA vs. IIC, IIX vs. IIX/IIB and IIB vs. IIB/IIX, we decided to pool them in four groups, as shown in Table 1: group 1 includes type I and IC fibres, group 2 includes IIA, IIC and I/IIA/IIX fibres, group 3 includes IIX and IIX/IIB fibres and group 4 includes IIB and IIB/IIX fibres. Fibre type I/IIA/IIX was included in group 2, since it showed similar kinetic values to the type IIA and IIC fibres, despite the fact that its content of MHC type I was larger than the content of MHC types IIa and IIx. Table 1 also shows the absolute and relative number of each fibre type. Two approaches have been followed to show the results: comparison between the groups and analysis of the distribution of the values for the main parameters of the Ca2+ transients.
Ca2+ transients and MHC composition in dissociated fibres from soleus and EDL muscles from adult mice
We were able to obtain both Ca2+ transients and MHC composition in 23 fibres from soleus and 16 from EDL. Representative Ca2+ transients obtained from dissociated soleus (a–d) and EDL (e–g) fibres are shown in Fig. 1A and B, respectively. The MHC bands obtained for the same fibre (a′–d′ and e′–g′) are also shown. Controls are from whole soleus (Fig. 1A) and EDL (Fig. 1B) muscles. It is important to note the following: (i) there is a clear and consistent difference between the time course of decay of Ca2+ transients from soleus and EDL fibres: all signals from soleus fibres are appreciably slower than those of EDL fibres (compare transients a and g, and d and e in Fig. 1C); (ii) Ca2+ transients from type IIA fibres are only slightly faster than those from type I (compare transients a and d in Fig. 1C), but noticeably slower than Ca2+ transients from fibres types either IIX or IIB.
Figure 1. Ca2+ transients (a–d and e–g) and myosin heavy chain composition (a′–d′ and e′–g′) of dissociated fibres from soleus (A) and extensor digitorum longus (B) muscles from adult mice.
Note that each pair of results (i.e. a and a′, b and b′, etc.) comes from the same fibre. Controls are from whole muscles. C shows selected comparisons between Ca2+ transients from different types of fibres. Bottom panel illustrates the striking difference between records a and g, coming from fibres type I and IIB, respectively. All records were normalized.
Table 2 shows the mean values of the different kinetic parameters of Ca2+ transients associated with single twitches of fibres from soleus and EDL muscles. In these fibres, the time course of decay of Ca2+ transients is well fitted by a double exponential function, with time constants τ1 and τ2. The magnitude of the components associated with the two time constants is defined as A1 and A2, respectively. The table clearly shows that the parameters associated with the two groups of muscle fibres are significantly different with the exception of the ΔF/F parameter.
Table 2.
Kinetic parameters of Ca2+ transients of soleus and EDL fibres from adult mice
| Muscle | n | ΔF/F | 10–90% rise time | Half-width | Decay time | Time constants (ms) |
A1 | A2 | ΔF/RT | |
|---|---|---|---|---|---|---|---|---|---|---|
| (ms) | (ms) | (ms) | τ1 | τ2 | (%) | (%) | ||||
| Soleus | 23 | 0.59±0.04 | 1.57±0.05 | 17.23±1.69 | 65.46±3.75 | 3.16±0.13 | 49.50±3.79 | 28.08±1.99 | 71.92±1.99 | 0.38±0.03 |
| Extensor digitorum longus | 16 | 0.61±0.03 | 1.15±0.04 | 4.35±0.27 | 16.50±1.13 | 1.61±0.08 | 10.52±0.98 | 35.75±1.80 | 64.25±1.80 | 0.54±0.03 |
| Soleus vs. extensor digitorum longus | P= 0.673 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P= 0.009 | P= 0.009 | P < 0.001 | |
Values are mean±s.e.m. A1, amplitude of fast component of decay; A2, amplitude of slow component of decay; ΔF/RT, ratio of amplitude to rise time. Comparisons were done using the two samples, independent Student's t test.
Table 3 shows that the values of the kinetic parameters of groups 1 and 4 are extreme, while those of groups 2 and 3 are intermediate. However, a closer look at the values and comparisons shows that groups 2 and 3 are closer to groups 1 and 4, respectively, than to each other, for almost all parameters. If we set apart fibres type I from type IC (both part of group 1) and compare them separately with group 2, type I fibres are different in a similar fashion as that shown in Table 3 (i.e. except for ΔF/F and τ2); however, type IC fibres only differ statistically with group 2 in rise time and amplitudes A1 and A2.
Table 3.
Kinetic parameters of Ca2+ transients of different fibre types of soleus and extensor digitorum longus from adult mice
| Fibre type | n | ΔF/F | 10–90% rise time | Half-width | Decay time | Time constants (ms) |
A1 (%) | A2 (%) | |
|---|---|---|---|---|---|---|---|---|---|
| (ms) | (ms) | (ms) | τ1 | τ2 | |||||
| Group 1 | 17 | 0.61±0.05 | 1.64±0.05 | 19.12±1.85 | 68.1±3.41 | 3.33±0.13 | 52.48±3.92 | 25.92±2.31 | 74.08±2.31 |
| Group 2 | 6 | 0.53±0.08 | 1.35±0.05 | 11.86±3.03 | 57.98±10.82 | 2.69±0.22 | 41.06±9.13 | 34.23±2.83 | 65.77±2.83 |
| Group 3 | 5 | 0.61±0.06 | 1.18±0.06 | 4.62±0.31 | 19.47±2.02 | 1.74±0.06 | 12.88±1.93 | 39.06±3.21 | 60.94±3.21 |
| Group 4 | 11 | 0.61±0.03 | 1.14±0.04 | 4.23±0.37 | 15.15±1.21 | 1.56±0.11 | 9.45±1.03 | 34.24±2.11 | 65.76±2.11 |
| Group 1 vs. 2 | P= 0.462 | P= 0.002 | P= 0.017 | P= 0.025 | P= 0.030 | P= 0.059 | P= 0.030 | P= 0.030 | |
| Group 1 vs. 3 | P= 0.695 | P= 0.001 | P= 0.001 | P= 0.001 | P= 0.001 | P= 0.001 | P= 0.030 | P= 0.030 | |
| Group 1 vs. 4 | P= 0.672 | P= 0.000 | P= 0.000 | P= 0.000 | P= 0.000 | P= 0.000 | P= 0.012 | P= 0.012 | |
| Group 2 vs. 3 | P= 0.715 | P= 0.054 | P= 0.006 | P= 0.006 | P= 0.006 | P= 0.006 | P= 0.273 | P= 0.273 | |
| Group 2 vs. 4 | P= 0.545 | P= 0.013 | P= 0.001 | P= 0.001 | P= 0.003 | P= 0.001 | P= 0.841 | P= 0.841 | |
| Group 3 vs. 4 | P= 0.733 | P= 0.413 | P= 0.193 | P= 0.126 | P= 0.027 | P= 0.126 | P= 0.308 | P= 0.308 | |
Values are mean±s.e.m. A1, amplitude of fast component of decay; A2, amplitude of slow component of decay. Groups as in Table 1. Comparisons were done using the Mann–Whitney test.
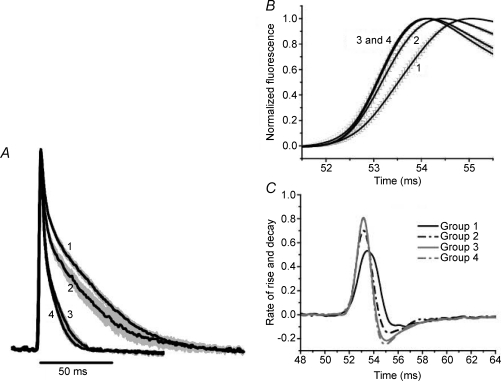
To look deeper into these observations and to check if a pattern of morphologies per fibre type could be easily recognized, we calculated the mean Ca2+ transients from fibres of every group and superimposed them in Fig. 2. This figure indicates that, in general, Ca2+ transients of mouse muscles can be ordered from slower to faster as: I > IIA > IIX > IIB. The characteristics of the Ca2+ transients numbered 1 and 2 correspond to the morphology previously called type I (MT-I), while those of the Ca2+ transients numbered 3 and 4 correspond to the one called morphology type II (MT-II) which has also been found in dissociated fibres from FDB of adult mice (Capote et al. 2005; Calderón et al. 2009). The use of MagFluo-4 AM and a high acquisition rate allowed us to resolve not only the clear differences in the decay phase (Fig. 2A) of the four groups, but also in their rising phase (Fig. 2B). It was consistently observed that the rising phase of group 2 transients, although close to groups 3 and 4, is not identical to them. Figure 2C shows that groups 3 and 4 have the highest rates of rising and decay, while group 1 has the lowest and group 2 has intermediate values.
Figure 2. Time course of mean Ca2+ transients of different fibre groups.
A, mean Ca2+ transients. Numbers represent groups as in Table 1. Note that the decay phase of the Ca2+ transients can be paired (slow pair, 1–2, and fast pair, 3–4) in close correspondence to the PV content (see the Fig. 5 of Füchtbauer et al. 1991). B shows a detail of the raising part of the mean Ca2+ transients in A, to stress the differences obtained in the 4 groups. Note that group 2, although closer to group 1 in the decay phase, is closer to groups 3 and 4 regarding the rising phase. C represents the differentiation of the records in A, and shows that groups 3 and 4 have the maximal rates of rise and decay, group 1 the minimal and group 2 intermediate rates.
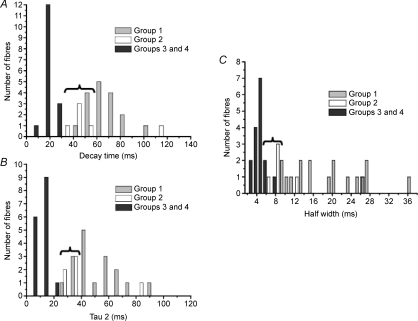
To further test the validity of this classification we carried out an analysis of the fibre distribution of selected Ca2+ transient parameters. Figure 3A and B show the distribution of the decay time and of the slower time constant of decay τ2, two parameters clearly related to the Ca2+ clearance mechanisms. The two histograms show a similar pattern of distribution of values of both parameters. There is a clear separation of the values corresponding to groups 3 and 4 and groups 1 and 2, while there is a great overlapping, indicated by the horizontal brace, for the values corresponding to groups 1 and 2. Figure 3C shows the distribution pattern of the half-width values. This parameter depends mostly on the activity of clearance mechanisms, but it may also be affected, to a lesser degree, by the kinetics of Ca2+ release. It can be seen that the half-width values of groups 3 and 4 are widely different from the values of group 1 fibres. The values of group 2 show some overlap with both groups 3 and 4, and group 1.
Figure 3. Histograms showing the distribution of decay time (A), second time constant of decay (τ2, B) and half-width (C) from Ca2+ transients measured in extensor digitorum longus (EDL) and soleus fibres from adult mice.
Note that there is a high overlap between fibre types I and IIA (braces) from soleus but no overlap is seen between fibres from EDL and those of soleus in A and B, not even with type IIA ones. C shows some overlap between group 2 with both groups 3 and 4 and group 1. Groups as in Table 1. See text for more details.
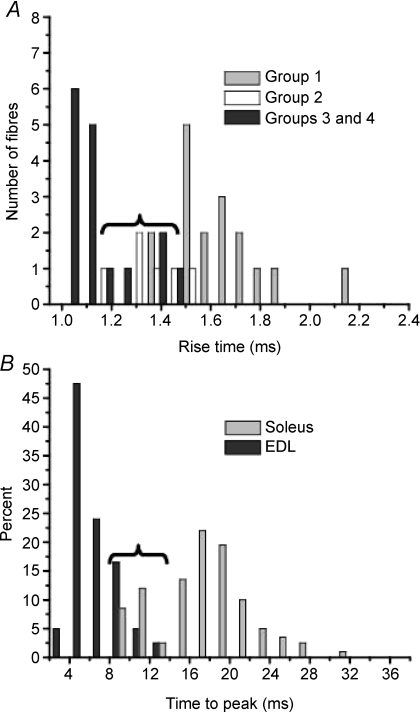
Figure 4A shows the values of the rise time for the same groups as above and Fig. 4B shows the per cent distribution of the time to peak (Tp) tension of fibres from fast and slow muscles from mouse, taken from a paper by Luff & Atwood (1972) and replotted for comparative purposes. Comparison of histograms A and B shows that there is a close correspondence between the distribution values of the rise time of Ca2+ transients for all groups and Tp of twitch tension records of fibres from fast and slow muscles. Opposite to what was seen in Fig. 3, in this case there is a great overlap between group 2 and groups 3 and 4, but a minimum overlap between group 2 and group 1.
Figure 4. Histograms showing the distribution of rise time (A), and time to peak (B) from Ca2+ transients (A) and tension records (B) measured in extensor digitorum longus (EDL) and soleus fibres from adult mice.
Note the similarity of the distribution of the variable of the Ca2+ transients and the one of the tension records: great overlap exists between fibres from EDL and type IIA (braces) from soleus but minimum overlap exist between fibres from EDL and type I from soleus. B was replotted from Fig. 2 of Luff & Atwood (1972) and is used with permission. Groups as in Table 1. See text for more details.
After generating a pattern for Ca2+ release and clearance for single twitch Ca2+ transients, we wanted to see if a pattern could also be uncovered regarding tetanic Ca2+ transients for every fibre type. As the rise time is that of a twitch, we focused on relaxation where clear differences were noted. Figure 5 shows the normalized decay phase of tetanic transients of 350 ms, at 100 Hz, obtained in five fibres classified by MHC typing as indicated. The times to half-relaxation were 63.3, 41.2, 45.6, 23.8 and 13.6 ms for fibre types I, IIA, I/IIA/IIX, IIX and IIB, respectively. As indicated in the inset, the decay of fibre type IIA can be fitted with a single exponential function with a τ1 of 62.4 ms, while the decay of fibre type IIB could be fitted with a sum of two exponential functions, with a rapid τ1 of 15.2 ms and a second very slow component with time constant τ2 of 1231 ms. We found that the tetanic decay from all fibres in groups 1 and 2 could be fitted with a single exponential function with a τ1 of 74.8 ms (n= 5) and the decay phase of all fibres from groups 3 and 4 was better fitted with a sum of two exponential with τ1 and τ2 of 13.8 and 1050.1 ms, respectively (n= 7).
Figure 5. Normalized tetanic Ca2+ transient decay phase of fibres types I, IIA, I/IIA/IIX, IIX and IIB.
Records from slow type I and fast type IIB fibres are the extremes, whiles those from types IIA and IIX seem to be intermediate, but closer to fibres type I and IIB, respectively, than to each other. This behaviour parallels that observed for the decay of twitch Ca2+ transients. The hybrid fibre type I/IIA/IIX behaves like a fibre type IIA, despite the fact that its predominant myosin heavy chain isoform was type I. The inset shows that the decay of a fibre type IIA is well fitted by a single exponential function (continuous black line on the dashed black trace), as it is the decay of fibres type I (not shown), while the decay of fibres type IIX (not shown) and IIB is better fitted with a sum of two exponentials (continuous black line on the dashed grey trace).
Discussion
As previously suggested by Close (1965) and recently discussed and updated by Reggiani & te Kronnie (2006), some correlation is expected between myofibrillar contractile properties (linked to MHC isoform composition) and contraction kinetics parameters (linked to proteins which control Ca2+ transient kinetics). However, to our knowledge, a direct test of this hypothesis had not been carried out. Moreover, since excitation–contraction coupling is a phenomenon involving many different proteins (and probably several modules of genes), evaluating Ca2+ transients in all fibre types gives us the opportunity to gain some insight into the question of whether there is a master switch gene, probably that of MHC, or if there are modules of genes that can be activated in an independent fashion to determine a muscle fibre phenotype.
We have approached these issues by evaluating both Ca2+ transients, using the fast Ca2+ dye MagFluo-4 AM (Caputo et al. 2004, 2005; Calderón et al. 2009; Hollingworth et al. 2009), and MHC isoform composition using SDS-PAGE, in the same fibre. The enzymatically dissociated muscles used made it possible to study a continuum of fibre types: I, IC, IIC, IIA, I/IIA/IIX, IIX, IIX/B, IIB/X and IIB.
Despite the fact that enzymatic dissociation of ‘short’ muscles was achieved several decades ago (Bekoff & Betz, 1977) and it is frequently used, ‘long’ muscles have been rarely used for enzymatic dissociation (Szentesi et al. 1997; Calderón et al. 2009) and no previous work has dealt directly with the types of fibres used for the experiments. Interestingly, hybrid fibres have long been studied in rats (Staron & Pette, 1993; Bottinelli et al. 1994; Stephenson, 2001) but little information on them exist in mice.
MHC composition in soleus and EDL mice muscles
Our results concerning MHC composition of whole soleus and EDL muscles in mice of the NMRI strain are in good agreement with previously published works (Maréchal & Beckers-Bleukx, 1993; Maréchal et al. 1995). Regarding the MHC composition of dissociated fibres, there are some interesting observations: (i) fibres from soleus cover the spectrum from type I to type I/IIA/IIX while EDL has fibres from type IIX to IIB and the proportion of fibre types is similar to the proportion of MHC isoforms in the whole muscles from which they were dissociated; (ii) we confirm the co-expression of up to three MHC isoforms in one fibre, as previously described for fibres of other species (Staron & Pette, 1993; Bottinelli et al. 1994; Stephenson, 2001).
Ca2+ transients and dynamic properties of mammalian skeletal muscle
In this work we report measurements of kinetic parameters of Ca2+ transients for the entire spectrum of fibre types, which are separated into groups 1 to 4, as shown in Table 1. Our results, both in terms of Ca2+ release and Ca2+ sequestration parameters, agree with previous reports on molecular and biochemical properties of the different fibre types.
Since the resting membrane potential, the action potential (AP) amplitude or maximum rate of rise of the AP are not different in EDL and soleus fibres from mouse (Luff & Atwood, 1972), it seems unlikely that the excitability of the membrane could play an important role in the differences found regarding the rising phase of single Ca2+ transients; however, some other factors could be involved. It has been shown that in FDB fibres from 10- to 15-day-old mice, the rising phase of Ca2+ transients is slower compared to adults, and this has been explained in part by the poor development of Ca2+ releasing units in young animals (Capote et al. 2005). Likewise, several authors have found that in different species, including mouse, fast fibres have a several-fold higher density of Ca2+ release units than the slow ones and type IIA are intermediate between I and IIB (Hollingworth & Marshall, 1981; Lamb & Walsh, 1987; Franzini-Armstrong et al. 1988; Damiani & Margreth, 1994; Renganathan et al. 1998; Appelt et al. 1989). Our results seem to parallel the percentage of junctional T-tubules and the content of ryanodine receptors (RyR) reported for fibre types I, IIA and IIB of guinea pig (compare Fig. 14 of Franzini-Armstrong et al. 1988 and our Fig. 4A). Furthermore, despite the fact that all fibre types share the RyR1 isoform with many similar biochemical characteristics (Lee et al. 1991; Damiani & Margreth, 1994), functional differences have been recognized in RyR1 from slow vs. fast muscles, such as a lower initial rate of Ca2+ release from passively loaded vesicles and a smaller open probability when the channel was incorporated in lipid bilayers (Lee et al. 1991; Morrissette et al. 2000). Although some role of RyR3 as a mediator of differences in rising time cannot be ruled out, it is not supported by the available literature in which a clear fibre-type expression pattern has not been found (Conti et al. 1996) and because the lack of RyR3 in soleus and EDL mouse muscles does not abolish the differences between them (Rossi et al. 2001). Proteins such as calsequestrin, triadin and junctin have a modulating function on Ca2+ release, and their differences regarding fast and slow muscles (Leberer & Pette 1986; Damiani & Margreth, 1994; Paolini et al. 2007) may explain some of our results. Paolini et al. (2007) found a smaller Ca2+ transient in FDB fibres lacking calsequestrin 1, and an increased Tp of twitch tension records in EDL muscles; however, Royer et al. (2008a,b); did not find differences in the kinetics of Ca2+ release in murine FDB fibres over-expressing or knocked-down of calsequestrin1. It might be, despite that, that fast and slow fibres have different ‘evacuability’, a relation of sarcoplasmic reticulum (SR) permeability and buffer capacity (Royer & Rios, 2009); both are affected by kinetics of calsequestrin and local SR Ca2+ depletion. An interesting point to be discussed is what would be the effect of a different sequestration capacity of an intact fibre on the rate of Ca2+ release. Baylor & Hollingworth (1988) found that increasing the buffering capacity of frog skeletal muscle fibres increases the peak rate of Ca2+ release, but reduces the amplitude of the Ca2+ transient. This suggests that, besides the effect of myoplasmic buffering, intrinsic properties of the releasing machinery are still responsible for the differences between fibre types.
Different authors have found Tp values of type I and IIB fibres to be extreme, while those of type IIA and IIX fibres to be intermediate (Luff & Atwood, 1972; Luff, 1981; Ranatunga & Thomas, 1990). Our results (Fig. 2 and Table 3) show that rise time and half-width from groups 1 and 4 are extremes while groups 2 and 3 have intermediate values. These results are also supported by the close correspondence between the distribution of rise time values of Ca2+ transients obtained in this work and the Tp values for twitch tension reported by Luff & Atwood (1972) in isolated fibres from mouse muscles (Fig. 4). In Fig. 4B the grey columns on the left are thought to be mainly fibres IIA. Actually, Close (1967) and Lewis et al. (1982) found a very similar distribution for soleus, and the fibres on the left of their distributions were considered to be type IIA fibres.
Regarding Ca2+ transient decay, it is known that type I and IIA fibres have much lower levels of PV than type IIX and IIB fibres in mouse (see Fig. 5 of Füchtbauer et al. 1991). Likewise, it has been shown, for different species, that the amount of SR Ca2+-ATPase (SERCA) is lower and differently regulated in slow muscles compared to fast ones (Leberer & Pette, 1986; Ferguson & Franzini-Armstrong, 1988; Periasamy & Kalyanasundaram, 2007). From these biochemical data it seemed that the Ca2+ decay of all fibre types could be graded from a slow group, which included type I and IIA (with low content of PV) to a fast one, which included IIX and IIB (with high content of PV). Interestingly, we have obtained the same pattern considering the values of the parameters related to Ca2+ decay, such as half-width, decay time, time constants of decay (τ1 and τ2) and the amplitudes A1 and A2. Groups 1 and 2 are slower than groups 3 and 4 (P < 0.01 in all cases, see Table 2).
These results are consistent with the fact that values of half-relaxation time for slow and fast muscles in mouse are quite different (Luff & Atwood, 1972; Maréchal et al. 1995; Luff, 1981) and muscles with a higher proportion of both type IIA and IIX fibres have intermediate values (Luff, 1981; Ranatunga & Thomas, 1990).
The fact that the pairing according to PV content correlated with the pairing regarding kinetics of decay of Ca2+ transients suggests that differences in relaxation between fibre types are given primarily because of differences in PV content, and the small difference remaining may be due to differences in SERCA content. In fact, Caputo et al. (1999) has shown that the SERCA has almost no role in the relaxation of a single transient in amphibian skeletal muscle and has suggested that an intracellular buffer, most probably PV, is directly responsible for it.
We have also shown differences in the kinetics and morphology of the decay of tetanic transients for the whole spectrum of fibre types. In fibres from groups 1 and 2, in which the low PV content is expected to be saturated after only a few stimuli, the SERCA seem to be the main factor involved in relaxation. However, in fibres from groups 3 and 4, a more complicated picture seems to appear. A rather rapid first component accounts for roughly 75–85% of the decay phase, while a very slow component accounts for the remaining 15–25%, for full relaxation. It seems very improbable that the first component could be only related to PV, since our tetanic stimulation is harsh enough to mobilize a great amount of Ca2+ and is likely to saturate almost all PV. However, we can neither rule out this possibility, nor the involvement of other factors such as the mitochondria or the Na+–Ca2+ exchanger in the relaxation of single and tetanic Ca2+ transients in the different types of fibres (Caputo & Bolaños, 2008; Manno et al. 2008). These issues remain open for further research.
Although some differences in the kinetics of Ca2+ transients between fast and slow fibres were previously reported (Carroll et al. 1997; Baylor & Hollingworth, 2003), these works studied a small number of fibres, no fibre typing was done and in some experiments a slow Ca2+ dye was used. All these limitations were overcome in this work by the use of a larger number (39) of fully dissociated fibres of soleus and EDL muscles from mouse. These fibres could be loaded with the fast Ca2+ dye MagFluo-4 AM, which seems to reliably track the time course of the Ca2+ transients (Caputo et al. 2004; Hollingworth et al. 2009), thus allowing us to recognize differences in both the rising and decaying phases of Ca2+ transients, and could be further typed for MHC content by SDS-PAGE.
Our results seem to lend support to the idea of the regulation of fibre phenotype by regulating modules or programs of genes, from which the possibility of greater variability may arise. It calls our attention, for instance, to the fact that type IIX and IIB fibres greatly overlapped in all variables (Fig. 2), such that they were pooled and represented as black bars in the histograms (Figs 3 and 4), despite the fact that they have different MHC isoforms. Type IIA fibres, although a fast MHC type, behave rather ‘out’ of this pattern regarding Ca2+ reuptake, and in general there is a variability that spans a rather large range taking into account the whole spectrum of fibres. However, the variability was always found within a range, which interestingly seems to be narrower for fast than for slow fibres, and fibres such as those of type IC are a kind of mixture of the properties of slow and fast fibres (as they are actually composed of slow type I and fast type IIa MHC). Taking all this into account, it seems that, at least to a certain degree, Ca2+ handling properties are related to MHC composition; however, the great variability within fibres with the same MHC and the great similarity of some variables among different fibre types seem to lend support to the fact that regulation of the phenotype of fibres may be done by modules or programs of genes, rather than by a single master switch gene. It would be interesting to evaluate the changes in Ca2+ transient kinetics related to processes known to induce fibre type transitions, taking the results presented here as a reference, to draw a more powerful conclusion on this issue.
In conclusion, we detail here, for the first time, the variability of adult mice Ca2+ transients along the whole continuum of fibre types. The structural and biochemical variability of the machinery related to Ca2+ handling correlates with the Ca2+ transient kinetics, regarding both Ca2+ release and clearance. The transient kinetics shows a continuum from the slowest records, obtained in the type I fibres, to the fastest ones, obtained in types IIX and IIB, while type IIA fibres are fast regarding Ca2+ release and slow regarding Ca2+ clearance. The differences in kinetics of the Ca2+ twitch and tetanic transients seem to underlie the different kinetics of contractile characteristics of slow and fast muscles. MagFluo-4 shows its usefulness in recognizing differences between slow and fast fibre types.
Acknowledgments
We wish to thank Dr Alfredo Mijares and Lic. Alis Guillén from Instituto Venezolano de Investigaciones Científicas (IVIC), and Drs Osvaldo Delbono and Carlo Reggiani for useful advice. Grant support was provided by Fondo Nacional de Ciencia, Tecnología e Innovación (FONACIT) project G-2001000637.
Glossary
Abbreviations
- BTS
N-benzyl-p-toluene sulphonamide
- DTT
dithiothreitol
- EDL
extensor digitorum longus
- EDTA
ethylenediaminetetraacetic acid
- FDB
flexor digitorum brevis
- Hepes
N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid
- MHC
myosin heavy chain
- MT-I
morphology type I
- MT-II
morphology type II
- PV
parvalbumin
- SDS-PAGE
polyacrylamide gel electrophoresis under denaturing conditions with sodium dodecyl sulfate
- SERCA
sarcoplasmic reticulum Ca2+ ATPase
- SR
sarcoplasmic reticulum
- Tp
time to peak
Author contributions
J.C.C. and C.C. contributed to the conception and design and J.C.C. conducted the experiments; they also contributed to the analysis and interpretation of the data and drafted the article. P.B. contributed to the analysis and interpretation of the data, critically reviewed the manuscript and made important intellectual contributions. These results are part of the doctoral thesis of J.C.C. performed under the supervision of C.C. All authors gave the final approval of the version to be published. All experiments were done at the Venezuelan Institute for Scientific Research (IVIC).
References
- Appelt D, Buenviaje B, Champ C, Franzini-Armstrong C. Quantitation of ‘junctional feet’ content in two types of muscle fibre from hind limb muscles of the rat. Tissue Cell. 1989;21:783–794. doi: 10.1016/0040-8166(89)90087-6. [DOI] [PubMed] [Google Scholar]
- Bär A, Pette D. Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett. 1988;235:153–155. doi: 10.1016/0014-5793(88)81253-5. [DOI] [PubMed] [Google Scholar]
- Baylor S, Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S, Hollingworth S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol. 2003;551:125–138. doi: 10.1113/jphysiol.2003.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff A, Betz W. Physiological properties of dissociated muscle fibres obtained from innervated and denervated adult rat muscle. J Physiol. 1977;271:25–40. doi: 10.1113/jphysiol.1977.sp011988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough E, Rennie E, Zhang F, Reiser P. Enhanced electrophoretic separation and resolution of myosin heavy chains in mammalian and avian skeletal muscles. Anal Biochem. 1996;233:31–35. doi: 10.1006/abio.1996.0003. [DOI] [PubMed] [Google Scholar]
- Bottinelli R. Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story? Pflügers Arch. 2001;443:6–17. doi: 10.1007/s004240100700. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Betto R, Schiaffino S, Reggiani C. Maximum shortening velocity and coexistence of myosin heavy chain isoforms in single skinned fast fibres of rat skeletal muscle. J Muscle Res Cell Motil. 1994;15:413–419. doi: 10.1007/BF00122115. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol. 2000;73:195–262. doi: 10.1016/s0079-6107(00)00006-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooke M, Kaiser K. Three ‘myosin adenosine triphosphatase’ systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Calderón JC, Bolaños P, Torres SH, Rodríguez-Arroyo G, Caputo C. Different fibre populations distinguished by their calcium transient characteristics in enzymatically dissociated murine flexor digitorum brevis and soleus muscles. J Muscle Res Cell Motil. 2009;30:125–137. doi: 10.1007/s10974-009-9181-1. [DOI] [PubMed] [Google Scholar]
- Capote J, Bolaños P, Schuhmeier R, Melzer W, Caputo C. Calcium transients in developing mouse skeletal muscle fibres. J Physiol. 2005;564:451–464. doi: 10.1113/jphysiol.2004.081034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C, Bolaños P. Effect of mitochondria poisoning by FCCP on Ca2+ signalling in mouse skeletal muscle fibres. Pflügers Arch. 2008;455:733–743. doi: 10.1007/s00424-007-0317-0. [DOI] [PubMed] [Google Scholar]
- Caputo C, Bolaños P, Escobar A. Fast calcium removal during single twitches in amphibian skeletal muscle fibres. J Muscle Res Cell Motil. 1999;20:555–567. doi: 10.1023/a:1005526202747. [DOI] [PubMed] [Google Scholar]
- Caputo C, Bolaños P, González A. Inactivation of Ca2+ transients in amphibian and mammalian muscle fibres. J Muscle Res Cell Motil. 2004;25:315–328. doi: 10.1007/s10974-004-4071-z. [DOI] [PubMed] [Google Scholar]
- Carroll S, Klein M, Schneider M. Decay of calcium transients after electrical stimulation in rat fast- and slow-twitch skeletal muscle fibres. J Physiol. 1997;501:573–588. doi: 10.1111/j.1469-7793.1997.573bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. The relation between intrinsic speed of shortening and duration of the active state of muscle. J Physiol. 1965;180:542–559. doi: 10.1113/jphysiol.1965.sp007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967;193:45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti A, Gorza L, Sorrentino V. Differential distribution of ryanodine receptor type 3 (RyR3) gene product in mammalian skeletal muscles. Biochem J. 1996;316:19–23. doi: 10.1042/bj3160019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E, Margreth A. Characterization study of the ryanodine receptor and of calsequestrin isoforms of mammalian skeletal muscles in relation to fibre types. J Muscle Res Cell Motil. 1994;15:86–101. doi: 10.1007/BF00130421. [DOI] [PubMed] [Google Scholar]
- Dubowitz V, Pearse A. A comparative histochemical study of oxidative enzyme and phophorylase activity in skeletal muscle. Histochemie. 1960;2:105–117. doi: 10.1007/BF00744575. [DOI] [PubMed] [Google Scholar]
- Engel W. The essentiality of histo- and cytochemical studies of skeletal muscle in the investigation of neuromuscular disease. Neurology. 1962;12:778–794. [PubMed] [Google Scholar]
- Eusebi F, Miledi R, Takahashi T. Calcium transients in mammalian muscles. Nature. 1980;284:560–561. doi: 10.1038/284560a0. [DOI] [PubMed] [Google Scholar]
- Ferguson D, Franzini-Armstrong C. The Ca2+ ATPase content of slow and fast twitch fibres of guinea pig. Muscle Nerve. 1988;11:561–570. doi: 10.1002/mus.880110607. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Ferguson D, Champ C. Discrimination between fast- and slow-twitch fibres of guinea pig skeletal muscle using the relative surface density of junctional transverse tubule membrane. J Muscle Res Cell Motil. 1988;9:403–414. doi: 10.1007/BF01774067. [DOI] [PubMed] [Google Scholar]
- Füchtbauer E, Rowlerson A, Gotz K, Friedrich G, Mabuchi K, Gergely J, et al. Direct correlation of parvalbumin levels with myosin isoforms and succinate dehydrogenase activity on frozen sections of rodent muscle. J Histochem Cytochem. 1991;39:355–361. doi: 10.1177/39.3.1825216. [DOI] [PubMed] [Google Scholar]
- Hämäläinen N, Pette D. Patterns of myosin isoforms in mammalian skeletal muscle fibres. Microsc Res Tech. 1995;30:381–389. doi: 10.1002/jemt.1070300505. [DOI] [PubMed] [Google Scholar]
- Hollingworth S, Gee K, Baylor S. Low-affinity Ca2+ indicators compared in measurements of skeletal muscle Ca2+ transients. Biophys J. 2009;97:1864–1872. doi: 10.1016/j.bpj.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth S, Marshall M. A comparative study of charge movement in rat and frog skeletal muscle fibres. J Physiol. 1981;321:583–602. doi: 10.1113/jphysiol.1981.sp014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Hollingworth S, Harkins A, Baylor S. Myoplasmic calcium transients in intact frog skeletal muscle fibres monitored with the fluorescent indicator furaptra. J Gen Physiol. 1991;97:271–301. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G, Walsh T. Calcium currents, charge movement and dihydropyridine binding in fast- and slow-twitch muscles of rat and rabbit. J Physiol. 1987;393:595–617. doi: 10.1113/jphysiol.1987.sp016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E, Pette D. Immunochemical quantification of sarcoplasmic reticulum Ca-ATPase, of calsequestrin and of parvalbumin in rabbit skeletal muscles. Eur J Biochem. 1986;156:489–496. doi: 10.1111/j.1432-1033.1986.tb09607.x. [DOI] [PubMed] [Google Scholar]
- Lee Y, Ondrias K, Duhl A, Ehrlich B, Kim D. Comparison of calcium release from sarcoplasmic reticulum of slow and fast twitch muscles. J Membrane Biol. 1991;122:155–163. doi: 10.1007/BF01872638. [DOI] [PubMed] [Google Scholar]
- Lewis D, Parry D, Rowlerson A. Isometric contractions of motor units and immunohistochemistry of mouse soleus muscle. J Physiol. 1982;325:393–401. doi: 10.1113/jphysiol.1982.sp014157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff A. Dynamic properties of the inferior rectus, extensor digitorum longus, diaphragm and soleus muscles of the mouse. J Physiol. 1981;313:161–171. doi: 10.1113/jphysiol.1981.sp013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff A, Atwood H. Membrane properties and contraction of single muscle fibres in the mouse. Am J Physiol. 1972;222:1435–1440. doi: 10.1152/ajplegacy.1972.222.6.1435. [DOI] [PubMed] [Google Scholar]
- Manno C, Bolaños P, Caputo C. Importance of NCX in the regulation of Ca2+ homeostasis in skeletal muscle. Biophys J. 2008;95:305a. [Google Scholar]
- Maréchal G, Beckers-Bleukx G. Force-velocity relation and isomyosins in soleus muscles from two strains of mice (C57 and NMRI) Pflügers Arch. 1993;424:478–487. doi: 10.1007/BF00374911. [DOI] [PubMed] [Google Scholar]
- Maréchal G, Coulton G, Beckers-Bleukx G. Mechanical power and myosin composition of soleus and extensor digitorum longus muscles of ky mice. Am J Physiol Cell Physiol. 1995;268:C513–C519. doi: 10.1152/ajpcell.1995.268.2.C513. [DOI] [PubMed] [Google Scholar]
- Morrissette J, Xu L, Nelson A, Meissner G, Block B. Characterization of RyR1-slow, a ryanodine receptor specific to slow-twitch skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1889–R1898. doi: 10.1152/ajpregu.2000.279.5.R1889. [DOI] [PubMed] [Google Scholar]
- Paolini C, Quarta M, Nori A, Boncompagni S, Canato M, Volpe P, et al. Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1. J Physiol. 2007;583:767–784. doi: 10.1113/jphysiol.2007.138024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- Ranatunga K, Thomas P. Correlation between shortening velocity, force-velocity relation and histochemical fibre-type composition in rat muscles. J Muscle Res Cell Motil. 1990;11:240–250. doi: 10.1007/BF01843577. [DOI] [PubMed] [Google Scholar]
- Reggiani C, te Kronnie T. RyR isoforms and fibre-type specific expression of proteins controlling intracellular calcium concentration in skeletal muscles. J Muscle Res Cell Motil. 2006;27:327–335. doi: 10.1007/s10974-006-9076-3. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Messi M, Delbono O. Overexpression of IGF-1 exclusively in skeletal muscle prevents age-related decline in the number of dihydropyridine receptors. J Biol Chem. 1998;273:28845–28851. doi: 10.1074/jbc.273.44.28845. [DOI] [PubMed] [Google Scholar]
- Rossi R, Bottinelli R, Sorrentino V, Reggiani C. Response to caffeine and ryanodine receptor isoforms in mouse skeletal muscle. Am J Physiol Cell Physiol. 2001;281:C585–C594. doi: 10.1152/ajpcell.2001.281.2.C585. [DOI] [PubMed] [Google Scholar]
- Royer L, Pouvreau S, Wang Y, Meissner G, Zhou J, Volpe P, et al. Functional and structural consequences of transiently increasing calsequestrin concentration by ∼700% in mouse skeletal muscle. Biophys J. 2008a;94:99–100a. [Google Scholar]
- Royer L, Pouvreau S, Wang Y, Meissner G, Zhou J, Nori A, et al. The effect of severe knock-down of calsequestrin 1 in adult mammalian muscle. Biophys J. 2008b;94:538a. [Google Scholar]
- Royer L, Rios E. Deconstructing calsequestrin. Complex buffering in the calcium store of skeletal muscle. J Physiol. 2009;587:3101–3111. doi: 10.1113/jphysiol.2009.171934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius C, Lu B, Acakpo-Satchivi L, Jacobsen R, Byrnes W, Leinwand L. Myosin heavy chains IIa and IId are functionally distinct in the mouse. J Cell Biol. 1998;141:943–953. doi: 10.1083/jcb.141.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Spangenburg E, Booth F. Molecular regulation of individual skeletal muscle fibre types. Acta Physiol Scand. 2003;178:413–424. doi: 10.1046/j.1365-201X.2003.01158.x. [DOI] [PubMed] [Google Scholar]
- Staron R, Pette D. The continuum of pure and hybrid myosin heavy chain-based fibre types in rat skeletal muscle. Histochemistry. 1993;100:149–153. doi: 10.1007/BF00572901. [DOI] [PubMed] [Google Scholar]
- Stephenson D, Lamb G, Stephenson G. Events of the excitation-contraction-relaxation (E-C-R) cycle in fast- and slow-twitch mammalian muscle fibres relevant to muscle fatigue. Acta Physiol Scand. 1998;162:229–245. doi: 10.1046/j.1365-201X.1998.0304f.x. [DOI] [PubMed] [Google Scholar]
- Stephenson G. Hybrid skeletal muscle fibres: a rare or common phenomenon? Proc Austr Physiol Pharmacol Soc. 2001;32:69–87. doi: 10.1046/j.1440-1681.2001.03505.x. [DOI] [PubMed] [Google Scholar]
- Szentesi P, Jacquemond V, Kovács L, Csernoch L. Intramembrane charge movement and sarcoplasmic calcium release in enzymatically isolated mammalian skeletal muscle fibres. J Physiol. 1997;502:371–384. doi: 10.1111/j.1469-7793.1997.371bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge R, Roy R. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]